Biodegradable Behaviors of Ultrafine-Grained ZE41A Magnesium Alloy in DMEM Solution
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Microstructure of the ECAP-Fabricated ZE41A Alloy


| ECAP Pass | Duration of Incubation Period (h) | Hydrogen Evolution Rate (mL/cm2/h) | Corrosion Rate (mm/y) | ||||
|---|---|---|---|---|---|---|---|
| Incubation Period | Fast Corrosion Period | Steady Period | Incubation Period | Fast Corrosion Period | Steady Period | ||
| 8 | 120 | 0.019 | 1.38 | 0.075 | 1.04 | 75.48 | 4.10 |
| 16 | 96 | 0.022 | 1.45 | 0.050 | 1.20 | 79.30 | 2.73 |
| 60 | 5 | 0.025 | 2.60 | 0.045 | 1.37 | 142.20 | 2.46 |
| ECAP Pass | 8 | 16 | 60 |
|---|---|---|---|
| Ecorr (mV SCE) | −1358.59 | −1352.57 | −1337.43 |
| Icorr (mA/cm−2) | 1.71 × 10−2 | 1.46 × 10−2 | 2.08 × 10−2 |
| CRi (mm/y) | 0.39 | 0.33 | 0.47 |


4. Conclusions
- (1)
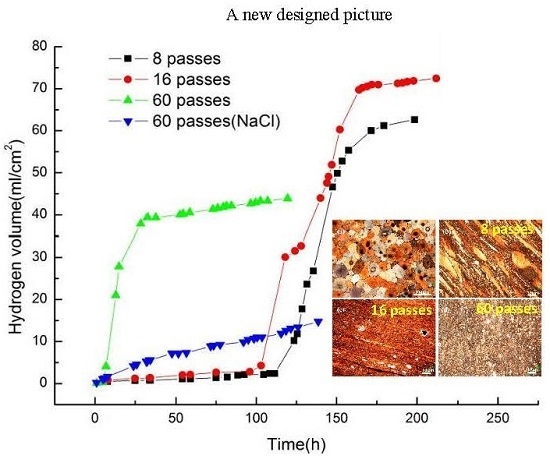
- The degradation process of the UFG Mg alloy was time-dependent in the simulated body fluid. The ZE41A alloy via different ECAP passes went through an incubation period, a fast corrosion period and then a steady period in DMEM solution. With the ECAP pass number increasing, the incubation period was shortened and the biodegradation rate at the steady corrosion period was decreased.
- (2)
- The finer grains and more homogeneous second phases after more ECAP passes can improve the bio-corrosion resistance of the magnesium alloy. It results in a higher resistance toward localized corrosion and a lower biodegradation rate during the steady corrosion period. The UFG alloy for 60 ECAP passes has excellent corrosion resistance in DMEM solution.
- (3)
- ECAP should be taken into consideration as an efficient technique to control the bio-corrosion rate of Mg alloys in the physiological environment and tailor the lifetime of the biodegradable implant, owing to its significant ability to acquire bulk UFG materials with homogeneous second phases.
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Lee, J.Y.; Han, G.S.; Yu, C.K. Effects of impurities on the biodegradation behaviour of pure magnesium. Met. Mater. Int. 2009, 15, 955–961. [Google Scholar] [CrossRef]
- Levesque, J.; Dube, D.; Fiset, M.; Mantovani, D. Materials and properties for coronary stents. Adv. Mater. Process. 2004, 162, 45–48. [Google Scholar]
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Mueller, W.D.; Nascimento, M.L.; de Mele, M.F.L. Critical discussion of the results from different corrosion studies of Mg and Mg alloys for biomaterial applications. Acta Biomater. 2010, 6, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G.J. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater.Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Alvarez-Lopez, M.; Pereda, M.D.; del Valle, J.A.; Fernandez-Lorenzo, M.; Garcia-Alonso, M.C.; Ruano, O.A.; Escudero, M.L. Corrosion behaviour of AZ31 magnesium alloy with different grain sizes in simulated biological fluids. Acta Biomater. 2010, 6, 1763–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.Q.; Shi, Y.N.; Zhang, M.X.; Lu, K. Plastic strain-induced grain refinement in the nanometer scale in a Mg alloy. Acta Mater. 2007, 55, 975–982. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Langdon, T.G. Principles of grain refinement and superplastic flow in magnesium alloys processed by ECAP. Mater. Sci. Eng. 2009, 501, 105–114. [Google Scholar] [CrossRef]
- Feng, X.M.; Ai, T.T. Microstructure evolution and mechanical behavior of AZ31 Mg alloy processed by equal-channel angular pressing. Trans. Nonferrous Metals Soc. China 2009, 19, 293–298. [Google Scholar] [CrossRef]
- Ding, R.; Chung, C.; Chiu, Y. Effect of ECAP on microstructure and mechanical properties of ZE41 magnesium alloy. Mater. Sci. Eng. 2010, 527, 3777–3784. [Google Scholar] [CrossRef]
- Huang, P.; Li, J.; Zhang, S.; Chen, C.; Han, Y.; Liu, N.; Xiao, Y.; Wang, H.; Zhang, M.; Yu, Q.; et al. Effects of lanthanum, cerium, and neodymium on the nuclei and mitochondria of hepatocytes: Accumulation and oxidative damage. Environ. Toxicol. Pharmacol. 2011, 31, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drucker, H.; Vogt, C.; Hort, N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yang, L. Microstructure, mechanical properties and bio-corrosion properties of Mg-Zn-Mn-Ca alloy for biomedical application. Mater. Sci. Eng. 2008, 497, 111–118. [Google Scholar] [CrossRef]
- Gao, J.H.; Guan, S.K.; Ren, Z.W.; Zhu, S.J.; Wang, B. Homogeneous corrosion of high pressure torsion treated Mg-Zn-Ca alloy in simulated body fluid. Mater. Lett. 2011, 65, 691–693. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion-a framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Abidin, N.I.Z.; Martin, D.; Atrens, A. Corrosion of high purity Mg, AZ91, ZE41 and Mg2Zn0.2Mn in Hank’s solution at room temperature. Corros. Sci. 2011, 53, 862–872. [Google Scholar] [CrossRef]
- Galiyev, A.; Kaibyshev, R.; Gottstein, G. Correlation of plastic deformation and dynamic recrystallization in magnesium alloy ZK60. Acta Materi. 2001, 49, 1199–1207. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. Influence of test solutions on in vitro studies of biomedical magnesium alloys. J. Electrochem. Soc. 2010, 157, C238–C243. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Zhang, F.; Ma, A.; Song, D.; Chen, J.; Liu, H.; Qiang, M. Biodegradable Behaviors of Ultrafine-Grained ZE41A Magnesium Alloy in DMEM Solution. Metals 2016, 6, 3. https://doi.org/10.3390/met6010003
Jiang J, Zhang F, Ma A, Song D, Chen J, Liu H, Qiang M. Biodegradable Behaviors of Ultrafine-Grained ZE41A Magnesium Alloy in DMEM Solution. Metals. 2016; 6(1):3. https://doi.org/10.3390/met6010003
Chicago/Turabian StyleJiang, Jinghua, Fan Zhang, Aibin Ma, Dan Song, Jianqing Chen, Huan Liu, and Mingshan Qiang. 2016. "Biodegradable Behaviors of Ultrafine-Grained ZE41A Magnesium Alloy in DMEM Solution" Metals 6, no. 1: 3. https://doi.org/10.3390/met6010003