Establishment of Heat Treatment Process for Modified 440A Martensitic Stainless Steel Using Differential Scanning Calorimetry and Thermo-Calc Calculation
Abstract
:1. Introduction
2. Materials and Experimental Procedure
| Element (wt. %) | C | Mn | P | S | Cr | Mo |
|---|---|---|---|---|---|---|
| Typical 440A | 0.60–0.75 | 1.00 | 0.35 max | 0.030 max | 16.0–18.0 | 0.075 |
| Modified 440A | 0.65 | 0.68 | 0.02 | 0.003 | 12.55 | 0.05 |
3. Results and Discussion
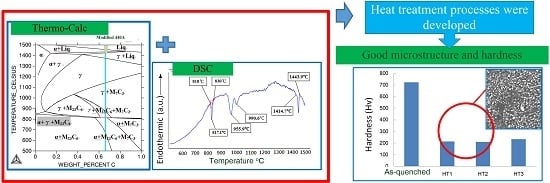
3.1. Thermo-Calc Prediction and DSC Analysis


3.2. Design of Annealing Heat Treatment Processes

3.3. Microstructure Analysis of As-Received, As-Quenched and Heat-Treated Materials




3.4. Microhardness under Various Heat Treatment Processes

4. Conclusions
- The combination of DSC and the Thermo-Calc calculation approach was used to determine various annealing heat treatment parameters (HT1, HT2 and HT3) for the air-quenched samples.
- After the HT1, HT2 and HT3 processes, the volume factions of the larger sized carbide (>5 µm) were 0.6%, 0.8% and 2.0%, respectively.
- After the HT1, HT2 and HT3 processes, hardness values were approximately 210–230 Hv.
- Considering the effects of the microstructure and hardness, the HT1, HT2 or soaking temperatures between HT1 and HT2, were the most recommended processes for the modified Grade 440A MSS.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fan, R.; Gao, M.; Ma, Y.; Zha, X.; Hao, X.; Liu, K. Effect of heat treatment and nitrogen on microstructure and mechanical properties of 1Cr12NiMo martensitic stainless steel. J. Mater. Sci. Technol. 2012, 28, 1059–1066. [Google Scholar] [CrossRef]
- Aksoy, M.; Yilmaz, O.; Korkut, M.H. The effect of strong carbide-forming elements on the adhesive wear resistance of ferritic stainless steel. Wear 2001, 249, 639–646. [Google Scholar] [CrossRef]
- Andres, C.G.; Caruana, G.; Alvarez, L.F. Control of M23C6 Carbides in 0.45C–13Cr martensitic stainless steel by means of three representative heat treatment parameters. Mater. Sci. Eng. A 1998, 241, 211–215. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, Y. Microstructure and mechanical properties of 0.63C–12.7Cr martensitic stainless steel. Taiwan Chung Hua J. Sci. Eng. 2009, 7, 41–46. [Google Scholar]
- Andersson, J.O.; Helander, T.; Hdghmd, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA computational tools. Mater. Sci. 2002, 26, 273–312. [Google Scholar]
- Zhang, J.; Singer, R.F. Hot tearing of nickel-based superalloys during directional solidification. Acta Mater. 2002, 50, 1869–1879. [Google Scholar] [CrossRef]
- Shi, Z.; Dong, J.; Zhang, M.; Zheng, L. Solidification characteristics and segregation behavior of Ni-based superalloy K418 for auto turbocharger turbine. J. Alloy. Compd. 2013, 571, 168–177. [Google Scholar] [CrossRef]
- ASTM Standard E562–11. Standard Test Method for Determining Volume Fraction by Systematic Manual Point Count; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-S.; Hsieh, P.-J. Establishment of Heat Treatment Process for Modified 440A Martensitic Stainless Steel Using Differential Scanning Calorimetry and Thermo-Calc Calculation. Metals 2016, 6, 4. https://doi.org/10.3390/met6010004
Wang H-S, Hsieh P-J. Establishment of Heat Treatment Process for Modified 440A Martensitic Stainless Steel Using Differential Scanning Calorimetry and Thermo-Calc Calculation. Metals. 2016; 6(1):4. https://doi.org/10.3390/met6010004
Chicago/Turabian StyleWang, Huei-Sen, and Pei-Ju Hsieh. 2016. "Establishment of Heat Treatment Process for Modified 440A Martensitic Stainless Steel Using Differential Scanning Calorimetry and Thermo-Calc Calculation" Metals 6, no. 1: 4. https://doi.org/10.3390/met6010004
APA StyleWang, H.-S., & Hsieh, P.-J. (2016). Establishment of Heat Treatment Process for Modified 440A Martensitic Stainless Steel Using Differential Scanning Calorimetry and Thermo-Calc Calculation. Metals, 6(1), 4. https://doi.org/10.3390/met6010004