Influence of Alloying Treatment and Rapid Solidification on the Degradation Behavior and Mechanical Properties of Mg
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Microstructures Characterization
2.4. Immersion Tests
2.5. Mechanical Properties
3. Results and Discussion
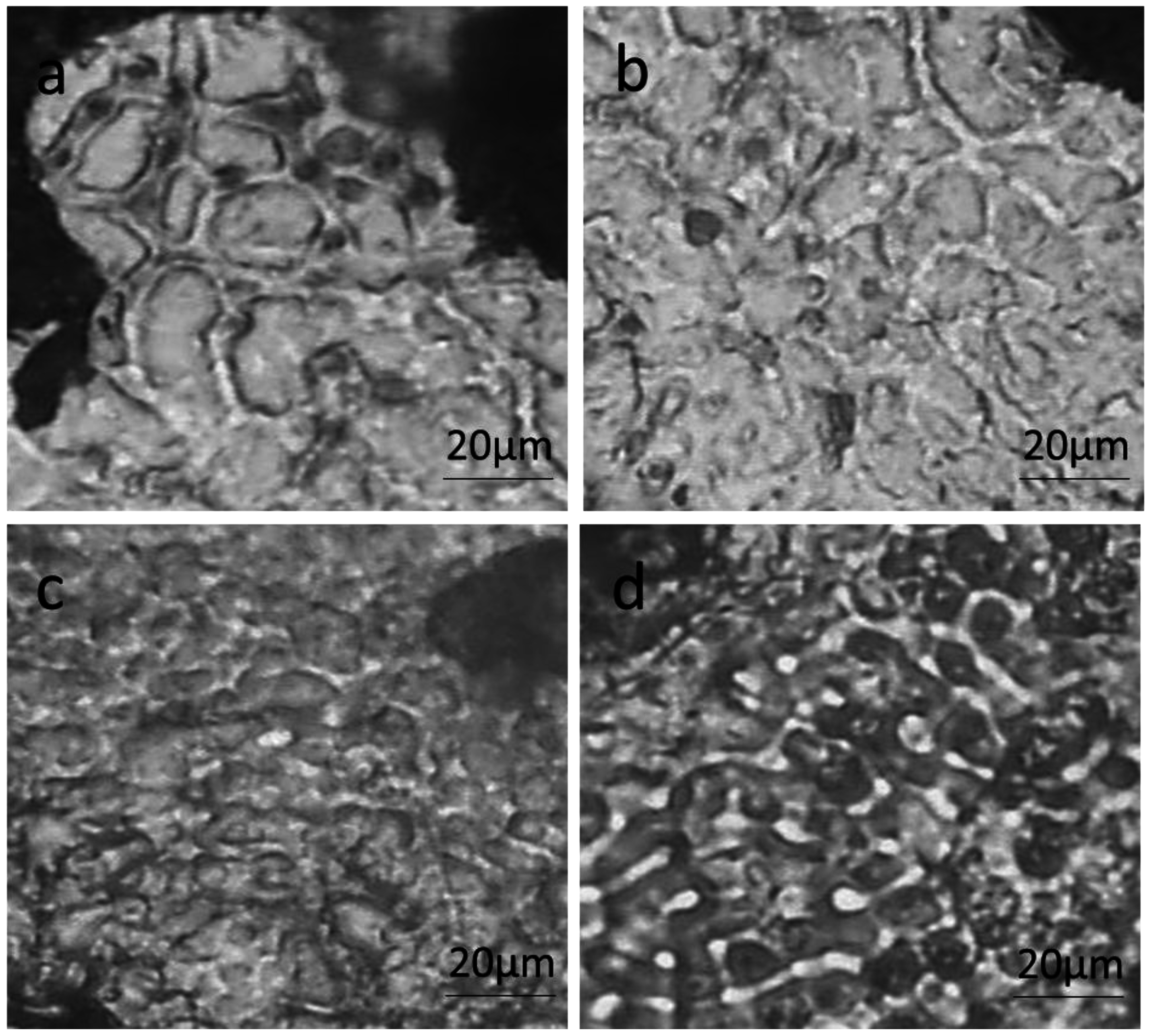
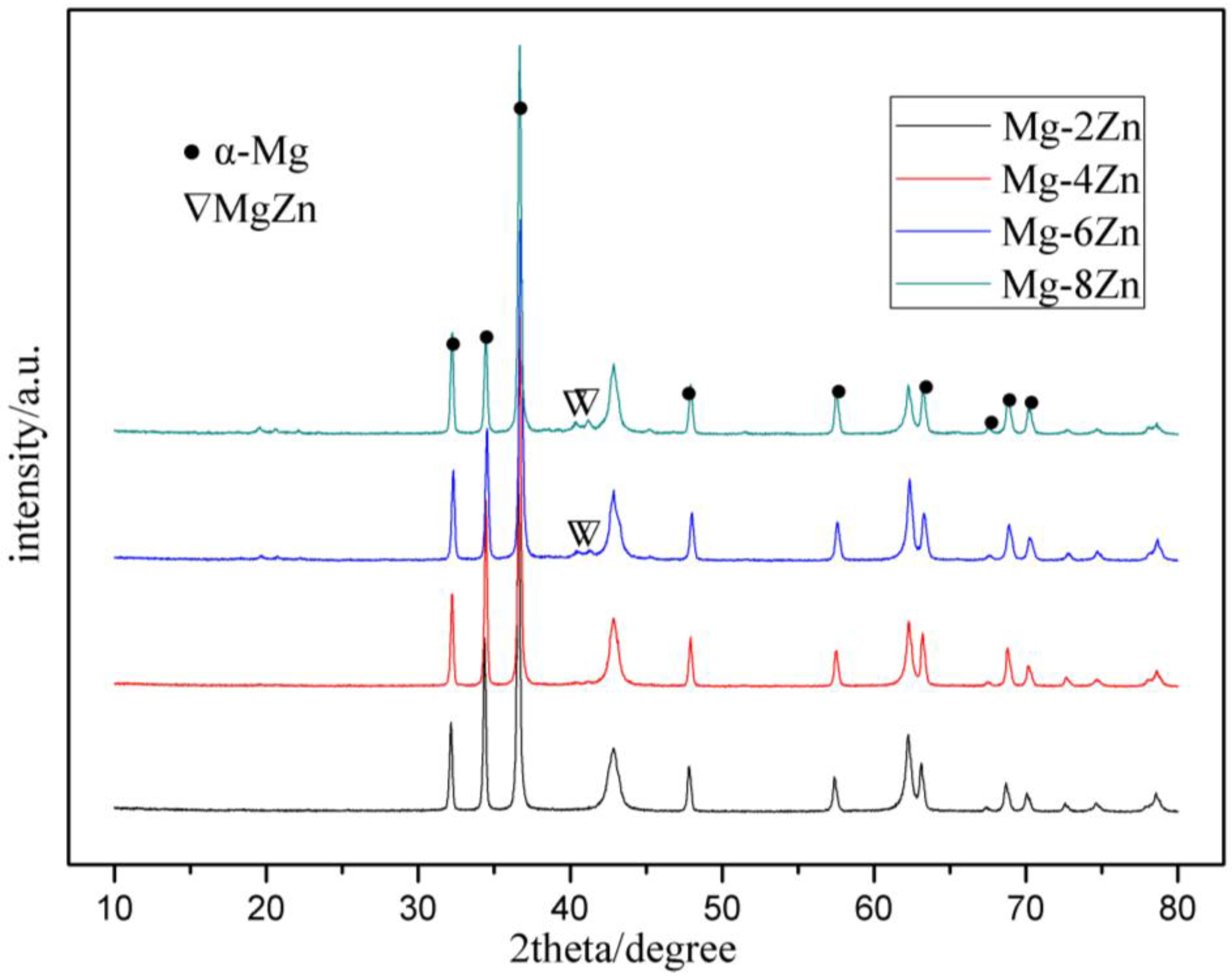
3.1. Microstructures
3.2. Degradation Behavior
3.3. Mechanical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jamesh, M.; Wu, G.S.; Zhao, Y.; Chu, P.K. Electrochemical corrosion behavior of biodegradable Mg-Y-RE and Mg-Zn-Zr alloys in Ringer’s solution and simulated body fluid. Corros. Sci. 2013, 69, 158–163. [Google Scholar] [CrossRef]
- Chou, D.; Hong, D.; Saha, P.; Ferrero, J.; Lee, B.; Tan, Z.Q.; Dong, Z.Y.; Kumt, P.N. In vitro and in vivo corrosion, cytocompatibility and mechanical properties of biodegradable Mg-Y-Ca-Zr alloys as implant materials. Acta Biomater. 2013, 9, 8518–8533. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.M.; Eifler, R.; Bach, F.W.; Maier, H.J. Magnesium degradation products: Effects on tissue and human metabolism. J. Biomed. Mater. Res. A 2014, 102, 3744–3753. [Google Scholar] [CrossRef] [PubMed]
- Tie, D.; Guan, R.; Liu, H.; Cipriano, A.; Liu, Y.; Wang, Q.; Huang, Y.; Hort, N. An in vivo study on the metabolism and osteogenic activity of bioabsorbable Mg-1Sr alloy. Acta Biomater. 2016, 29, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Hampp, C.; Angrisani, N.; Reifenrath, J. Evaluation of the biocompatibility of two magnesium alloys as degradable implant materials in comparison to titanium as non-resorbable material in the rabbit. Mater. Sci. Eng. C 2013, 33, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Song, G.L. Effect of alloying elements on corrosion behaviour of binary magnesium alloys. In Proceedings of the Annual Conference Proceedings: Australasian Corrosion Association, Brisbane, Australia, 10–13 November 2013; pp. 1–12.
- Esmaily, M.; Navid, M.S.; Mortazavib, N.; Svensson, J.E.; Halvarsson, M.; Wessén, M.; Jarfors, A.E.W.; Johansson, L.G. Microstructural characterization of the Mg-Al alloy AM50 produced by a newly developed rheo-casting process. Mater. Charact. 2014, 95, 50–64. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, W.; Tian, P.; Meng, F.; Zhu, H.; Jiang, X.; Liu, X.; Chu, P.K. Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials 2014, 35, 6882–6897. [Google Scholar] [CrossRef] [PubMed]
- Boehlert, C.J.; Knittel, K. The microstructure, tensile properties, and creep behavior of Mg-Zn alloys containing 0–4.4 wt. % Zn. Mater. Sci. Eng. A 2006, 417, 315–321. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, C.; Zhang, Y.; Tao, H.; He, Y.; Jiang, Y. In vitro degradation, hemolysis and MC3T3-E1 cell adhesion of biodegradable Mg-Zn alloy. Mater. Sci. Eng. C 2009, 29, 1907–1912. [Google Scholar] [CrossRef]
- Song, B.; Zhao, X.; Li, S.; Han, C.; Wei, Q.; Wen, S.; Shi, Y. Differences in microstructure and properties between selective laser melting and traditional manufacturing for fabrication of metal parts: A review. Front. Mech. Eng. 2015, 10, 111–125. [Google Scholar] [CrossRef]
- Ng, C.C.; Savalani, M.M.; Lau, M.L. Microstructure and mechanical properties of selective laser melted magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Krakhmalev, P.; Yadroitsava, I. Selective laser melting of Ti6Al4V alloy for biomedical applications: Temperature monitoring and microstructural evolution. J. Alloys Compd. 2014, 583, 404–409. [Google Scholar] [CrossRef]
- Shahzad, M.; Waqas, H.; Qureshi, A.H.; Wagner, L. The roles of Zn distribution and eutectic particles on microstructure development during extrusion and anisotropic mechanical properties in a Mg-Zn-Zr alloy. Mater. Sci. Eng. A 2015, 620, 50–57. [Google Scholar] [CrossRef]
- McKeown, J.T.; Zweiacker, K.; Liu, C.; Coughlin, D.R.; Clarke, A.J.; Baldwin, J.K.; Gibbs, J.W.; Roehling, J.D.; Imhoff, S.D.; Gibbs, P.J. Time-Resolved In Situ Measurements During Rapid Alloy Solidification: Experimental Insight for Additive Manufacturing. JOM 2016, 68, 985–999. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Bian, H.; Zhou, Y.; Shuai, C. System development, formability quality and microstructure evolution of selective laser-melted magnesium. Virtual Phys. Prototyp. 2016, 11, 173–181. [Google Scholar] [CrossRef]
- Freni, A.; Dawoud, B.; Bonaccorsi, L.; Chmielewski, S.; Frazzica, A.; Calabrese, L.; Restuccia, G. Characterization of Zeolite-Based Coatings for Adsorption Heat Pumps; Springer: Messina, Italy, 2015; p. 81. [Google Scholar]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg-Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Vrancken, B.; Thijs, L.; Kruth, J.P. Microstructure and mechanical properties of a novel β titanium metallic composite by selective laser melting. Acta Mater. 2014, 68, 150–158. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, M.; Gao, J.; Hu, J.; Zhang, Y. Corrosion process of pure magnesium in simulated body fluid. Mater. Lett. 2008, 62, 2181–2184. [Google Scholar] [CrossRef]
- Wang, H.; Estrin, Y.; Fu, H.M.; Song, G.L.; Zuberova, Z. The effect of pre-processing and grain structure on the bio-corrosion and fatigue resistance of magnesium alloy AZ31. Adv. Eng. Mater. 2007, 9, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Shan, D.; Chen, R. Effect of second phases on the corrosion behaviour of wrought Mg-Zn-Y-Zr alloy. Corros. Sci. 2010, 52, 1830–1837. [Google Scholar] [CrossRef]
- Rad, H.R.B.; Hamzah, E.; Lotfabadi, A.F. Microstructure and bio-corrosion behavior of Mg-Zn and Mg-Zn-Ca alloys for biomedical applications. Mater. Corros. 2014, 65, 1178–1187. [Google Scholar]
- Hodgskinson, R.; Currey, J.D.; Evans, G.P. Hardness, an indicator of the mechanical competence of cancellous bone. J. Orthop. Res. 1989, 7, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Peng, J.; Peng, Y. The effect of addition of Nd and Ce on the microstructure and mechanical properties of ZM21 Mg alloy. J. Magnes. Alloy. 2013, 1, 94–100. [Google Scholar] [CrossRef]
- Okuda, H.; Horiuchi, T.; Tsukamoto, T.; Ochiai, S.; Yamasaki, M.; Kawamura, Y. Evolution of long-period stacking ordered structures on annealing as-cast Mg85Y9Zn6 alloy ingot observed by synchrotron radiation small-angle scattering. Scr. Mater. 2013, 68, 575–578. [Google Scholar] [CrossRef] [Green Version]






| Laser Power (W) | Laser Scan Speed (mm/s) | Layer Thickness (mm) | Spot Diameter (μm) |
|---|---|---|---|
| 70 | 100 | 0.1–0.2 | 50 |
| Sample | Mg-2Zn | Mg-4Zn | Mg-6Zn | Mg-8Zn |
|---|---|---|---|---|
| Intensity | 320 | 400 | 455 | 486 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wu, P.; Wang, Q.; Yang, Y.; Peng, S.; Zhou, Y.; Shuai, C.; Deng, Y. Influence of Alloying Treatment and Rapid Solidification on the Degradation Behavior and Mechanical Properties of Mg. Metals 2016, 6, 259. https://doi.org/10.3390/met6110259
Chen J, Wu P, Wang Q, Yang Y, Peng S, Zhou Y, Shuai C, Deng Y. Influence of Alloying Treatment and Rapid Solidification on the Degradation Behavior and Mechanical Properties of Mg. Metals. 2016; 6(11):259. https://doi.org/10.3390/met6110259
Chicago/Turabian StyleChen, Jian, Ping Wu, Qiyuan Wang, Youwen Yang, Shuping Peng, Yuanzhuo Zhou, Cijun Shuai, and Youwen Deng. 2016. "Influence of Alloying Treatment and Rapid Solidification on the Degradation Behavior and Mechanical Properties of Mg" Metals 6, no. 11: 259. https://doi.org/10.3390/met6110259





