Formation and Corrosion Resistance of Micro-Arc Oxidation Coating on Equal-Channel Angular Pressed AZ91D Mg Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
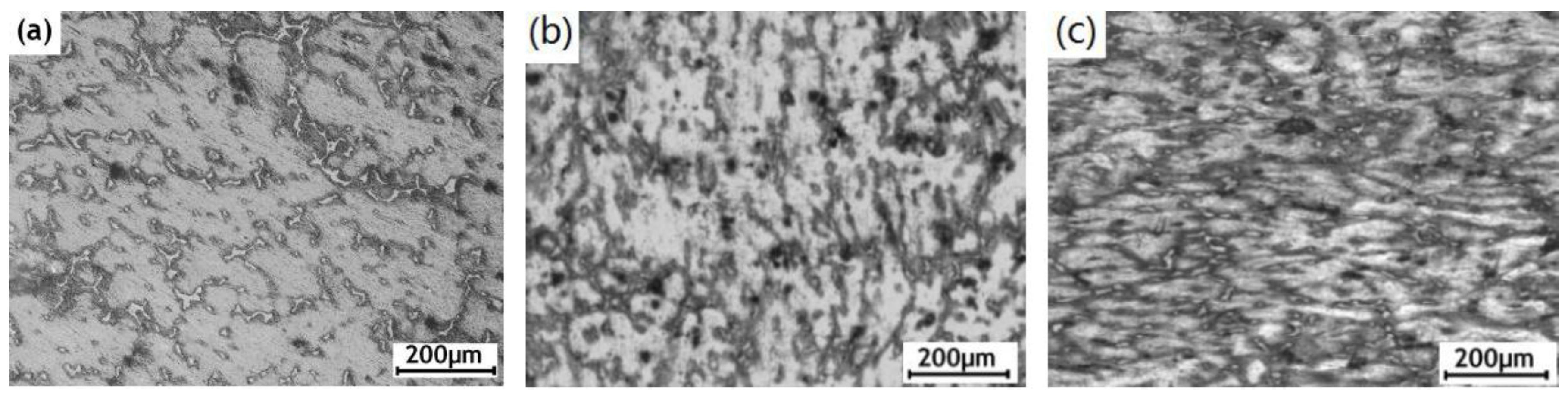
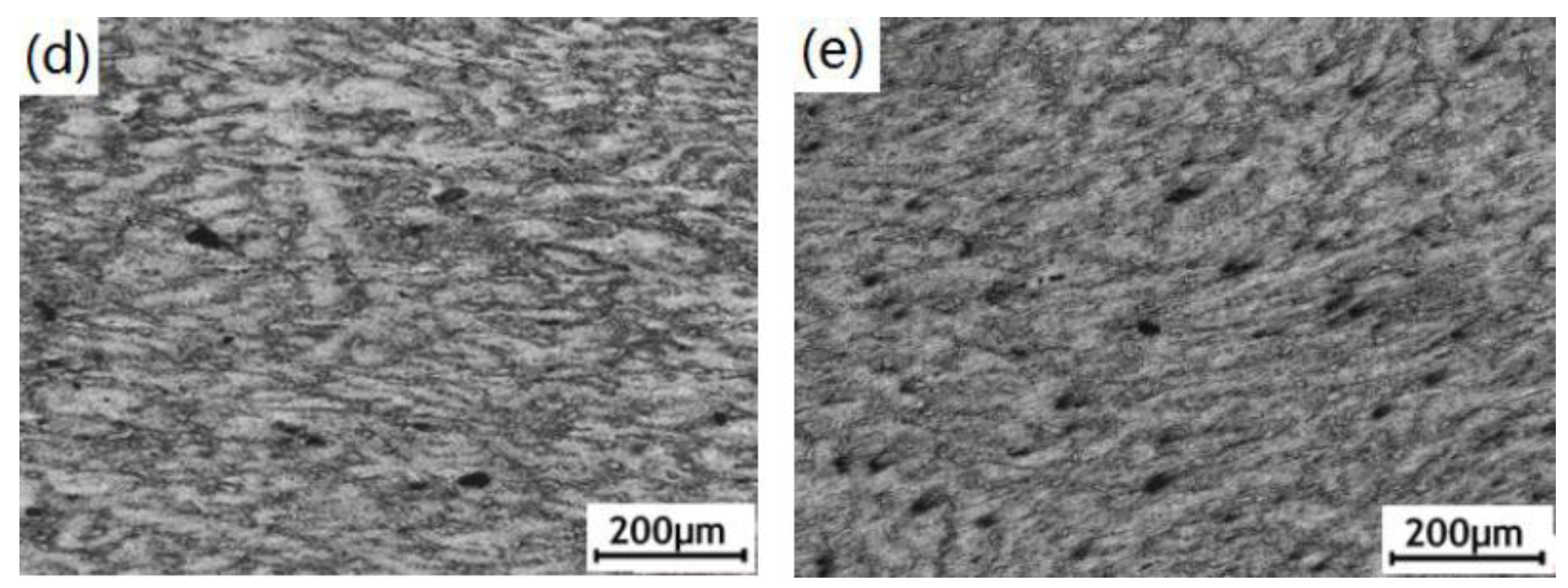
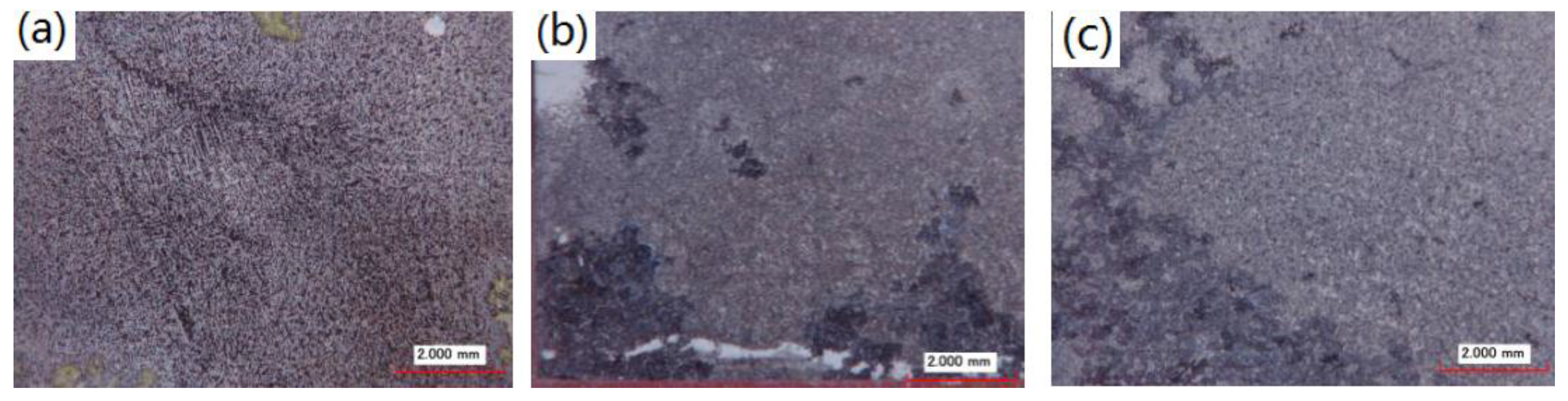
3.1. Microstructure Observation of the ECAPed AZ91D Alloy
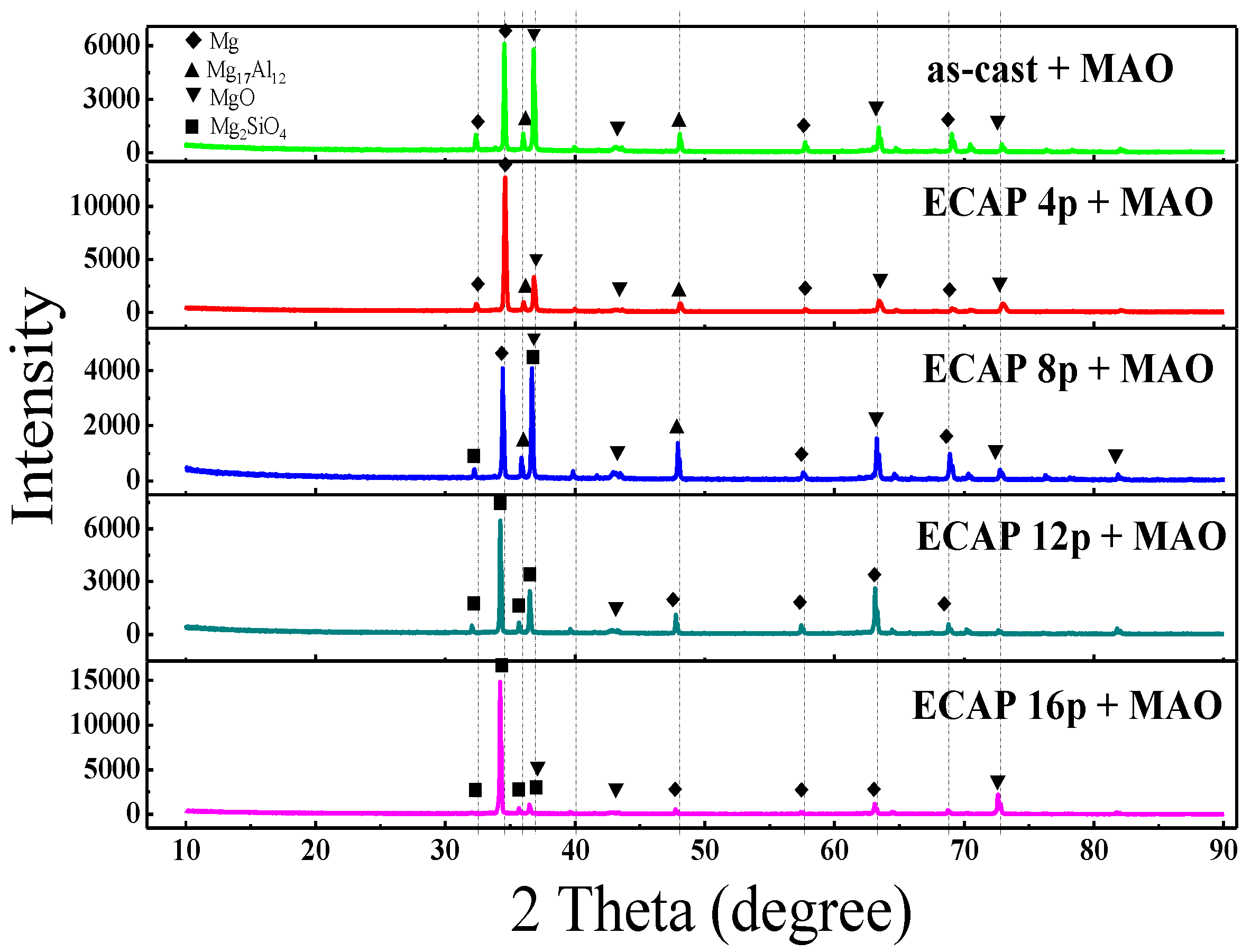
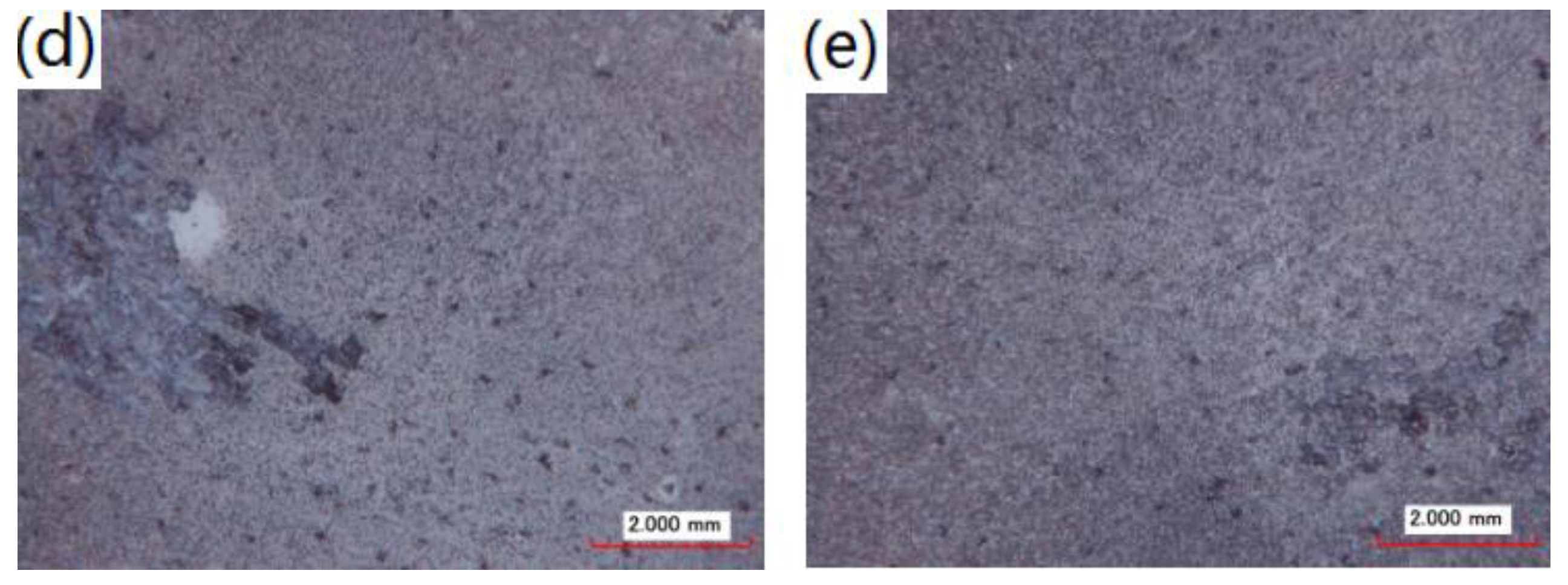
3.2. Formation and Morphologies of the MAO Coatings
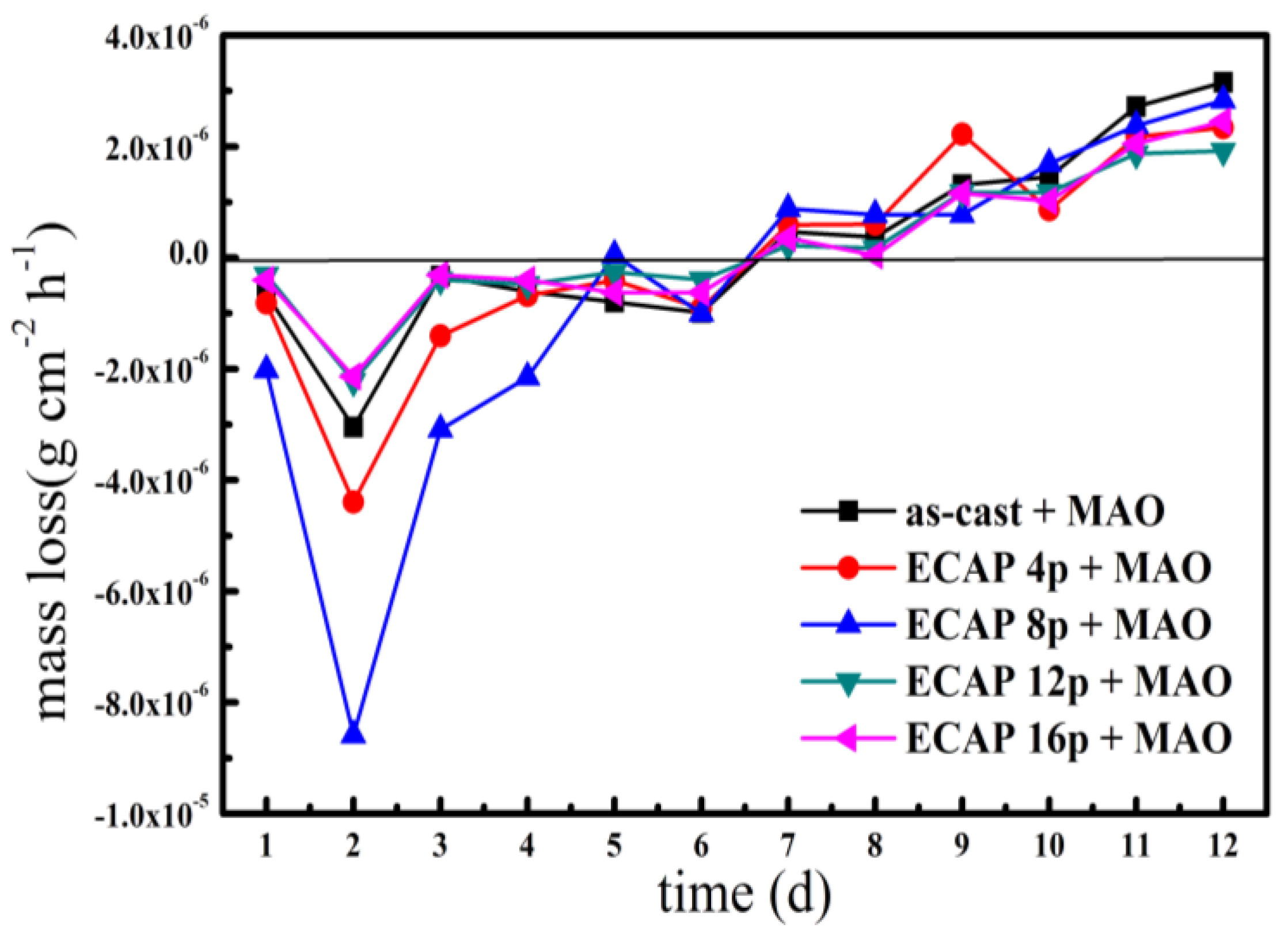
3.3. Corrosion Behavior in the Constant Immersion Test
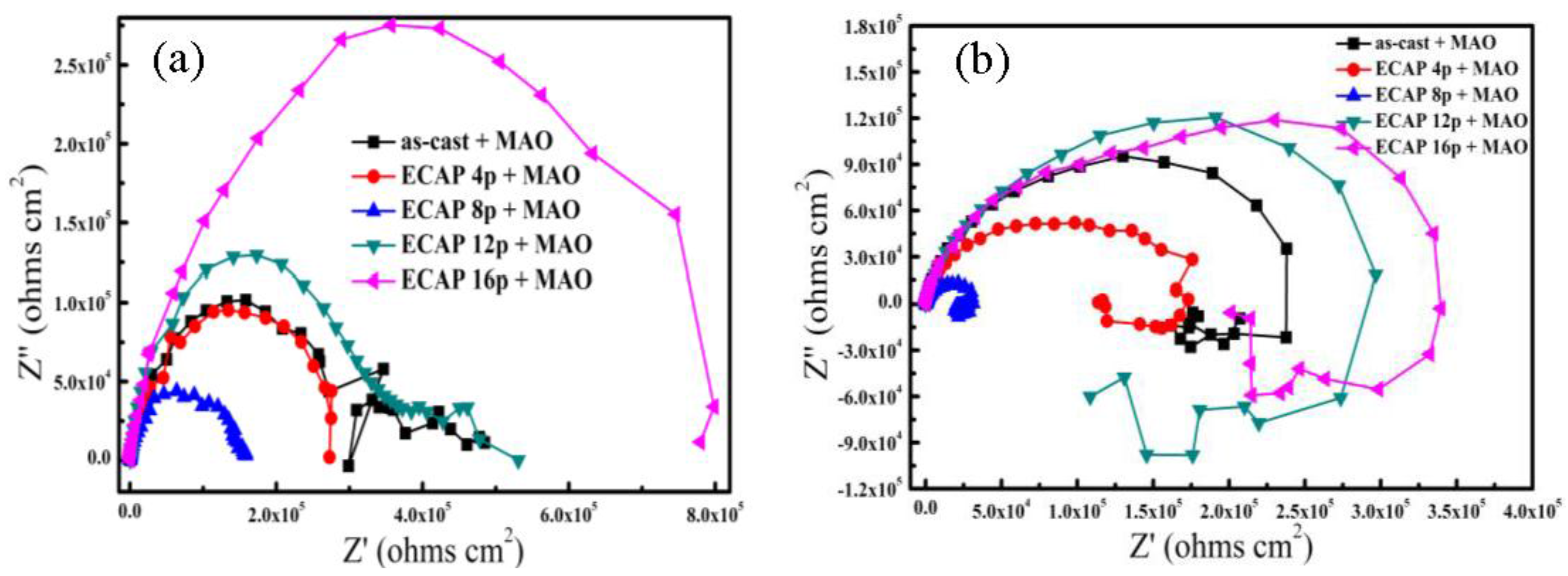
3.4. Electrochemical Corrosion Behaviors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mordike, B.L.; Ebert, T. Magnesium properties-applications-potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Chang, L.R.; Cao, F.H.; Cai, J.S.; Liu, W.J.; Zhang, Z.; Zhang, J.Q. Influence of electric parameters on MAO of AZ91D magnesium alloy using alternative square-wave power source. Trans. Nonferr. Met. Soc. China 2011, 21, 307–316. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Ma, A.B.; Gou, X.F.; Jiang, J.H.; Song, D.; Zhu, Y.T. Superior mechanical properties of ZK60 mg alloy processed by equal channel angular pressing and rolling. Mater. Sci. Eng. A 2015, 630, 45–50. [Google Scholar]
- Máthis, K.; Gubicza, J.; Nam, N.H. Microstructure and mechanical behavior of AZ91 Mg alloy processed by equal channel angular pressing. J. Alloy. Compd. 2005, 394, 194–199. [Google Scholar] [CrossRef]
- Stolyarov, V.V.; Zhu, Y.T.; Alexandrov, I.V.; Lowe, T.C.; Valiev, R.Z. Grain refinement and properties of pure Ti processed by warm ECAP and cold rolling. Mater. Sci. Eng. A 2003, 343, 43–50. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, I.; Kim, J.; Kim, Y.S.; Semiatin, S.L. Microstructure development during equal-channel angular pressing of titanium. Acta Mater. 2003, 51, 983–996. [Google Scholar] [CrossRef]
- Chen, B.; Lin, D.; Jin, L.; Zeng, X.; Lu, C. Equal-channel angular pressing of magnesium alloy AZ91 and its effects on microstructure and mechanical properties. Mater. Sci. Eng. A 2008, 483–484, 113–116. [Google Scholar] [CrossRef]
- Minárik, P.; Král, R.; Janeček, M. Effect of ECAP processing on corrosion resistance of AE21 and AE42 magnesium alloys. Appl. Surf. Sci. 2013, 281, 44–48. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.B.; Jiang, J.H.; Lin, P.H.; Yang, D.H.; Fan, J.F. Corrosion behavior of bulk ultra-fine grained AZ91D magnesium alloy fabricated by equal-channel angular pressing. Corros. Sci. 2011, 53, 362–373. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.H.; Zhao, Y.H.; Ma, A.B.; Wen, D.J.; Zhu, Y.T. Effect of equal-channel angular pressing and aging on corrosion behavior of ZK60 magnesium alloy. Trans. Nonferr. Met. Soc. China 2015, 25, 3909–3920. [Google Scholar] [CrossRef]
- Fan, X.Z.; Wang, Y.; Zhou, B.L.; Gu, L.J.; Huang, W.Z.; Cao, X.Q. Preparation and corrosion resistance of MAO/Ni–P composite coat on Mg alloy. Appl. Surf. Sci. 2013, 277, 272–280. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yan, C.W.; Wang, F.H.; Lou, H.Y.; Cao, C. Study on the environmentally friendly anodizing of AZ91D magnesium alloy. Surf. Coat. Technol. 2002, 161, 36–43. [Google Scholar] [CrossRef]
- Laleh, M.; Kargar, F.; Rouhaghdam, A.S. Formation of a compact oxide layer on AZ91D magnesium alloy by microarc oxidation via addition of cerium chloride into the MAO electrolyte. J. Coat. Technol. Res. 2011, 8, 765–771. [Google Scholar] [CrossRef]
- Chen, C.Z.; Dong, Q.; Wang, D.G. Microstructure and element distributions of ceramic-like coatings on the AZ91 alloy by micro-arc oxidation. Surf. Rev. Lett. 2006, 13, 63–68. [Google Scholar] [CrossRef]
- Krishna, L.R.; Poshal, G.; Sundararajan, G. Influence of electrolyte chemistry on morphology and corrosion resistance of micro arc oxidation coatings deposited on magnesium. Metall. Mater. Trans. A 2010, 41, 3499–3508. [Google Scholar] [CrossRef]
- Jin, F.; Chu, P.; Xu, G.; Zhao, J.; Tang, D.; Tong, H. Structure and mechanical properties of magnesium alloy treated by micro-arc discharge oxidation using direct current and high-frequency bipolar pulsing modes. Mater. Sci. Eng. A 2006, 435–436, 123–126. [Google Scholar]
- Chen, J.; Zeng, R.C.; Huang, W.J.; Zheng, Z.Q.; Wang, Z.L.; Wang, J. Characterization and wear resistance of macro-arc oxidation coating on magnesium alloy AZ91 in simulated body fluids. Trans. Nonferr. Met. Soc. China 2008, 18, S261–S364. [Google Scholar] [CrossRef]
- Jiang, J.; Zhou, Q.; Yu, J.; Ma, A.; Song, D.; Lu, F.; Zhang, L.; Yang, D.; Chen, J. Comparative analysis for corrosion resistance of micro-arc oxidation coatings on coarse-grained and ultra-fine grained AZ91D Mg Alloy. Surf. Coat. Tech. 2013, 216, 259–266. [Google Scholar]
- Song, D.; Ma, A.; Jiang, J.; Lin, P.; Yang, D.; Fan, J. Corrosion behavior of equal-channel-angular-pressed pure magnesium in NaCl aqueous solution. Corros. Sci. 2010, 52, 481–490. [Google Scholar] [CrossRef]
- American Society for Testing and Materials International. G31, Recommended Practice for Laboratory Immersion Corrosion Testing of Metals; Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 1977; p. 722. [Google Scholar]
- Malik, K.N.B. Discontinuous and continuous precipitation in magnesium-aluminium type alloys. J. Alloy. Compd. 2009, 477, 870–876. [Google Scholar] [CrossRef]
- Segal, V.M. Materials processing by simple shear. Mater. Sci. Eng. A 1995, 197, 157–164. [Google Scholar]
- Tong, W.P.; Tao, N.R.; Wang, Z.B.; Lu, J.; Lu, K. Nitriding iron at lower temperatures. Science 2003, 299, 686–688. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zheng, M.Y.; Wu, K. Microarc oxidation coating formed on SiCw/AZ91 magnesium matrix composite and its corrosion resistance. Mater. Lett. 2005, 59, 1727–1731. [Google Scholar] [CrossRef]
- Apelfeld, A.V.; Bespalova, O.V.; Borisov, A.M.; Dunkin, O.N.; Goryaga, N.G.; Kulikauskas, V.S.; Romanovsky, E.A.; Semenov, S.V.; Souminov, I.V. Application of the particle backscattering methods for the study of new oxide protective coatings at the surface of Al and Mg alloys. Nucl. Instrum. Methods Phys. Res. B 2000, 161–163, 553–557. [Google Scholar]
- Song, Y.L.; Liu, Y.H.; Yu, S.R.; Zhu, X.Y.; Wang, Q. Plasma electrolytic oxidation coating on AZ91 magnesium alloy modified by neodymium and its corrosion resistance. Appl. Surf. Sci. 2008, 254, 3014–3020. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhang, J.; Zhang, Z. Characteristics of anodic coatings oxidized to different voltage on AZ91D Mg alloy by micro-arc oxidization technique. Mater. Corros. 2005, 56, 88–92. [Google Scholar] [CrossRef]
- Duan, H.P.; Yan, C.W.; Wang, F.H. Effect of electrolyte additives on performance of plasma electrolytic oxidation films formed on magnesium alloy AZ91D. Electrochim. Acta 2007, 52, 3785–3793. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, S.; An, P.; Luo, J. Study on the deterioration process of a chromium-free conversion coating on AZ91D magnesium alloy in NaCl solution. Appl. Surf. Sci. 2006, 253, 468–475. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, A.; Lu, F.; Zhou, Q.; Jiang, J.; Song, D.; Chen, J.; Zheng, Y. Formation and Corrosion Resistance of Micro-Arc Oxidation Coating on Equal-Channel Angular Pressed AZ91D Mg Alloy. Metals 2016, 6, 308. https://doi.org/10.3390/met6120308
Ma A, Lu F, Zhou Q, Jiang J, Song D, Chen J, Zheng Y. Formation and Corrosion Resistance of Micro-Arc Oxidation Coating on Equal-Channel Angular Pressed AZ91D Mg Alloy. Metals. 2016; 6(12):308. https://doi.org/10.3390/met6120308
Chicago/Turabian StyleMa, Aibin, Fumin Lu, Qi Zhou, Jinghua Jiang, Dan Song, Jianqing Chen, and Yuxi Zheng. 2016. "Formation and Corrosion Resistance of Micro-Arc Oxidation Coating on Equal-Channel Angular Pressed AZ91D Mg Alloy" Metals 6, no. 12: 308. https://doi.org/10.3390/met6120308




