Effect of Immersion in Simulated Body Fluid on the Mechanical Properties and Biocompatibility of Sintered Fe–Mn-Based Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Preparation
2.2. Press and Sinter
2.3. Immersion Testing
2.4. Cytotoxicity Testing
2.5. Characterisation
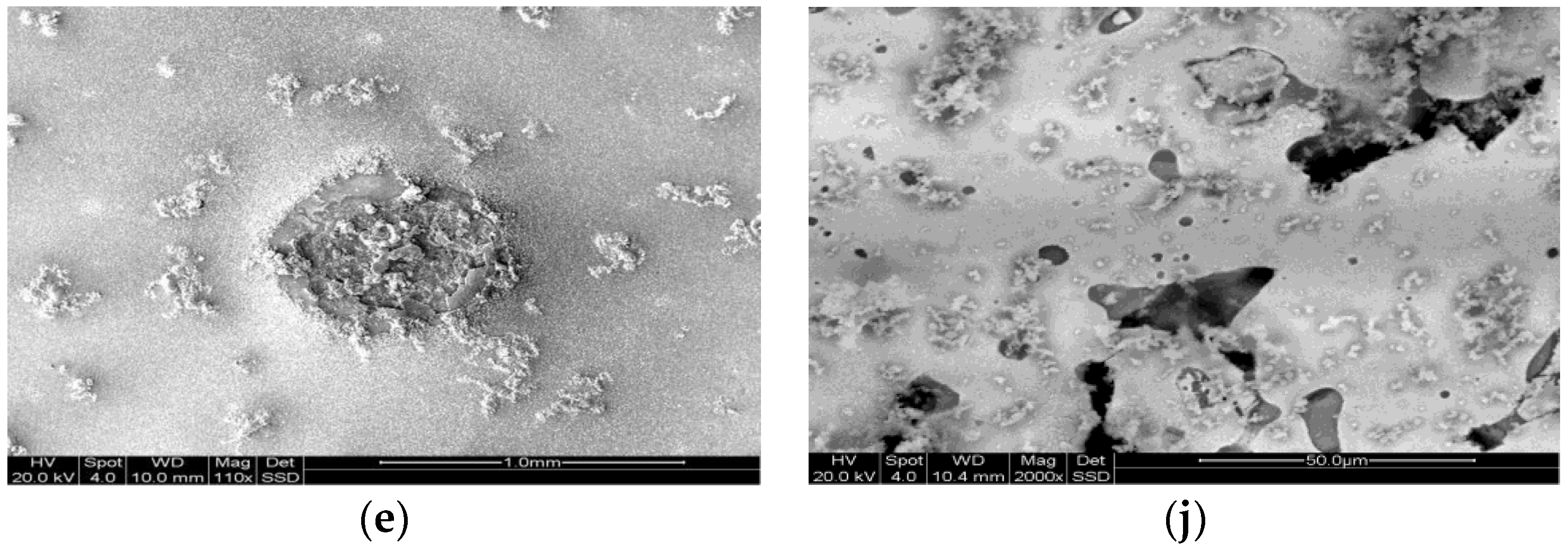
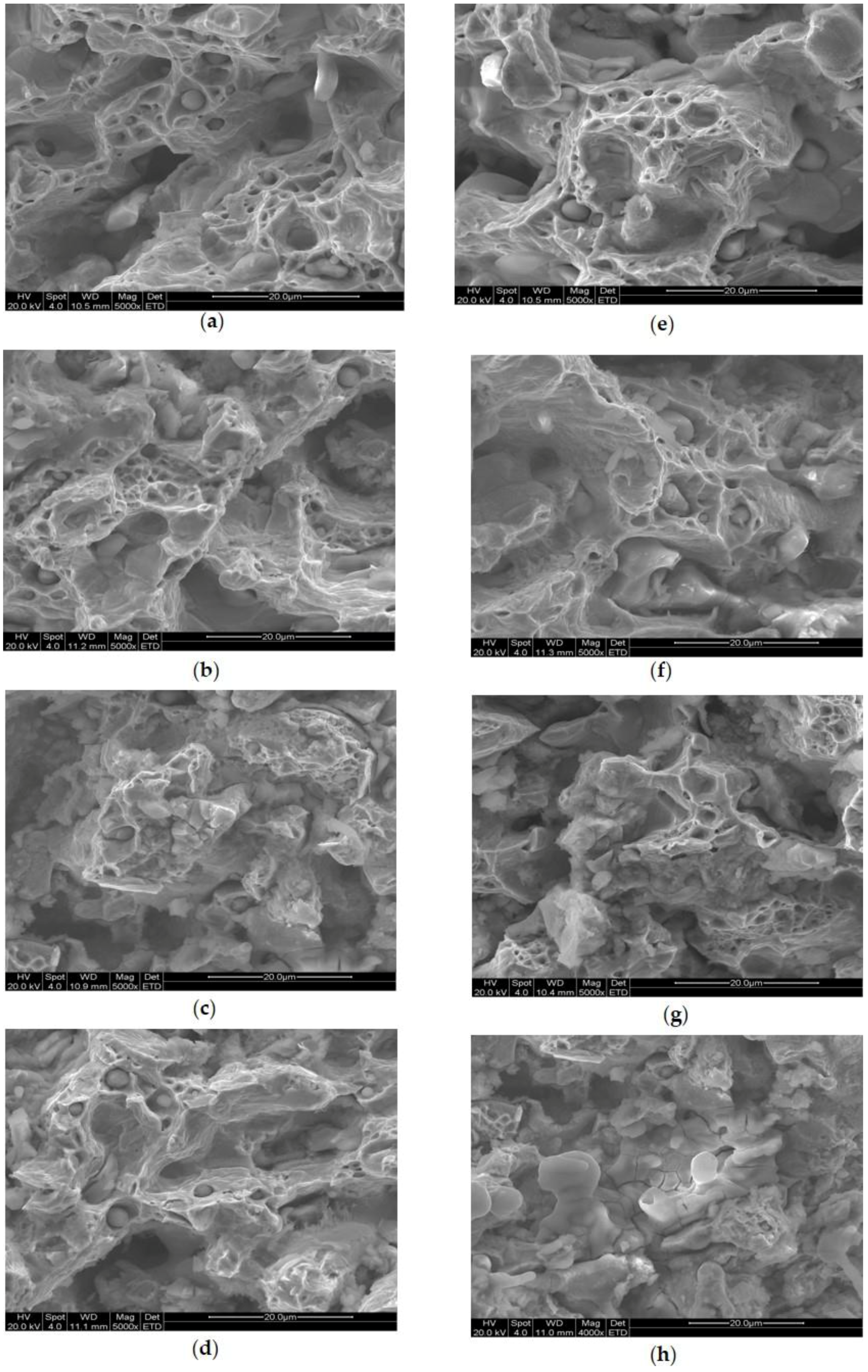
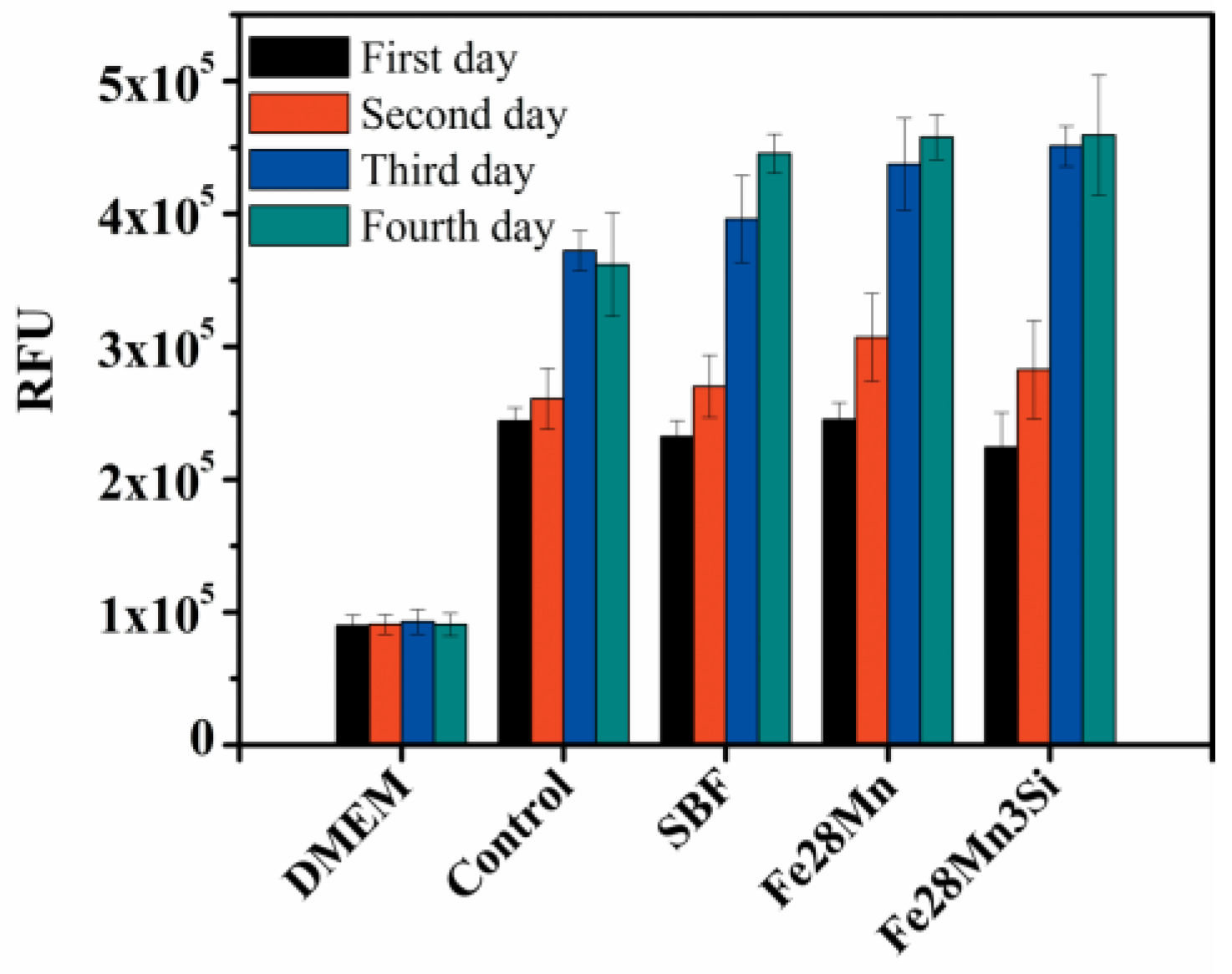
3. Results and Discussion
3.1. Chemical Composition of the Sintered Fe–Mn-Based Alloys
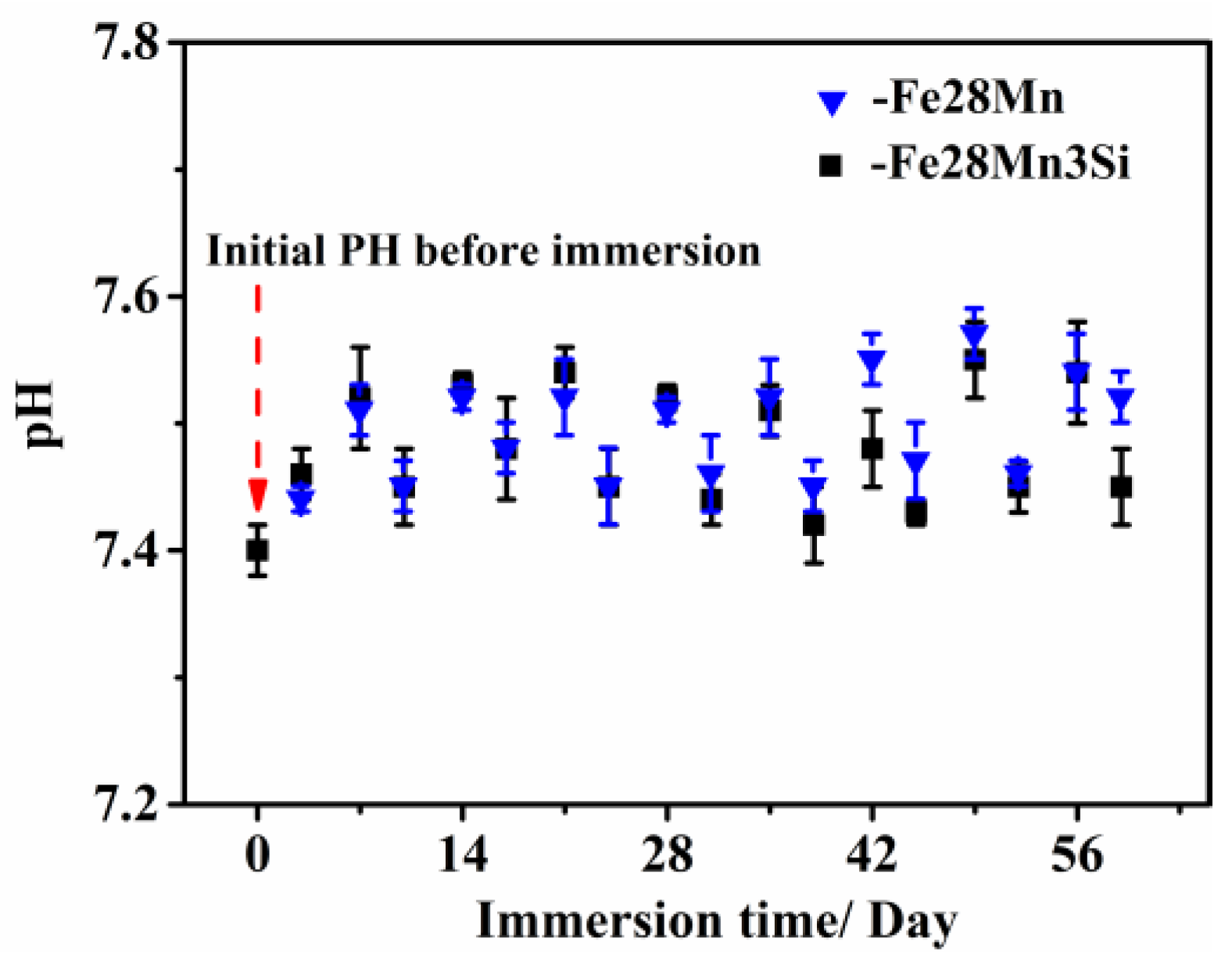
3.2. pH Monitoring
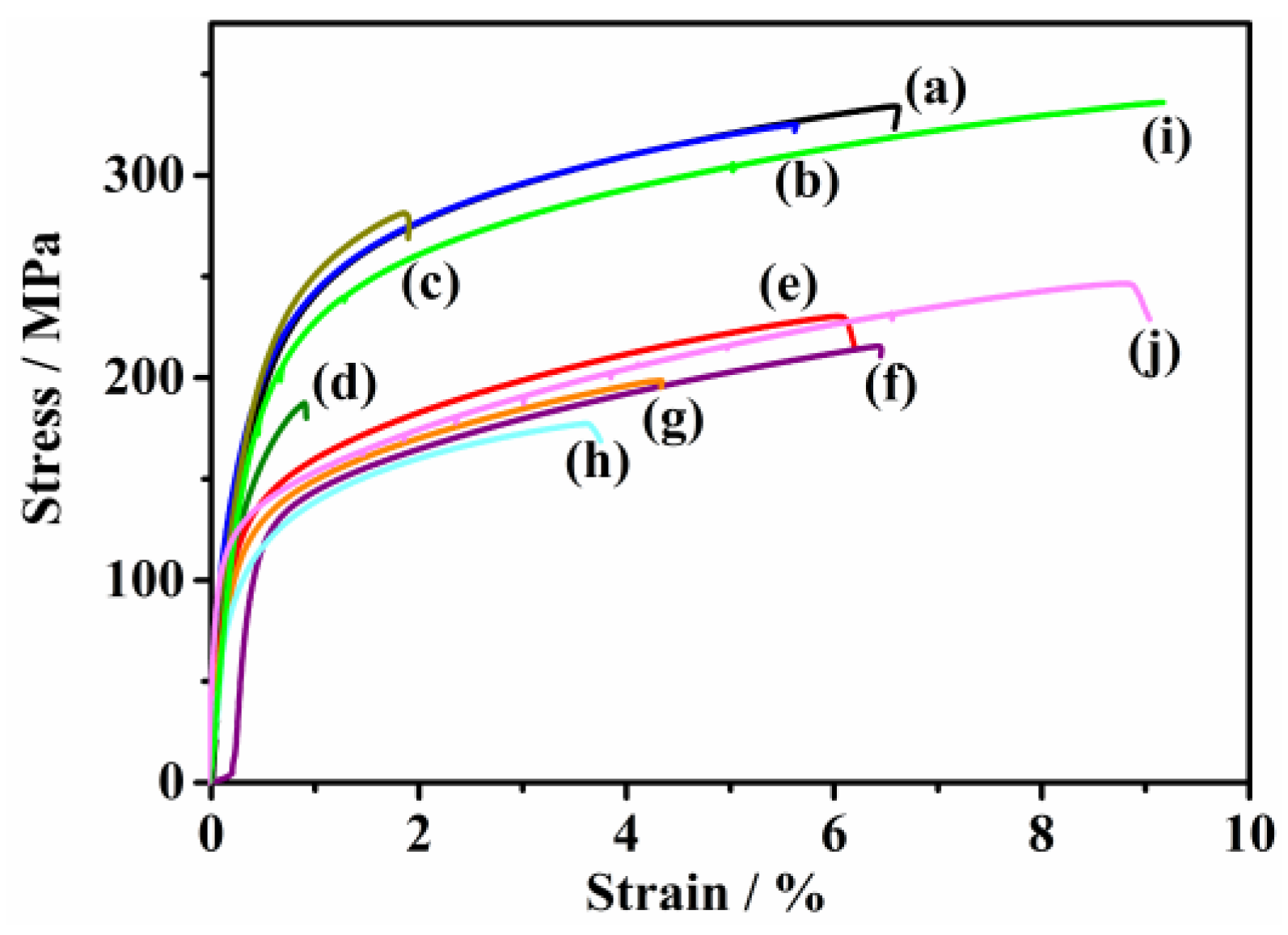
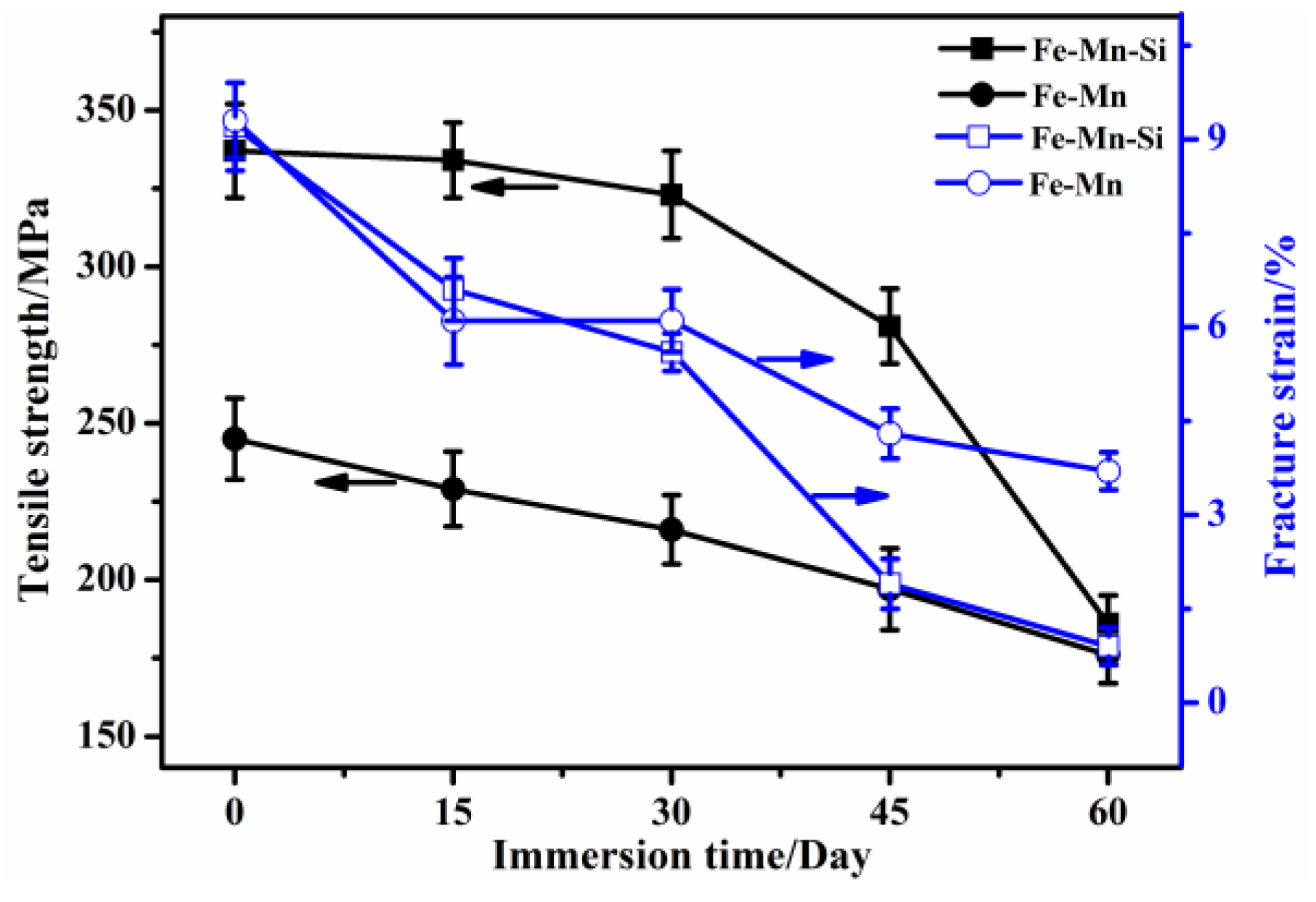
3.3. Tensile Properties of the Immersed Fe–Mn-Based Alloys
3.4. Biocompatibility of the Sintered Fe–Mn-Based Alloys
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hermawan, H.; Dubé, D.; Mantovani, D. Developments in metallic biodegradable stents. Acta Biomater. 2010, 6, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Electroformed pure iron as a new biomaterial for degradable stents: In vitro degradation and preliminary cell viability studies. Acta Biomater. 2010, 6, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hodgson, M.A.; Cao, P. A comparative study of powder metallurgical (PM) and wrought Fe–Mn–Si alloys. Mater. Sci. Eng. A 2015, 630, 116–124. [Google Scholar] [CrossRef]
- Schinhammer, M.; Gerber, I.; Hänzi, A.C.; Uggowitzer, P.J. On the cytocompatibility of biodegradable Fe–based alloys. Mater. Sci. Eng. C 2013, 33, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Yang, Y.; Virtanen, S.; Boccaccini, A. Iron and iron-based alloys for temporary cardiovascular applications. J. Mater. Sci. Mater. Med. 2015, 26, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hodgson, M.A.; Cao, P. Microstructure and degradation behavior of forged Fe–Mn–Si alloys. Int. J. Mod. Phys. B 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Song, G.; Song, S. A possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Mintz, G.S.; Hoffmann, R.; Mehran, R.; Pichard, A.D.; Kent, K.M.; Satler, L.F.; Popma, J.J.; Leon, M.B. In-stent restenosis: The Washington Hospital Center experience. Amer. J. Cardiol. 1998, 81, 7E–13E. [Google Scholar] [CrossRef]
- Hermawan, H.; Purnama, A.; Dube, D.; Couet, J.; Mantovani, D. Fe–Mn alloys for metallic biodegradable stents: Degradation and cell viability studies. Acta Biomater. 2010, 6, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Moravej, M.; Dubé, D.; Fiset, M.; Mantovani, D. Degradation behaviour of metallic biomaterials for degradable stents. Adv. Mater. Res. 2007, 15, 113–118. [Google Scholar] [CrossRef]
- Tang, Y.C.; Katsuma, S.; Fujimoto, S.; Hiromoto, S. Electrochemical study of Type 304 and 316L stainless steels in simulated body fluids and cell cultures. Acta Biomater. 2006, 2, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Oak, J.J.; Inoue, A. Attempt to develop Ti-based amorphous alloys for biomaterials. Mater. Sci. Eng. A 2007, 449, 220–224. [Google Scholar] [CrossRef]
- Takaichi, A.; Nakamoto, T.; Joko, N.; Nomura, N.; Tsutsumi, Y.; Migita, S.; Doi, H.; Kurosu, S.; Chiba, A.; Wakabayashi, N. Microstructures and mechanical properties of Co–29Cr–6Mo alloy fabricated by selective laser melting process for dental applications. J. Mech. Behav. Biomed. 2013, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Nasab, M.B.; Hassan, M.R. Metallic biomaterials of knee and hip-A review. Trends Biomater. Artif. Organs 2010, 24, 69–82. [Google Scholar]
- Milošev, I.; Pišot, V.; Campbell, P. Serum levels of cobalt and chromium in patients with Sikomet metal–metal total hip replacements. J. Orthop. Res. 2005, 23, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Wegener, B.; Sievers, B.; Utzschneider, S.; Müller, P.; Jansson, V.; Rößler, S.; Nies, B.; Stephani, G.; Kieback, B.; Quadbeck, P. Microstructure, cytotoxicity and corrosion of powder-metallurgical iron alloys for biodegradable bone replacement materials. Mater. Sci. Eng. B 2011, 176, 1789–1796. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Moravej, M.; Mantovani, D. Biodegradable metals for cardiovascular stent application: Interests and new opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef] [PubMed]
- Andani, M.T.; Moghaddam, N.S.; Haberland, C.; Dean, D.; Miller, M.J.; Elahinia, M. Metals for bone implants. Part 1. Powder metallurgy and implant rendering. Acta Biomater. 2014, 10, 4058–4070. [Google Scholar] [CrossRef] [PubMed]
- Hanks, C.T.; Wataha, J.C.; Sun, Z. In vitro models of biocompatibility: A review. Dent. Mater. 1996, 12, 186–193. [Google Scholar] [CrossRef]
- Říhová, B. Biocompatibility of biomaterials: Hemocompatibility, immunocompatiblity and biocompatibility of solid polymeric materials and soluble targetable polymeric carriers. Adv. Drug Del. Rev. 1996, 21, 157–176. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M. Biodegradable Fe–based alloys for use in osteosynthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Drynda, A.; Hassel, T.; Bach, F.W.; Peuster, M. In vitro and in vivo corrosion properties of new iron–manganese alloys designed for cardiovascular applications. J. Biomed. Mater. Res. B 2015, 103, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Oriňaková, R.; Oriňak, A.; Giretová, M.; Medvecký, L.U.; Kupková, M.; Hrubovčáková, M.; Maskal’ová, I.; Macko, J.; Kal’avský, F. A study of cytocompatibility and degradation of iron-based biodegradable materials. J. Biomater. Appl. 2016, 30, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.; Northup, S.; Anderson, J. Biological testing of biomaterials. In Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Elsevier: San Diego, CA, USA, 2004; pp. 355–360. [Google Scholar]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Dubé, D.; Mantovani, D. Degradable metallic biomaterials: Design and development of Fe–Mn alloys for stents. J. Biomed. Mater. Res. A 2010, 93, 1–11. [Google Scholar] [CrossRef] [PubMed]
- International Standardization Organization. International Standardization Organization. ISO 10993-5: Biological evaluation of medical devices. In Part 5: Tests for in Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Xu, Z.; Hodgson, M.A.; Cao, P. Effects of Mechanical Milling and Sintering Temperature on the Densification, Microstructure and Tensile Properties of the Fe–Mn–Si Powder Compacts. J. Mater. Sci. Technol. 2016, 32, 1161–1170. [Google Scholar] [CrossRef]
- Šalak, A.; Selecká, M.; Bureš, R. Manganese in ferrous powder metallurgy. Powder Metall. Prog. 2001, 1, 41–58. [Google Scholar]
- Kirkland, N.; Birbilis, N.; Staiger, M. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Schinhammer, M.; Hofstetter, J.; Wegmann, C.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. On the immersion testing of degradable implant materials in simulated body fluid: Active pH regulation using CO2. Adv. Eng. Mater. 2013, 15, 434–441. [Google Scholar] [CrossRef]
- Holmes, J.; Queeney, R. Fatigue crack initiation in a porous steel. Powder Metall. 1985, 28, 231–235. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, X.; Fu, D.; Zhang, L.; Li, C.; Cui, Z. Stress-strain behavior of porous NiTi alloys prepared by powders sintering. Mater. Sci. Eng. A 2005, 408, 264–268. [Google Scholar] [CrossRef]
- Kubicki, B. Stress Concentration at Pores in Sintered Materials. Powder Metall. 1995, 38, 295–298. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.; Ruan, L. In vitro investigation of Fe30Mn6Si shape memory alloy as potential biodegradable metallic material. Mater. Lett. 2011, 65, 540–543. [Google Scholar] [CrossRef]
- Cerit, M.; Genel, K.; Eksi, S. Numerical investigation on stress concentration of corrosion pit. Eng. Fail. Anal. 2009, 16, 2467–2472. [Google Scholar] [CrossRef]
- Anderson, T.L.; Anderson, T. Fracture Mechanics: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Gong, S.; Wang, H.; Sun, Q.; Xue, S.-T.; Wang, J.-Y. Mechanical properties and in vitro biocompatibility of porous zein scaffolds. Biomaterials 2006, 27, 3793–3799. [Google Scholar] [CrossRef] [PubMed]
- Čapek, J.; Kubásek, J.; Vojtěch, D.; Jablonská, E.; Lipov, J.; Ruml, T. Microstructural, mechanical, corrosion and cytotoxicity characterization of the hot forged FeMn30 (wt %) alloy. Mater. Sci. Eng. C 2016, 58, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zheng, Y. In vitro study on newly designed biodegradable Fe-X composites (X = W, CNT) prepared by spark plasma sintering. J. Biomed. Mater. Res. B 2013, 101, 485–497. [Google Scholar] [CrossRef] [PubMed]
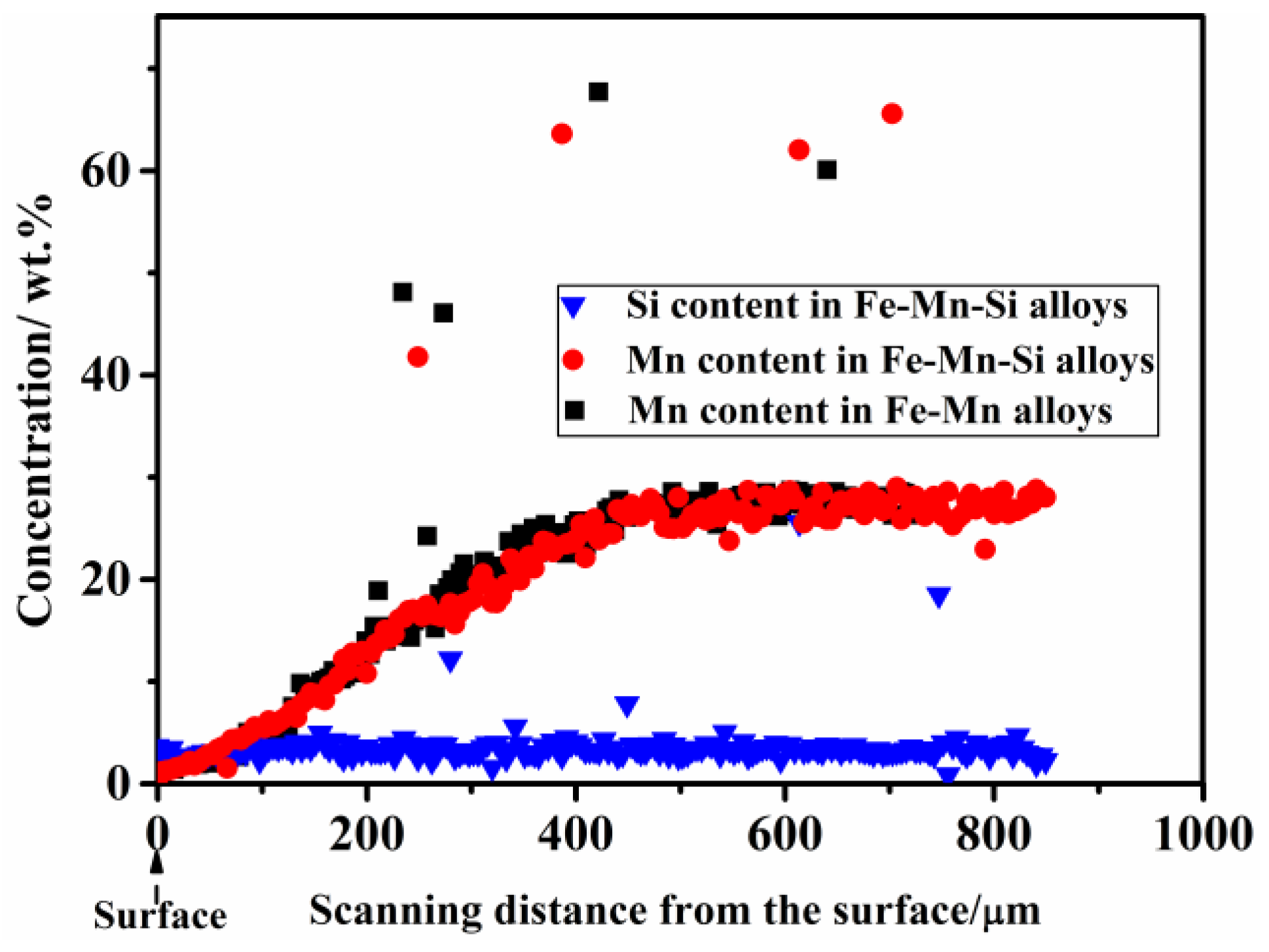


| Type of Samples | Thickness of Mn DR a (μm) | Chemical Composition (wt %) b | |||
|---|---|---|---|---|---|
| Mn | Si | O | Fe | ||
| Fe–Mn | 450 ± 38 | 27.5 ± 0.6 | — | 0.3 ± 0.1 | Bal. |
| Fe–Mn–Si | 480 ± 35 | 27.4 ± 0.5 | 3.1 ± 0.2 | 0.3 ± 0.1 | Bal. |
| Type of Samples | Immersion Time (Day) | Chemical Composition (wt %) | ||||||
|---|---|---|---|---|---|---|---|---|
| O | P | Ca | C | Mn | Si | Fe | ||
| Fe–Mn | 15 | 5.3 ± 0.1 | 2.5 ± 0.1 | 0.8 ± 0.1 | 2.6 ± 0.1 | 23.9 ± 0.5 | - | Bal. |
| 30 | 9.6 ± 0.2 | 2.8 ± 0.2 | 1.2 ± 0.2 | 3 ± 0.4 | 23.5 ± 0.7 | - | Bal. | |
| 45 | 15.8 ± 0.3 | 5.1 ± 0.2 | 2.1 ± 0.2 | 3.5 ± 0.3 | 22.8 ± 0.6 | - | Bal. | |
| 60 | 18.5 ± 0.7 | 4.5 ± 0.6 | 2.1 ± 0.3 | 3.3 ± 0.6 | 23.2 ± 0.8 | - | Bal. | |
| Fe–Mn–Si | 15 | 5.1 ± 0.2 | 2.9 ± 0.1 | 1.5 ± 0.1 | 3.5 ± 0.2 | 24 ± 0.5 | 2.3 ± 0.2 | Bal. |
| 30 | 10.8 ± 0.5 | 3.3 ± 0.4 | 1.4 ± 0.3 | 2.8 ± 0.3 | 23.1 ± 0.6 | 2 ± 0.4 | Bal. | |
| 45 | 23.7 ± 0.4 | 9.9 ± 0.3 | 3 ± 0.2 | 4 ± 0.4 | 17.5 ± 0.2 | 1.2 ± 0.2 | Bal. | |
| 60 | 25.9 ± 0.9 | 9.5 ± 0.5 | 2.7 ± 0.2 | 3.5 ± 0.2 | 18 ± 0.4 | 1.5 ± 0.5 | Bal. | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Hodgson, M.A.; Cao, P. Effect of Immersion in Simulated Body Fluid on the Mechanical Properties and Biocompatibility of Sintered Fe–Mn-Based Alloys. Metals 2016, 6, 309. https://doi.org/10.3390/met6120309
Xu Z, Hodgson MA, Cao P. Effect of Immersion in Simulated Body Fluid on the Mechanical Properties and Biocompatibility of Sintered Fe–Mn-Based Alloys. Metals. 2016; 6(12):309. https://doi.org/10.3390/met6120309
Chicago/Turabian StyleXu, Zhigang, Michael A. Hodgson, and Peng Cao. 2016. "Effect of Immersion in Simulated Body Fluid on the Mechanical Properties and Biocompatibility of Sintered Fe–Mn-Based Alloys" Metals 6, no. 12: 309. https://doi.org/10.3390/met6120309
APA StyleXu, Z., Hodgson, M. A., & Cao, P. (2016). Effect of Immersion in Simulated Body Fluid on the Mechanical Properties and Biocompatibility of Sintered Fe–Mn-Based Alloys. Metals, 6(12), 309. https://doi.org/10.3390/met6120309







