Effects of Reflow Time on the Interfacial Microstructure and Shear Behavior of the SAC/FeNi-Cu Joint
Abstract
:1. Introduction
2. Experiments
3. Results and Discussion
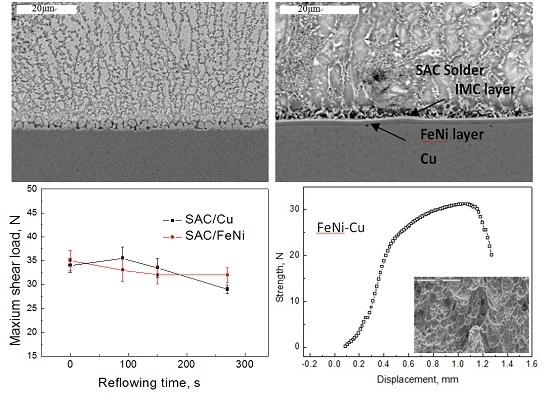
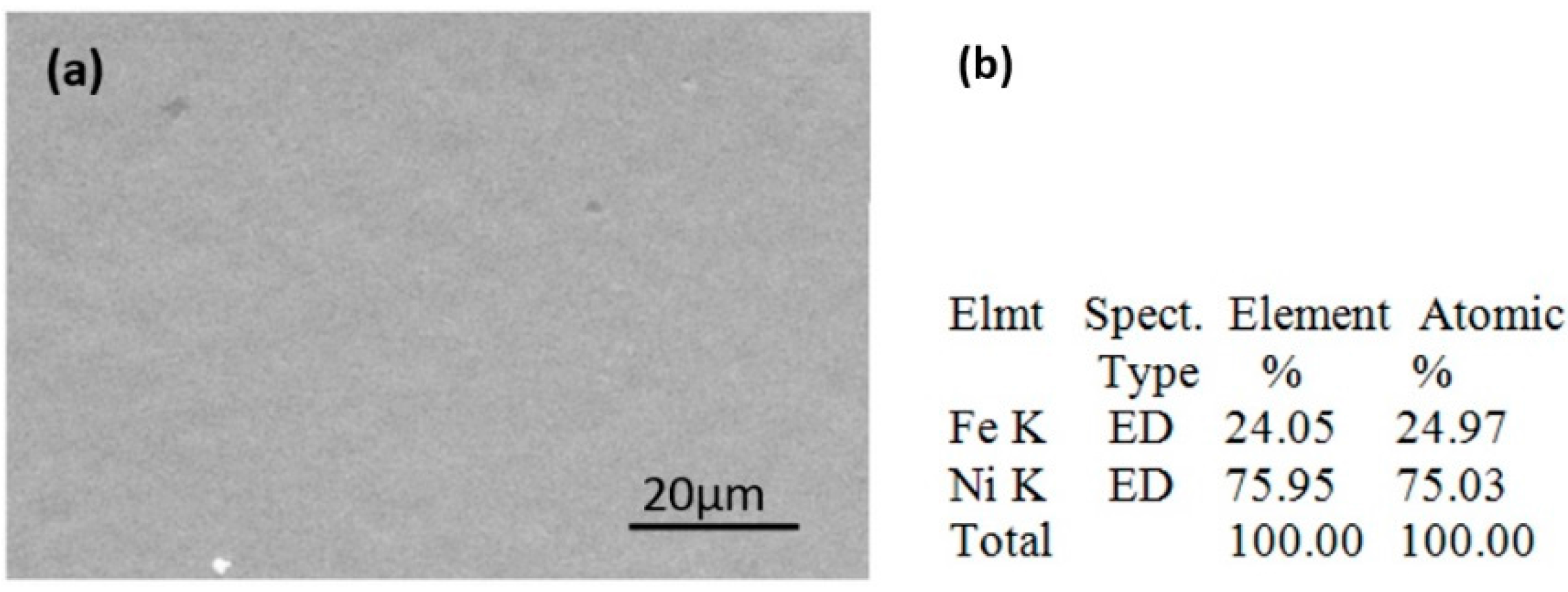
3.1. Interfacial Microstructure After Reflow
3.2. Interfacial Microstructures After Reflowing for Different Time
3.3. IMCs Growth Depending on Reflow Time
3.4. Shear Properties and the Fracture Behavior
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, C.P.; Luo, S.J.; Zhang, Z.Q. Microelectronics: Flip the Chip. Science 2000, 290, 2269–2270. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.E.; Yang, S.C.; Kao, C.R. Interfacial reaction issues for lead-free electronic solders. J. Mater. Sci. 2007, 18, 155–174. [Google Scholar]
- Laurila, T.; Vuorinen, V.; Kivilahti, J.K. Interfacial reactions between lead-free solders and common base materials. Mater. Sci. Eng. R 2005, 49, 1–60. [Google Scholar] [CrossRef]
- Ghosh, G. Interfacial microstructure and the kinetics of interfacial reaction in diffusion couples between Sn-Pb solder and Cu/Ni/Pd metallization. Acta Mater. 2014, 53, 205–211. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; Lai, Q.; Li, C.; Shang, J.K. Evolution of solder wettability with growth of interfacial compounds on tinned FeNi plating. J. Mater. Sci. Mater. Electron. 2011, 22, 1234–1238. [Google Scholar] [CrossRef]
- He, M.; Chen, Z.; Qi, G.J. Solid state interfacial reaction of Sn-37Pb and Sn-3.5Ag solders with Ni-P under bump metallization. Acta Mater. 2004, 52, 2047–2056. [Google Scholar] [CrossRef]
- Guo, J.J.; Zhang, L.; Xian, A.P.; Shang, J.K. Solderability of Electrodeposited Fe-Ni Alloys with Eutectic SnAgCu Solder. J. Mater. Sci. Technol. 2007, 23, 811–816. [Google Scholar]
- Dariavach, N.; Callahan, P.; Liang, J.; Fournelle, R. Intermetallic growth kinetics for Sn-Ag, Sn-Cu, and Sn-Ag-Cu lead-free solders on Cu, Ni, and Fe-42Ni substrates. J. Electron. Mater. 2006, 35, 1581–1592. [Google Scholar] [CrossRef]
- Zhu, Q.S.; Guo, J.J.; Shang, P.J.; Wang, J.G.; Shang, J.K. Effects of Aging on Interfacial Microstructure and Reliability Between SnAgCu Solder and FeNi/Cu UBM. Adv. Eng. Mater. 2010, 12, 497–503. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Yan, J.; Du, J.; Gan, G.; Yi, J. Direct Electrodeposition of Fe-Ni Alloy Films on Silicon Substrate. Rare Met. Mater. Eng. 2014, 43, 2966–2968. [Google Scholar]
- Hwang, C.W.; Suganuma, K.; Saiz, E.; Tomsia, A.P. Wetting and interface integrity of Sn-Ag-Bi solder/Fe-42% Ni alloy system. Trans. JWRI 2001, 30, 167–172. [Google Scholar]
- Suganuma, K. Advances in lead-free electronics soldering. Curr. Opin. Solid State Mater. Sci. 2001, 5, 55–64. [Google Scholar] [CrossRef]
- Hwang, C.W.; Kim, K.S.; Suganuma, K. Interfaces in lead-free solder. J. Electron. Mater. 2003, 32, 1249–1256. [Google Scholar] [CrossRef]
- Hwang, C.W.; Suganuma, K.; Lee, J.G.; Mori, H. Interface microstructure between Fe-42Ni alloy and pure Sn. J. Mater. Res. 2003, 18, 1202–1210. [Google Scholar] [CrossRef]
- Ozaki, H.; Yamamoto, T.; Sano, T.; Hirose, A.; Kobayashi, K.F.; Ishio, M.; Shiomi, K.; Hashimoto, A. Formation of micro joints with high-melting point through rapid reaction between Sn-3.5Ag solder and Ni-Fe alloy substrate. In Proceedings of Materials Science & Technology Conference and Exhibition (MS & T′06), Cincinnati, OH, USA, 15–19 October 2006; pp. 1415–1424.
- Yen, Y.W.; Hsiao, H.M.; Lin, S.W.; Lin, C.K.; Lee, C. Interfacial reaction in Sn/Fe-xNi couples. J. Electron. Mater. 2011, 41, 144–152. [Google Scholar] [CrossRef]
- Yen, Y.W.; Syu, R.S.; Chen, C.M.; Jao, C.C.; Chen, G.D. Interfacial reactions of Sn-58Bi and Sn-0.7Cu lead-free solders with Alloy 42 substrate. Microelectron. Reliab. 2014, 54, 233–238. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, T.Y.; Chen, Y.X.; Wang, J.X. Current induced interfacial microstructure and strength weakening of SAC/FeNi-Cu connection. Appl. Mech. Mater. 2014, 651–653, 11–15. [Google Scholar] [CrossRef]
- Li, J.F.; Agyakwa, P.A.; Johnson, C.M. Interfacial reaction in Cu/Sn/Cu system during the transient liquid phase soldering process. Acta Mater. 2011, 59, 1198–1211. [Google Scholar] [CrossRef]
- Vuorinen, V.; Yu, H.; Laurila, T.; Kivilahti, J.K. Formation of Intermetallic Compounds Between Liquid Sn and Various CuNix Metallizations. J. Electron. Mater. 2008, 37, 792–805. [Google Scholar] [CrossRef]






| Markers | Elements Composition (at. %) | Possible Phases | ||||
|---|---|---|---|---|---|---|
| Fe | Ni | Cu | Ag | Sn | ||
| 1 | 0.40 * | 15.87 | 39.98 | 0.13 * | 43.63 | (Cu,Ni)6Sn5 |
| 2 | 0.00 * | 11.28 | 23.89 | 2.52 | 62.31 | (Cu,Ni)6Sn5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wu, X.; Wang, X.; Huang, H. Effects of Reflow Time on the Interfacial Microstructure and Shear Behavior of the SAC/FeNi-Cu Joint. Metals 2016, 6, 109. https://doi.org/10.3390/met6050109
Chen Y, Wu X, Wang X, Huang H. Effects of Reflow Time on the Interfacial Microstructure and Shear Behavior of the SAC/FeNi-Cu Joint. Metals. 2016; 6(5):109. https://doi.org/10.3390/met6050109
Chicago/Turabian StyleChen, Yunxia, Xulei Wu, Xiaojing Wang, and Hai Huang. 2016. "Effects of Reflow Time on the Interfacial Microstructure and Shear Behavior of the SAC/FeNi-Cu Joint" Metals 6, no. 5: 109. https://doi.org/10.3390/met6050109