The Effect of Iron Content on Glass Forming Ability and Thermal Stability of Co–Fe–Ni–Ta–Nb–B–Si Bulk Metallic Glass
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- The critical casting thicknesses of the alloys decrease with iron content due to the increase in liquidus temperatures.
- The critical casting thicknesses of the alloys show a very good correlation with reduced glass transition temperature, Tg/Tl and Tx/(Tl − Tg).
- Thermal properties and microhardnesses of the alloys do not change with iron content because of the fact that cohesive energies of the Co–Co- and Fe–Fe bonds are almost the same.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Inoue, A.; Shen, B.L.; Koshiba, H.; Kato, H.; Yavari, A.R. Ultra-high strength above 5000 MPa and soft magnetic properties of Co–Fe–Ta–B bulk glassy alloys. Acta Mater. 2004, 52, 1631–1637. [Google Scholar] [CrossRef]
- Liu, D.Y.; Sun, W.S.; Zhang, H.F.; Hu, Z.Q. Preparation, thermal stability and magnetic properties of Fe–Co–Ni–Zr–Mo–B bulk metallic glass. Intermetallic 2004, 12, 1149–1152. [Google Scholar] [CrossRef]
- Song, D.S.; Kim, J.H.; Fleury, E.; Kim, W.T.; Kim, D.H. Synthesis of ferromagnetic Fe-based bulk glassy alloys in the Fe–Nb–B–Y system. J. Alloy. Compd. 2005, 389, 159–164. [Google Scholar] [CrossRef]
- Lee, S.; Kato, H.; Kubota, T.; Yubuta, K.; Makino, A.; Inoue, A. Excellent Thermal Stability and Bulk Glass Forming Ability of Fe–B–Nb–Y Soft Magnetic Metallic Glass. Trans. Jpn. Inst. Met. 2008, 49, 506–512. [Google Scholar] [CrossRef]
- Tiberto, P.; Piccin, R.; Lupu, N.; Chiriac, H.; Baricco, M. Magnetic properties of Fe–Co-based bulk metallic glasses. J. Alloy. Compd. 2009, 483, 608–612. [Google Scholar] [CrossRef]
- Jia, F.; Zhang, W.; Zhang, X.; Xie, G.; Kimura, H.; Makino, A.; Inoue, A. Effect of Co concentration on thermal stability and magnetic properties of (Fe,Co)–Nb–Gd–B glassy alloys. J. Alloy. Compd. 2010, 504S, 129–131. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, A.; Man, Q.; Shen, B. (Co1−xFex)68B21.9Si5.1Nb5 bulk glassy alloys with high glass-forming ability, excellent soft-magnetic properties and super high fracture strength. Intermetallics 2012, 23, 63–67. [Google Scholar] [CrossRef]
- Han, J.J.; Wang, C.; Kou, S.; Liu, X. Thermal stability, crystallization behavior, Vickers hardness and magnetic properties of Fe−Co−Ni−Cr−Mo−C−B−Y bulk metallic glasses. Trans. Nonferr. Met. Soc. China 2013, 23, 148–155. [Google Scholar] [CrossRef]
- Li, J.W.; He, A.N.; Shen, B.L. Effect of Tb addition on the thermal stability, glass-forming ability and magnetic properties of Fe–B–Si–Nb bulk metallic glass. J. Alloy. Compd. 2014, 586, 46–49. [Google Scholar] [CrossRef]
- Li, J.W.; Estevez, D.; Jiang, K.M.; Yang, W.M.; Man, Q.K.; Chang, C.T.; Wang, X.M. Electronic-structure origin of the glass-forming ability and magnetic properties in Fe–RE–B–Nb bulk metallic glasses. J. Alloy. Compd. 2014, 617, 332–336. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.L.; Chang, C.T. Super-high strength of over 4000 MPa for Fe-based bulk glassy alloys in [(Fe1−xCox)0.75B0.2Si0.05]96Nb4 system. Acta Mater. 2004, 52, 4093–4099. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.L.; Chang, C.T. Fe- and Co-based bulk glassy alloys with ultrahigh strength of over 4000 MPa. Intermetallics 2006, 14, 936–944. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Huang, X.M.; Chen, L.Y.; Ge, M.Y.; Jiang, Q.K.; Nie, X.P.; Jiang, J.Z. Catching Fe-based bulk metallic glass with combination of high glass forming ability, ultrahigh strength and good plasticity in Fe–Co–Nb–B system. Mater. Sci. Eng. A 2009, 517, 246–248. [Google Scholar] [CrossRef]
- Dun, C.; Liu, H.; Shen, B. Enhancement of plasticity in Co–Nb–B ternary bulk metallic glasses with ultrahigh strength. J. Non Cryst. Solids 2012, 358, 3060–3064. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Guan, S.; Zhu, S.; Li, R.; Zhang, T. Effects of boron content on the glass-forming ability and mechanical properties of Co–B–Ta glassy alloys. J. Alloy. Compd. 2014, 617, 7–11. [Google Scholar] [CrossRef]
- Yazici, Z.Ö.; Hitit, A.; Yalcin, Y.; Ozgul, M. Effects of Minor Cu and Si Additions on Glass Forming Ability and Mechanical Properties of Co-Fe-Ta-B Bulk Metallic Glass. Met. Mater. Int. 2016, 22, 50–57. [Google Scholar] [CrossRef]
- Li, S.; Wei, Q.; Li, Q.; Jiang, B.; Chen, Y.; Sun, Y. Development of Fe-based bulk metallic glasses as potential biomaterials. Mater. Sci. Eng. C 2015, 52, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.A.C.; Ribeiro, D.V.; Kiminami, C.S. Corrosion resistance of Fe-Cr-based amorphous alloys: An overview. J. Non Cryst. Solids 2016, 442, 56–66. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, Q.; Li, Q.; Jiang, B.; Chen, Y.; Sun, Y. Development of Co-based bulk metallic glasses as potential biomaterials. Mater. Sci. Eng. C 2016, 69, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Zheng, Y.F. Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Men, H.; Pang, S.J.; Zhang, T. Thermal stability and microhardness of new Co-based bulk metallic glasses. Mater. Sci. Eng. A 2007, 449–451, 538–540. [Google Scholar] [CrossRef]
- Hitit, A.; Talaş, Ş.; Kara, R. Effects of silicon and chromium additions on glass forming ability and microhardness of Co-based bulk metallic glasses. Indian J. Eng. Mater. Sci. 2014, 21, 111–115. [Google Scholar]
- Li, J.; Law, J.Y.; Ma, H.; He, A.; Man, Q.; Men, H.; Huo, J.; Chang, C.; Wang, X.; Li, R.W. Magnetocaloric effect in Fe–Tm–B–Nb metallic glasses near room temperature. J. Non Cryst. Solids 2015, 425, 114–117. [Google Scholar] [CrossRef]
- Fornell, J.; González, S.; Rossinyol, E.; Suriñach, S.; Baro, M.D.; Louzguine-Luzgin, D.V.; Perepezko, J.H.; Sort, J.; Inoue, A. Enhanced mechanical properties due to structural changes induced by devitrification in Fe–Co–B–Si–Nb bulk metallic glass. Acta Mater. 2010, 58, 6256–6266. [Google Scholar] [CrossRef]
- Hitit, A.; Geçgin, M.; Öztürk, P. Effect of Annealing on Microstructure and Microhardness of Co–Fe–Ni–Ta–B–Si Bulk Metallic Glass. J. Mater. Sci. Technol. 2015, 31, 148–152. [Google Scholar] [CrossRef]
- Çolak, F. Synthesis and Characterization of Nickel, Silicon and Niobium Containing Cobalt-Iron Based Bulk Metallic Glasses. Ph.D. Thesis, Afyon Kocatepe University, Afyonkarahisar, Turkey, 2011. [Google Scholar]
- Turnbull, D. Under What Conditions can a Glass be Formed? Contemp. Phys. 1969, 10, 473–478. [Google Scholar] [CrossRef]
- Kittel, C. Energy Bands. In Introduction to Solid State Physics, 8th ed.; Stuart, C., Patricia, M.F., Martin, B., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2005; Volume 7, pp. 161–182. [Google Scholar]

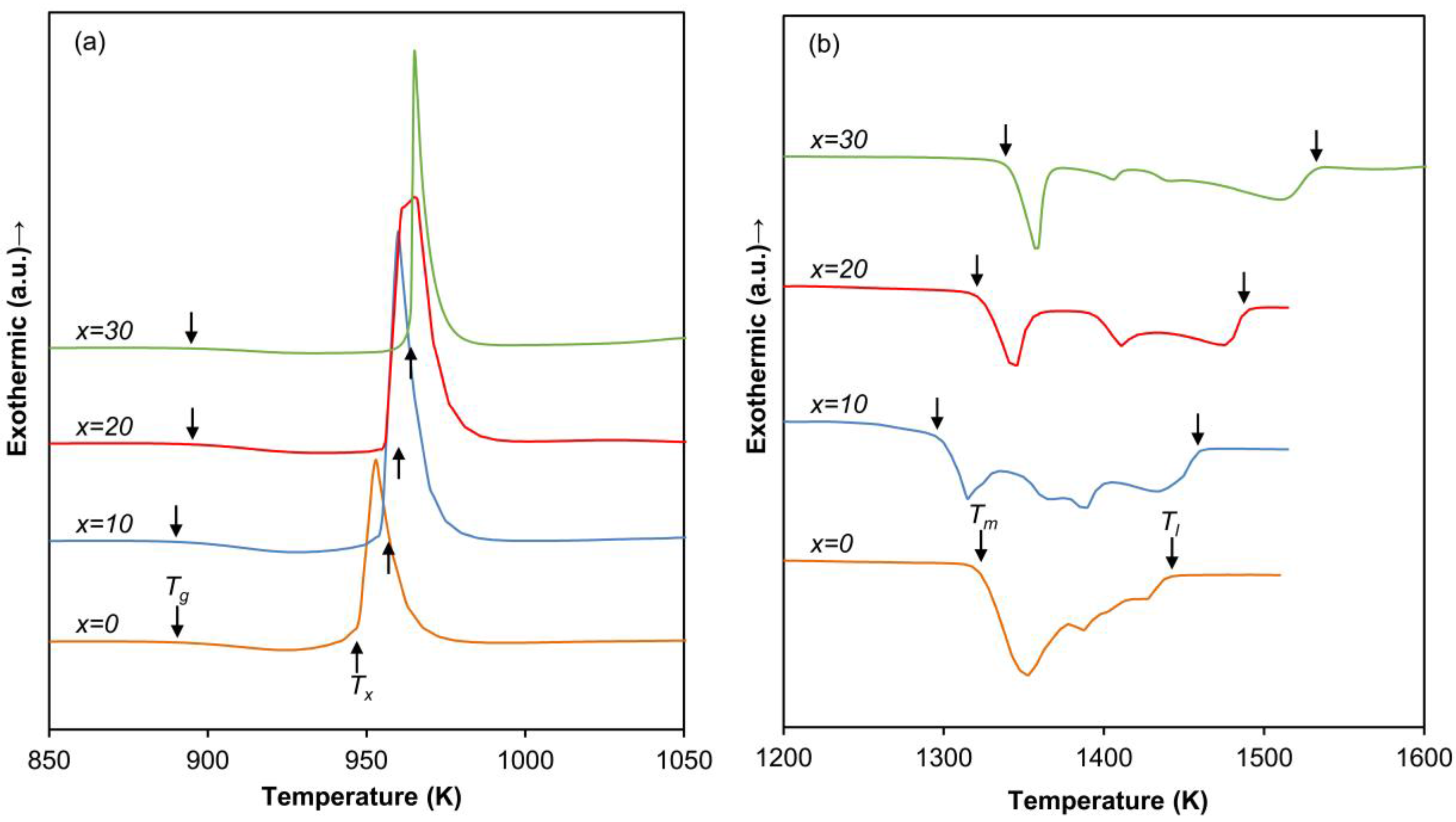
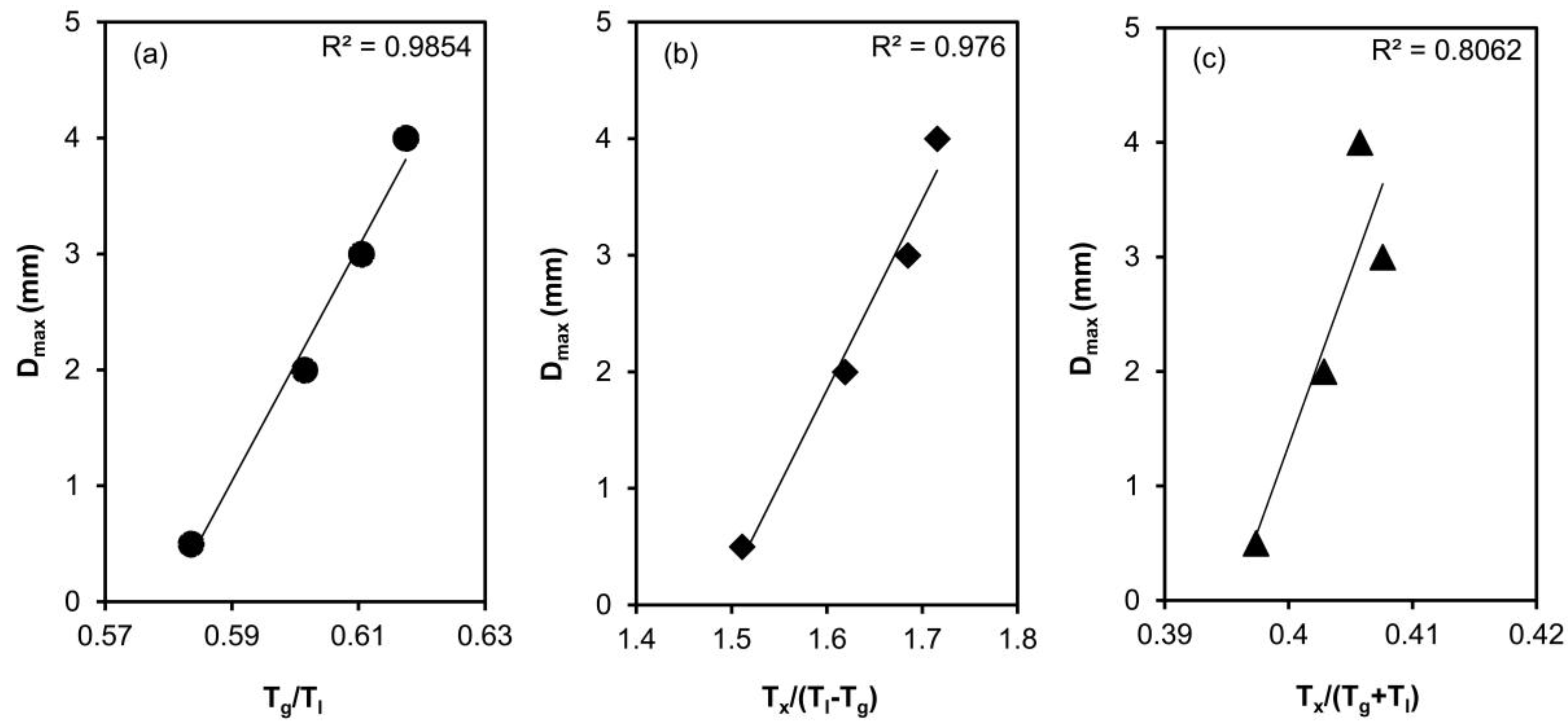
| Alloy | Tg (K) | Tx (K) | Tm (K) | Tl (K) | Tg/Tl | Tx/(Tl − Tg) | Tx/(Tg + Tl) | Dmax (mm) | Hv |
|---|---|---|---|---|---|---|---|---|---|
| Co41Fe20Ni2Ta2.75Nb2.75B26.5Si5 [26] | 891 | 947 | 1323 | 1443 | 0.617 | 1.716 | 0.406 | 4 | 1197 |
| Co31Fe30Ni2Ta2.75Nb2.75B26.5Si5 | 890 | 957 | 1295 | 1458 | 0.610 | 1.685 | 0.408 | 3 | 1242 |
| Co21Fe40Ni2Ta2.75Nb2.75B26.5Si5 | 895 | 960 | 1318 | 1488 | 0.601 | 1.619 | 0.403 | 2 | 1240 |
| Co11Fe50Ni2Ta2.75Nb2.75B26.5Si5 | 894 | 964 | 1343 | 1532 | 0.584 | 1.511 | 0.397 | 0.5 | 1238 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitit, A.; Şahin, H. The Effect of Iron Content on Glass Forming Ability and Thermal Stability of Co–Fe–Ni–Ta–Nb–B–Si Bulk Metallic Glass. Metals 2017, 7, 7. https://doi.org/10.3390/met7010007
Hitit A, Şahin H. The Effect of Iron Content on Glass Forming Ability and Thermal Stability of Co–Fe–Ni–Ta–Nb–B–Si Bulk Metallic Glass. Metals. 2017; 7(1):7. https://doi.org/10.3390/met7010007
Chicago/Turabian StyleHitit, Aytekin, and Hakan Şahin. 2017. "The Effect of Iron Content on Glass Forming Ability and Thermal Stability of Co–Fe–Ni–Ta–Nb–B–Si Bulk Metallic Glass" Metals 7, no. 1: 7. https://doi.org/10.3390/met7010007
APA StyleHitit, A., & Şahin, H. (2017). The Effect of Iron Content on Glass Forming Ability and Thermal Stability of Co–Fe–Ni–Ta–Nb–B–Si Bulk Metallic Glass. Metals, 7(1), 7. https://doi.org/10.3390/met7010007





