Microscopic Analysis and Electrochemical Behavior of Fe-Based Coating Produced by Laser Cladding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
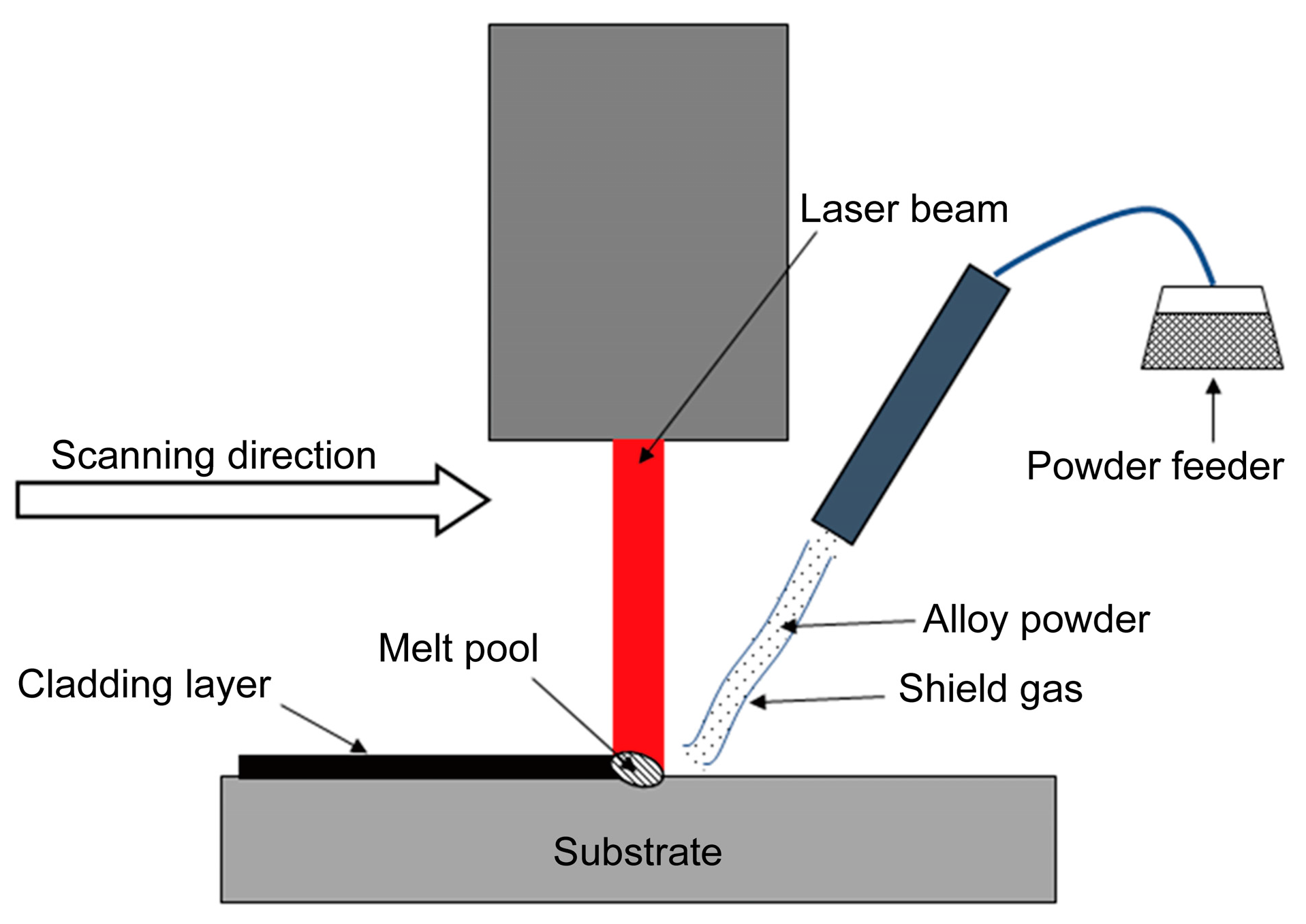
2.2. Laser Cladding Processing
2.3. Microscopic Analysis
2.4. Electrochemical Studies
3. Results and Discussion
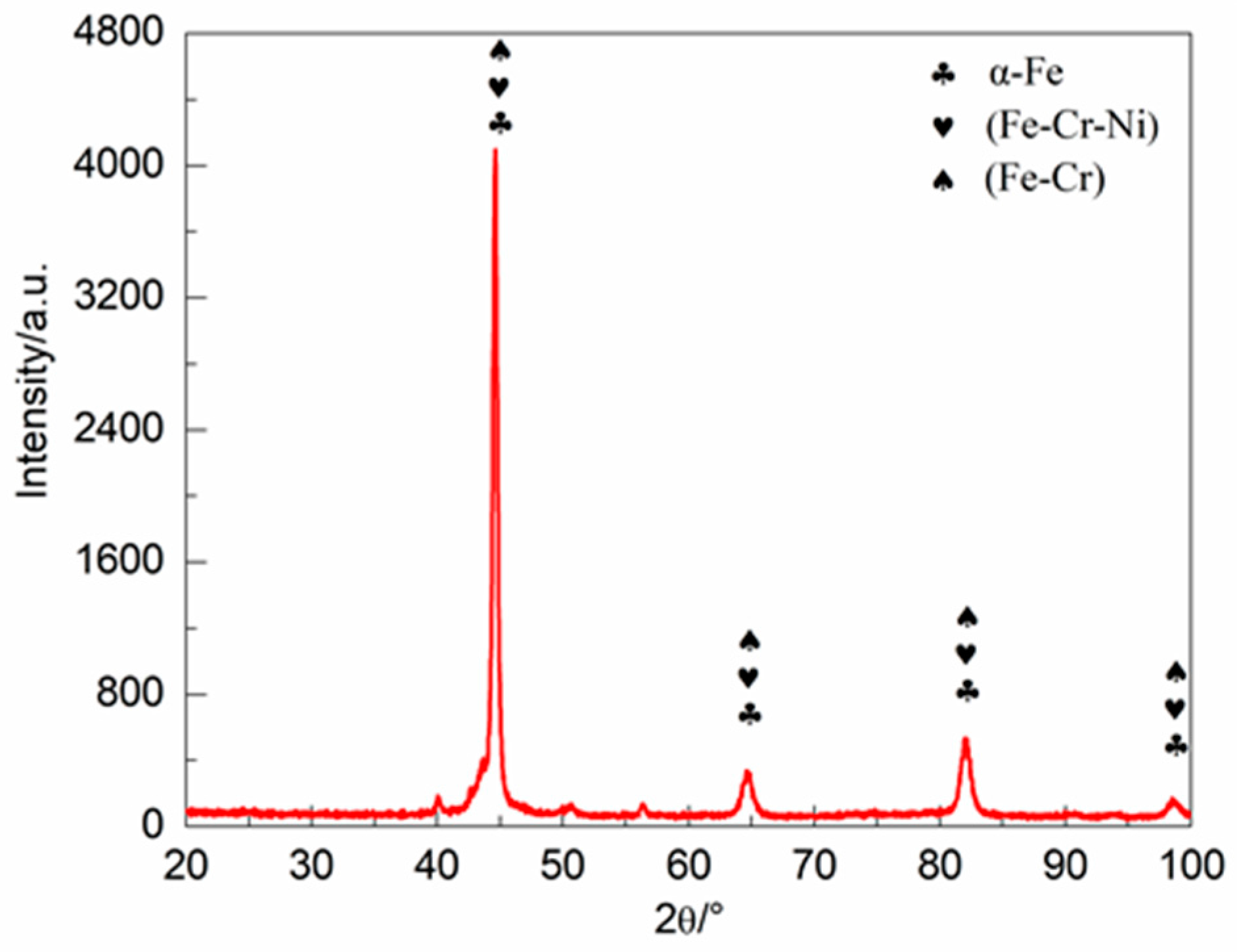
3.1. Phase Composition
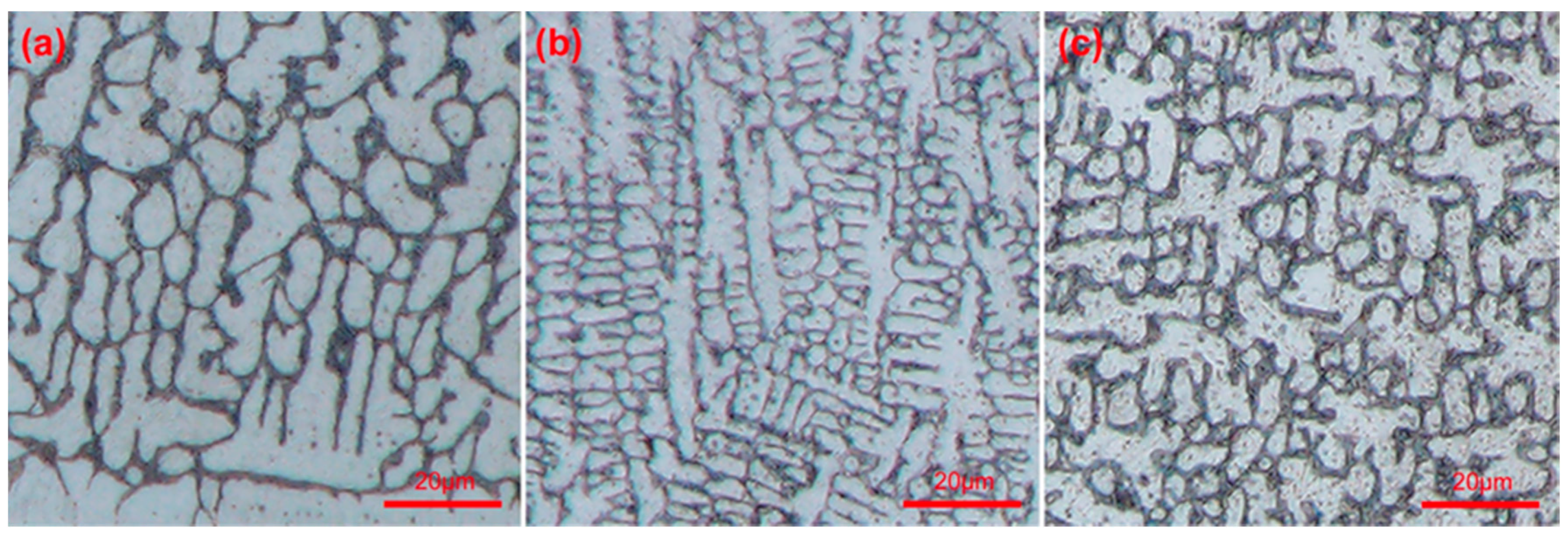
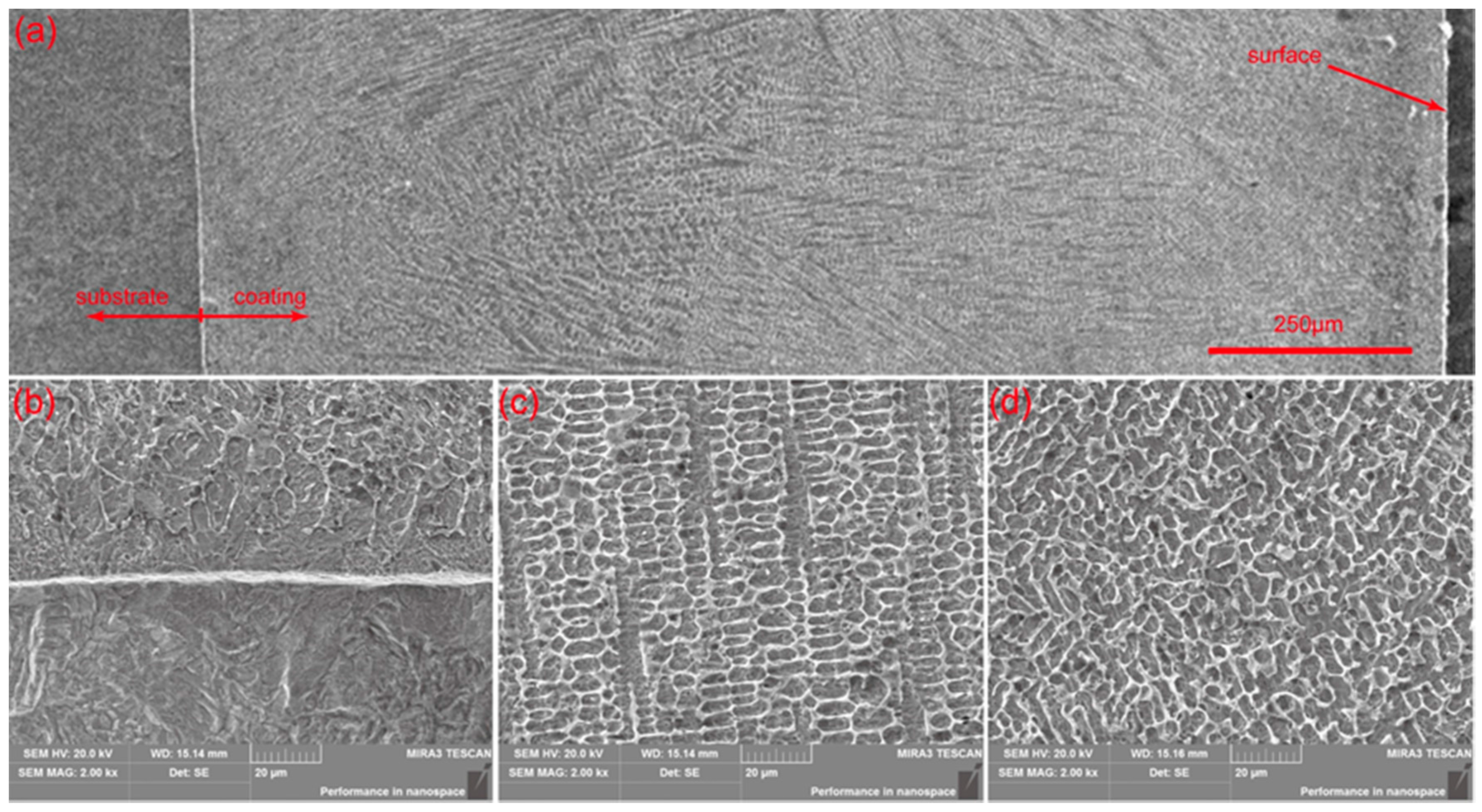
3.2. Microstructure
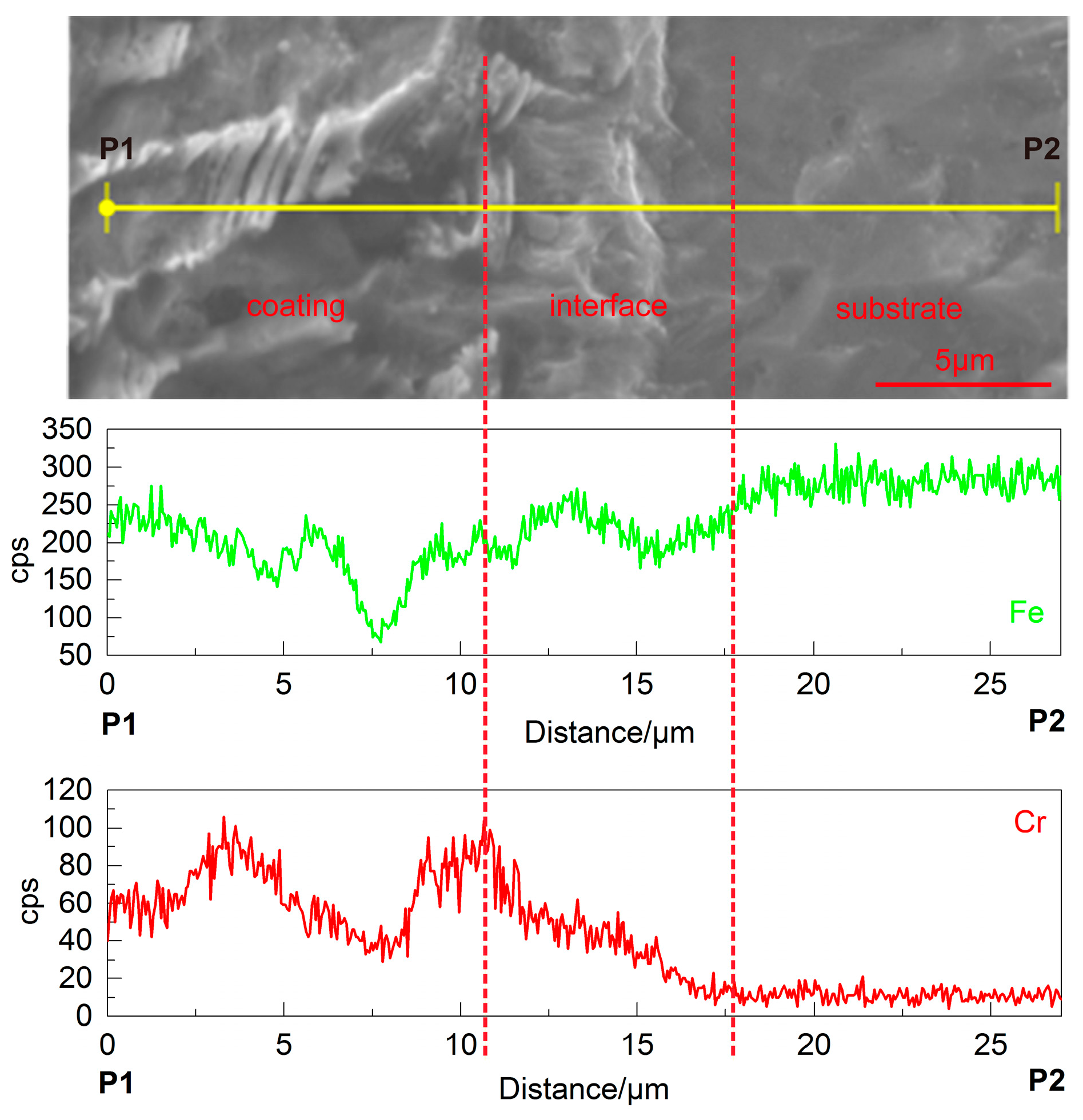
3.3. Energy Dispersive Spectrometer (EDS) Line Analysis
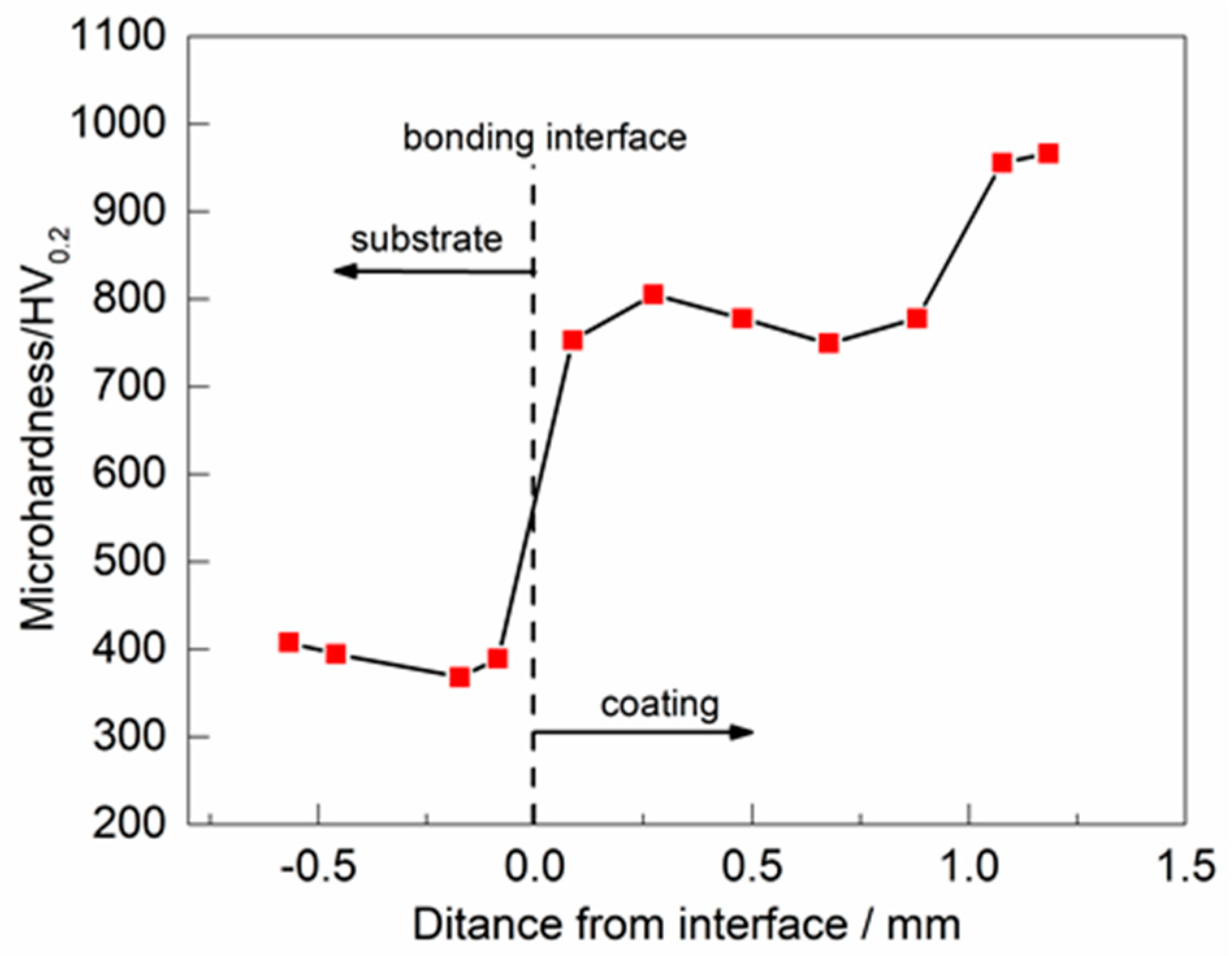
3.4. Microhardness
3.5. Electrochemical Analysis
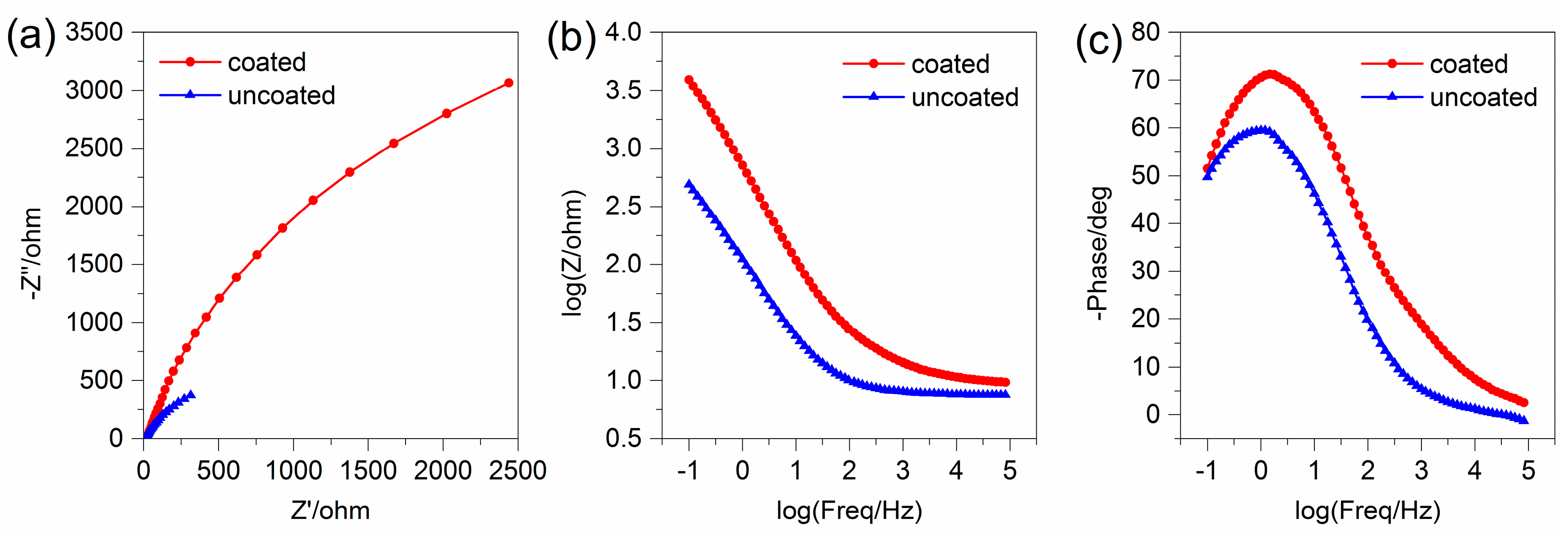
3.5.1. Electrochemical Impedance Spectroscopy
3.5.2. Potentiodynamic Polarization Curve
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liang, G.; Shi, C.; Zhou, Y.; Mao, D. Effect of Ultrasonic Treatment on the Solidification Microstructure of Die-Cast 35CrMo Steel. Metals 2016, 6, 260. [Google Scholar] [CrossRef]
- Zhang, J.W.; Lu, L.T.; Shiozawa, K.; Zhou, W.N.; Zhang, W.H. Effect of nitrocarburizing and post-oxidation on fatigue behavior of 35CrMo alloy steel in very high cycle fatigue regime. Int. J. Fatigue 2011, 33, 880–886. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Q.; Li, S.; Qin, Z. Microstructure, corrosion and tribological properties of Ti(CN) multilayer coatings on 35CrMo steel by CVD. Rare Met. 2014, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Hao, J.; Han, Z.; Yu, G.; He, X.; Yang, H. Microstructural evolution and bonding characteristic in multi-layer laser cladding of NiCoCr alloy on compacted graphite cast iron. J. Mater. Process. Technol. 2016, 232, 153–164. [Google Scholar] [CrossRef]
- Xu, G.; Kutsuna, M.; Liu, Z.; Yamada, K. Comparison between diode laser and TIG cladding of Co-based alloys on the SUS403 stainless steel. Surf. Coat. Technol. 2006, 201, 1138–1144. [Google Scholar] [CrossRef]
- Xu, P.Y.; Liu, Y.C.; Yi, P.; Fan, C.F.; Li, C.K. Research on variation and stress status of graphite in laser cladding process of grey cast iron. Mater. Sci. Technol.-Lond. 2014, 30, 1728–1734. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Z. Physical-mechanical and electrochemical corrosion behaviors of additively manufactured Cr-Ni-based stainless steel formed by laser cladding. Mater. Des. 2016, 100, 254–262. [Google Scholar] [CrossRef]
- Fu, Z.K.; Ding, H.H.; Wang, W.J.; Liu, Q.Y.; Guo, J.; Zhu, M.H. Investigation on microstructure and wear characteristic of laser cladding Fe-based alloy on wheel/rail materials. Wear 2015, 330–331, 592–599. [Google Scholar] [CrossRef]
- Liu, Q.; Janardhana, M.; Hinton, B.; Brandt, M.; Sharp, K. Laser cladding as a potential repair technology for damaged aircraft components. Int. J. Struct. Integr. 2011, 3, 314–331. [Google Scholar] [CrossRef]
- Sun, S.D.; Leary, M.; Liu, Q.; Brandt, M. Evaluation of microstructure and fatigue properties in laser cladding repair of ultrahigh strength AerMet® 100 steel. J. Laser Appl. 2015, 27, S29202. [Google Scholar] [CrossRef]
- Kattire, P.; Paul, S.; Singh, R.; Yan, W. Experimental characterization of laser cladding of CPM 9V on H13 tool steel for die repair applications. J. Manuf. Process. 2015, 20, 492–499. [Google Scholar] [CrossRef]
- Krishna, N.G.; Thinaharan, C.; George, R.P.; Parvathavarthini, N.; Mudali, U.K. Surface modification of type 304 stainless steel with duplex coatings for corrosion resistance in sea water environments. Surf. Eng. 2015, 31, 39–47. [Google Scholar] [CrossRef]
- Shi, J.; Geng, G. Electrochemical Behavior of Fine-Grained Steel in Alkaline Solutions in the Presence of Chlorides. J. Mater. Civ. Eng. 2017, 29, 04017039. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Qin, X.; Wang, Y.; Zhang, J.; Huang, S. Parameter optimization and experimental study of the sprocket repairing using laser cladding. Int. J. Adv. Manuf. Technol. 2017, 91, 3967–3975. [Google Scholar] [CrossRef]
- Hu, G.; Meng, H.; Liu, J. Microstructure and corrosion resistance of induction melted Fe-based alloy coating. Surf. Coat. Technol. 2014, 251, 300–306. [Google Scholar] [CrossRef]
- Farina, A.P.; Cecchin, D.; Soares, R.G.; Botelho, A.L.; Ferreira Koyama Takahashi, J.M.; Mazzetto, M.O.; Mesquita, M.F. Evaluation of Vickers hardness of different types of acrylic denture base resins with and without glass fibre reinforcement. Gerodontology 2012, 29, E155–E160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, Y.; Zou, Z.; Wu, D. Microstructure and properties of Fe-based composite coating by laser cladding Fe–Ti–V–Cr–C–CeO2 powder. Opt. Laser Technol. 2015, 65, 119–125. [Google Scholar] [CrossRef]
- Giourntas, L.; Hodgkiess, T.; Galloway, A.M. Comparative study of erosion-corrosion performance on a range of stainless steels. Wear 2015, 332, 1051–1058. [Google Scholar] [CrossRef] [Green Version]
- Skal, S.; Kerroum, Y.; Guenbour, A.; Bellaouchou, A.; Tabyaoui, H. Effect of abrasive particles on electrochemical behaviour of passive film formed on Alloy 59 in contaminated phosphoric acid. J. Mater. Environ. Sci. 2017, 8, 3234–3246. [Google Scholar]


| Laser Power (W) | Scan Velocity (mm∙s−1) | Spot Diameter (mm) | Overlap Ratio | Shielding Gas |
|---|---|---|---|---|
| 1350 | 10 | 5 | 50% | Ar |
| Parameters | Uncoated | Coated |
|---|---|---|
| Rs/Ω·cm−2 | 7.537 | 10.04 |
| Y1/Ω−1·cm−2·sn | 8.21 × 10−4 | 1.38 × 10−4 |
| n1 | 0.813 | 0.785 |
| Rp1/Ω·cm−2 | 3.791 | 20.12 |
| Rp2/Ω·cm−2 | 1719 | 1.02 × 10−4 |
| Y2/Ω−1·cm−2·sn | 1.63 × 10−3 | 1.71 × 10−4 |
| n2 | 0.6879 | 0.8554 |
| Samples | Ecorr (V) | Icorr (μA∙cm−2) | Rp (Ω∙cm2) |
|---|---|---|---|
| coated | −0.910 | 7.63 | 5.1 × 103 |
| uncoated | −0.922 | 34.39 | 1.03 × 103 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhou, Y.; Shi, C.; Mao, D. Microscopic Analysis and Electrochemical Behavior of Fe-Based Coating Produced by Laser Cladding. Metals 2017, 7, 435. https://doi.org/10.3390/met7100435
Chen J, Zhou Y, Shi C, Mao D. Microscopic Analysis and Electrochemical Behavior of Fe-Based Coating Produced by Laser Cladding. Metals. 2017; 7(10):435. https://doi.org/10.3390/met7100435
Chicago/Turabian StyleChen, Jinlin, Yajun Zhou, Chen Shi, and Daheng Mao. 2017. "Microscopic Analysis and Electrochemical Behavior of Fe-Based Coating Produced by Laser Cladding" Metals 7, no. 10: 435. https://doi.org/10.3390/met7100435




