Corrosion-Resistant High-Entropy Alloys: A Review
Abstract
:1. Introduction
2. Review
2.1. Environmental and Alloying Effects on Corrosion Behavior
2.1.1. Corrosion Behavior in Chloride-Containing Solutions
2.1.2. Corrosion Behavior in Acid Solutions
2.1.3. Corrosion Behavior in High-Temperature High-Pressure Water
2.2. Corrosion Resistance of HEA Coatings
2.2.1. Coating by Laser Cladding
2.2.2. Coating by Magnetron Sputtering
2.3. Heat-Treatment Effect
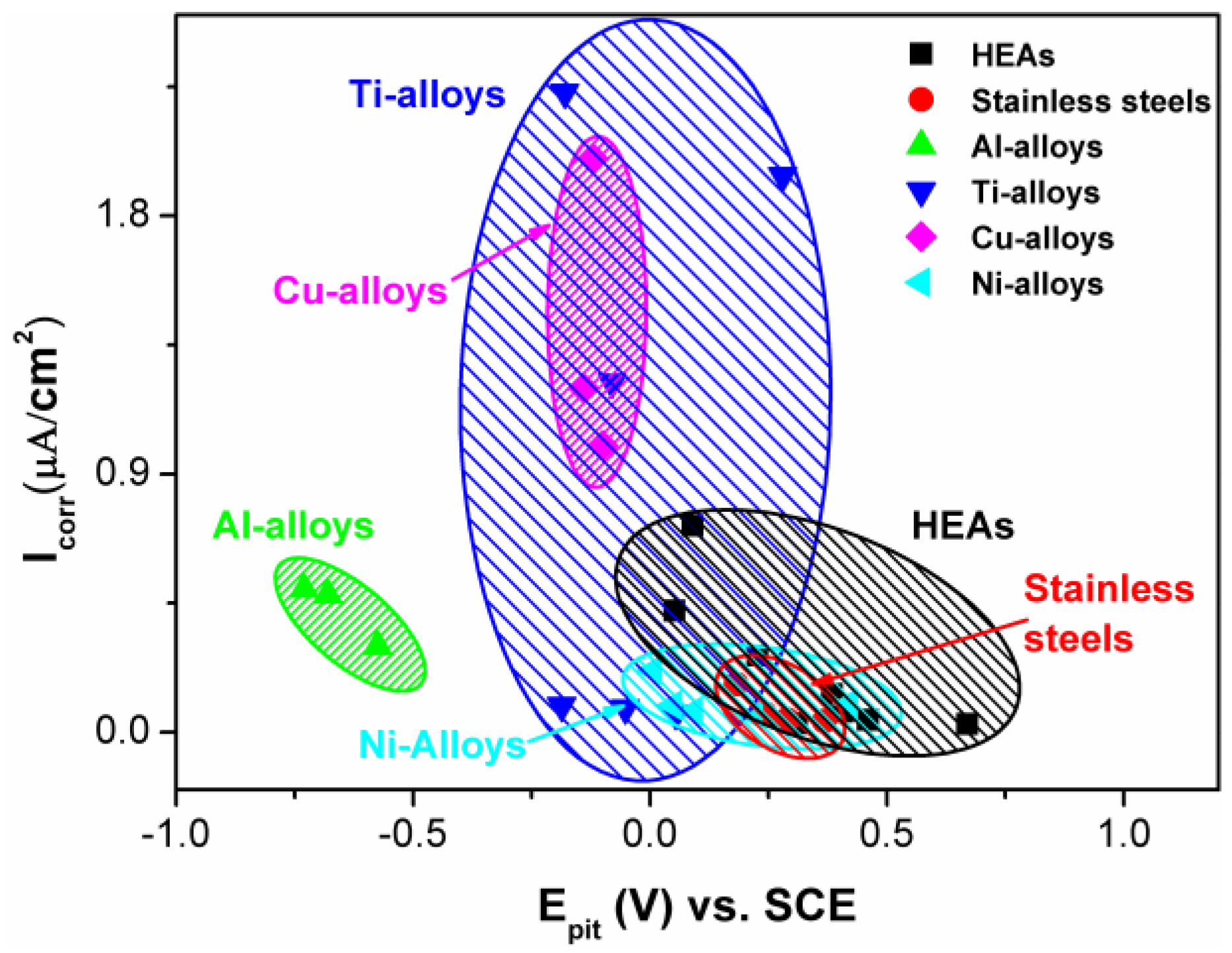
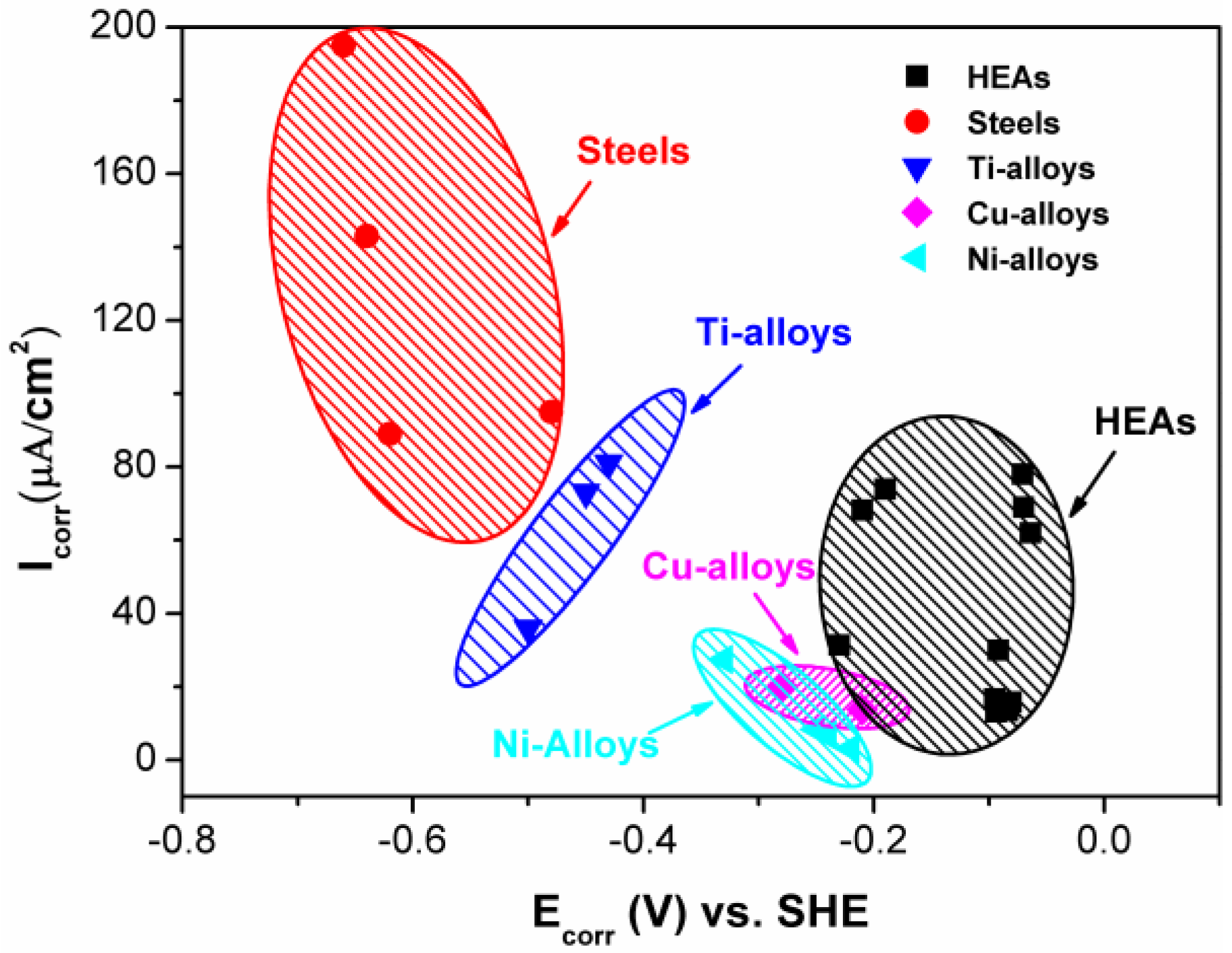
2.4. Comparison between HEAs and Conventional Alloys
3. Discussion
4. Suggested Future Work
- For the aspect of the comprehensive characterization of corrosion behavior, high-resolution observation of the oxide film formation during the electro-corrosion test should be carried out. The features of HEAs are the disordered chemical environment and the usually formed nanoscale participates due to the slow diffusion rate of the elements. However, the current research results mainly provide microscale characterization and cannot explain the basic reason behind why HEAs possess novel corrosion-resistant properties. Thus, the high-resolution investigation, including the ex-situ and in-situ transmission electron microscopy (TEM) study of passive films, nanoscale atomic-force microscopy (AFM)/scanning transmission electron microscopy (STEM) observations, and depth-profile studies of passive films that have nano-thicknesses, could provide an atomistic understanding and is a prerequisite for the control of electrochemical surface processes.
- Since the homogenization of microstructures and elemental distributions can remarkably improve the corrosion resistance, a certain heat treatment and rapid cooling rate during the formation of coatings could improve the corrosion behavior through the attainment of uniform microstructures. However, guidance for the development of these uniform microstructures is absent. Therefore, both equilibrium and non-equilibrium thermodynamics calculations to predict the phase formation/transformation need to be conducted.
- Through coatings, firstly, the problem of the higher cost of HEAs could be resolved. Secondly, the satisfactory corrosion-resistant property could be retained with the addition of strengthening elements such as Al. Thus, insufficient mechanical properties that stop the HEAs from being used could be solved. Therefore, the study of how to synthesize high-quality HEA coatings is one of the major research directions for exploring the application of HEAs as corrosion-resistant materials. The effect of processing parameters and alloying should be taken into consideration.
- HEAs exhibit promising potential for applications in industry. However, the synergistic effects of service environments have not been widely considered, including the effects of residual stress, stress-corrosion cracking, and corrosion fatigue. Those investigations are indispensable for real-world applications.
5. Conclusions
- The surface passive films, which could provide the protection for the underlying alloys from dissolution, are of importance to the corrosion behavior. The additions of Al, Cu, Mo, and/or B increase the degree of elemental segregation, and lead to the formation of non-uniform passive films, which could induce galvanic corrosion or the localized break down of the passive films. Therefore, these elemental additions decrease the corrosion resistance in salt water and/or acid. In salt water, the anodic treatment, addition of Mo, and inclusion of the inhibitors could either improve the protection of the passive film or resist the penetration of Cl− anions, thus improving the corrosion resistance.
- Some HEAs possess novel corrosion behavior in high-temperature, high-pressure water environments, thus indicating that HEAs have the capacity of being applied in supercritical service environments.
- The HEA coatings synthesized by laser cladding, electro-spark deposition, and magnetron sputtering showed superior corrosion resistance. Attributed to the rapid-cooling process and high-entropy effect, the microstructure and elemental distribution of the coatings are more homogeneous than the bulk materials. The reduced formation of weak points in the passive film and galvanic cell improve the corrosion resistance.
- During the heat treatment of HEAs at elevated temperatures, the high-entropy effect would homogenize the microstructures and compositions, and remove the elemental segregations, therefore improving the corrosion resistance. However, the heat treatment temperature should be selected suitably under the guidance of phase formation rules to avoid the formation of undesired participates.
- The comparison of the corrosion behavior between HEAs and conventional alloys indicates that HEAs are good candidates as corrosion-resistant alloys in various aqueous environments. However, to gain an in-depth understanding of corrosion behavior, the high resolution observations of corrosion processes are suggested for future work. Moreover, the phase-formation rules under both equilibrium and non-equilibrium conditions need to be developed to guide the heat treatment and coating processes.
Acknowledgments
Conflicts of Interest
References
- Koch, G.H.; Brongers, M.P.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Cost and Preventive Strategies in the United States; U.S. Federal Highway Administration: Washington, DC, USA, 2001.
- Cramer, S.D.; Covino, B.S., Jr. ASM Handbook, Volume 13A—Corrosion: Fundamentals, Testing, and Protection; ASM International: Novelty, OH, USA, 2003. [Google Scholar]
- Nilsson, J.O.; Wilson, A. Influence of isothermal phase transformations on toughness and pitting corrosion of super duplex stainless steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar] [CrossRef]
- Cvijović, Z.; Radenković, G. Microstructure and pitting corrosion resistance of annealed duplex stainless steel. Corros. Sci. 2006, 48, 3887–3906. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Han, J.; Wu, H.C.; Yang, B.; Wang, X.T. Effect of sigma phase precipitation on the mechanical and wear properties of Z3CN20.09M cast duplex stainless steel. Nucl. Eng. Des. 2013, 259, 1–7. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Gao, M.C. Chapter 11: Design of high-entropy alloys. In High-Entropy Alloys: Fundamentals and Applications; Gao, M.C., Yeh, J.W., Liaw, P.K., Zhang, Y., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Gao, M.C.; Zhang, B.; Guo, S.M.; Qiao, J.W.; Hawk, J.A. High-entropy alloys in hexagonal close-packed structure. Metall. Mater. Trans. A 2016, 47, 3322–3332. [Google Scholar] [CrossRef]
- Feuerbacher, M.; Heidelmann, M.; Thomas, C. Hexagonal high-entropy alloys. Mater. Res. Lett. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A novel low-density, high-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2015, 3, 95–99. [Google Scholar] [CrossRef]
- Diao, H.; Santodonato, L.J.; Tang, Z.; Egami, T.; Liaw, P.K. Local structures of high-entropy alloys (HEAs) on atomic scales: An overview. JOM 2015, 67, 2321–2325. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Duval, T.; Hung, U.D.; Yeh, J.W.; Shih, H.C. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Hong, U.T.; Shih, H.C.; Yeh, J.W.; Duval, T. Electrochemical kinetics of the high entropy alloys in aqueous environments—A comparison with type 304 stainless steel. Corros. Sci. 2005, 47, 2679–2699. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, L.; He, W.; Liaw, P. Alloying and processing effects on the aqueous corrosion behavior of high-entropy alloys. Entropy 2014, 16, 895–911. [Google Scholar] [CrossRef]
- Qiu, Y.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion characteristics of high entropy alloys (heas). J. Mater. Sci. Technol. 2015, 31, 1235–1243. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Cheng, C.-Q.; Shang, J.-L.; Wang, R.; Li, P.; Zhao, J. Oxidation behavior of high-entropy alloys AlxCoCrFeNi (x = 0.15, 0.4) in supercritical water and comparison with HR3C steel. Trans. Nonferr. Met. Soc. China 2015, 25, 1341–1351. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of AlxCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pittng behavior. Corros. Sci. under review.
- Senkov, O.N.; Senkova, S.V.; Woodward, C. Effect of aluminum on the microstructure and properties of two refractory high-entropy alloys. Acta Mater. 2014, 68, 214–228. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, M.A.; Yuan, T.; Wang, G.Y.; Yeh, J.W.; Tsai, C.W.; Chuang, A.; Liaw, P.K. Fatigue behavior of Al0.5CoCrCuFeNi high entropy alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Tang, Z.; Yuan, T.; Tsai, C.-W.; Yeh, J.-W.; Lundin, C.D.; Liaw, P.K. Fatigue behavior of a wrought Al0.5CoCrCuFeNi two-phase high-entropy alloy. Acta Mater. 2015, 99, 247–258. [Google Scholar] [CrossRef]
- Seifi, M.; Li, D.; Yong, Z.; Liaw, P.K.; Lewandowski, J.J. Fracture toughness and fatigue crack growth behavior of as-cast high-entropy alloys. JOM 2015, 67, 2288–2295. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mao, M.M.; Wang, J.; Gludovatz, B.; Zhang, Z.; Mao, S.X.; George, E.P.; Yu, Q.; Ritchie, R.O. Nanoscale origins of the damage tolerance of the high-entropy alloy CrMnFeCoNi. Nat. Commun. 2015, 6, 10143. [Google Scholar] [CrossRef] [PubMed]
- Santodonato, L.J.; Zhang, Y.; Feygenson, M.; Parish, C.M.; Gao, M.C.; Weber, R.J.; Neuefeind, J.C.; Tang, Z.; Liaw, P.K. Deviation from high-entropy configurations in the atomic distributions of a multi-principal-element alloy. Nat. Commun. 2015, 6, 5964. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ma, M.; Liu, W.; Li, L.; Zhong, M.; Liu, Y.; Wu, Q. Synthesis and characterization of high-entropy alloy FeCoNiCuCr by laser cladding. Adv. Mater. Sci. Eng. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.; Jiao, H. Microstructure and properties of 6FeNiCoSiCrAlTi high-entropy alloy coating prepared by laser cladding. Appl. Surf. Sci. 2011, 257, 2259–2263. [Google Scholar] [CrossRef]
- Cheng, J.B.; Liang, X.B.; Xu, B.S. Effect of Nb addition on the structure and mechanical behaviors of CoCrCuFeNi high-entropy alloy coatings. Surf. Coat. Technol. 2014, 240, 184–190. [Google Scholar] [CrossRef]
- Shon, Y.; Joshi, S.S.; Katakam, S.; Shanker Rajamure, R.; Dahotre, N.B. Laser additive synthesis of high entropy alloy coating on aluminum: Corrosion behavior. Mater. Lett. 2015, 142, 122–125. [Google Scholar] [CrossRef]
- Li, Q.H.; Yue, T.M.; Guo, Z.N.; Lin, X. Microstructure and corrosion properties of AlCoCrFeNi high entropy alloy coatings deposited on AISI 1045 steel by the electrospark process. Metall. Mater. Trans. A 2012, 44, 1767–1778. [Google Scholar] [CrossRef]
- An, Z.; Jia, H.; Wu, Y.; Rack, P.D.; Patchen, A.D.; Liu, Y.; Ren, Y.; Li, N.; Liaw, P.K. Solid-solution CrCoCuFeNi high-entropy alloy thin films synthesized by sputter deposition. Mater. Res. Lett. 2015, 3, 203–209. [Google Scholar] [CrossRef]
- Dou, D.; Li, X.C.; Zheng, Z.Y.; Li, J.C. Coatings of FeAlCoCuNiV high entropy alloy. Surf. Eng. 2016, 32, 766–770. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Z.; Dou, D.; Li, J. Microstructure and properties of coating of FeAlCuCrCoMn high entropy alloy deposited by direct current magnetron sputtering. Mater. Res. 2016, 19, 802–806. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Zhang, C.H.; Guan, M.; Tan, J.Z. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Xiang, C.; Fu, H.; Wang, J.; Han, E.-H.; Zhang, Z. Corrosion behavior of several high-entropy alloys in high temperature high pressurewater. J. Chin. Soc. Corros. Prot. 2016, 36, 108–112. [Google Scholar]
- Presuel-Moreno, F.; Jakab, M.A.; Tailleart, N.; Goldman, M.; Scully, J.R. Corrosion-resistant metallic coatings. Mater. Today 2008, 11, 14–23. [Google Scholar] [CrossRef]
- Lee, C.P.; Chang, C.C.; Chen, Y.Y.; Yeh, J.W.; Shih, H.C. Effect of the aluminium content of AlxCrFe1.5MnNi0.5 high-entropy alloys on the corrosion behaviour in aqueous environments. Corros. Sci. 2008, 50, 2053–2060. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Lee, T.-D.; Chen, S.-K.; Chang, Y.-S. Electrochemical passive properties of alxcocrfeni (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Lee, C.P.; Chen, Y.Y.; Hsu, C.Y.; Yeh, J.W.; Shih, H.C. Enhancing pitting corrosion resistance of AlxCrFe1.5MnNi0.5 high-entropy alloys by anodic treatment in sulfuric acid. Thin Solid Films 2008, 517, 1301–1305. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Chiang, W.-C.; Wu, J.-K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Chou, Y.L.; Yeh, J.W.; Shih, H.C. The effect of molybdenum on the corrosion behaviour of the high-entropy alloys Co1.5CrFeNi1.5Ti0.5Mox in aqueous environments. Corrosi. Sci. 2010, 52, 2571–2581. [Google Scholar] [CrossRef]
- Chou, Y.L.; Yeh, J.W.; Shih, H.C. Effect of inhibitors on the critical pitting temperature of the high-entropy alloy Co1.5CrFeNi1.5Ti0.5Mo0.1. J. Electrochem. Soc. 2011, 158, C246–C251. [Google Scholar] [CrossRef]
- Chou, Y.L.; Wang, Y.C.; Yeh, J.W.; Shih, H.C. Pitting corrosion of the high-entropy alloy Co1.5CrFeNi1.5Ti0.5Mo0.1 in chloride-containing sulphate solutions. Corros. Sci. 2010, 52, 3481–3491. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.X.; Li, D.M.; Shi, L.; Cai, B.; Wang, M.X. Corrosion behavior of CuCrFeNiMn high entropy alloy system in 1 M sulfuric acid solution. Mater. Corros. 2011, 828–834. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE International: Houston, TX, USA, 1974. [Google Scholar]
- Lee, C.P.; Chen, Y.Y.; Hsu, C.Y.; Yeh, J.W.; Shih, H.C. The effect of boron on the corrosion resistance of the high entropy alloys Al0.5CoCrCuFeNiBx. J. Electrochem. Soc. 2007, 154, C424–C430. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.-Z. Synthesis and characterization of FeCoNiCrCu high-entropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Ye, X.; Ma, M.; Cao, Y.; Liu, W.; Ye, X.; Gu, Y. The property research on high-entropy alloy alxfeconicucr coating by laser cladding. Phys. Procedia 2011, 12, 303–312. [Google Scholar] [CrossRef]
- Qiu, X.W.; Zhang, Y.P.; Liu, C.G. Effect of Ti content on structure and properties of Al2CrFeNiCoCuTix high-entropy alloy coatings. J. Alloys Compd. 2014, 585, 282–286. [Google Scholar] [CrossRef]
- Qiu, X.-W.; Liu, C.-G. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Qiu, X.-W.; Zhang, Y.-P.; He, L.; Liu, C.-G. Microstructure and corrosion resistance of AlCrFeCuCo high entropy alloy. J. Alloys Compd. 2013, 549, 195–199. [Google Scholar] [CrossRef]
- Lin, C.-M.; Tsai, H.-L.; Bor, H.-Y. Effect of aging treatment on microstructure and properties of high-entropy Cu0.5CoCrFeNi alloy. Intermetallics 2010, 18, 1244–1250. [Google Scholar] [CrossRef]
- Lin, C.-M.; Tsai, H.-L. Evolution of microstructure, hardness, and corrosion properties of high-entropy Al0.5CoCrFeNi alloy. Intermetallics 2011, 19, 288–294. [Google Scholar] [CrossRef]
- Feng, R.; Gao, M.; Lee, C.; Mathes, M.; Zuo, T.; Chen, S.; Hawk, J.; Zhang, Y.; Liaw, P. Design of light-weight high-entropy alloys. Entropy 2016, 18, 333. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Diao, H.; Gao, M.C.; Tang, Z.; Poplawsky, J.D.; Liaw, P.K. Understanding phase stability of Al-Co-Cr-Fe-Ni high entropy alloys. Mater. Des. 2016, 109, 425–433. [Google Scholar] [CrossRef]
- Kwok, C.T.; Cheng, F.T.; Man, H.C. Synergistic effect of cavitation erosion and corrosion of various engineering alloys in 3.5% NaCl solution. Mater. Sci. Eng. A 2000, 290, 145–154. [Google Scholar] [CrossRef]
- Delgado-Alvarado, C.; Sundaram, P.A. A study of the corrosion behavior of gamma titanium aluminide in 3.5 wt % nacl solution and seawater. Corros. Sci. 2007, 49, 3732–3741. [Google Scholar] [CrossRef]
- Al-Fozan, S.A.; Malik, A.U. Effect of seawater level on corrosion behavior of different alloys. Desalination 2008, 228, 61–67. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Y.; Zhao, J.; Wang, F. Electrochemical corrosion performance in 3.5% NaCl of the electrodeposited Nanocrystalline Ni films with and without dispersions of Cr nanoparticles. Electrochim. Acta 2006, 51, 4922–4927. [Google Scholar] [CrossRef]
- Ezuber, H.; El-Houd, A.; El-Shawesh, F. A study on the corrosion behavior of aluminum alloys in seawater. Mater. Des. 2008, 29, 801–805. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, N.; Yang, B. Effect of ferrite on pitting corrosion of Fe20Cr9Ni cast austenite stainless steel for nuclear power plant pipe. Corros. Eng. Sci. Technol. 2014, 50, 330–337. [Google Scholar] [CrossRef]
- Sarkar, P.P.; Kumar, P.; Manna, M.K.; Chakraborti, P.C. Microstructural influence on the electrochemical corrosion behaviour of dual-phase steels in 3.5% NaCl solution. Mater. Lett. 2005, 59, 2488–2491. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the TaFel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, S.H.; Kim, J.G.; Yoon, J.B. Corrosion behaviour of copper containing low alloy steels in sulphuric acid. Corros. Sci. 2012, 54, 174–182. [Google Scholar] [CrossRef]
- Park, S.-A.; Lee, S.-H.; Kim, J.-G. Effect of chromium on the corrosion behavior of low alloy steel in sulfuric acid. Met. Mater. Int. 2012, 18, 975–987. [Google Scholar] [CrossRef]
- Baik, S.-Y. The study of corrosion behavior for solution and aging heat treated Ti alloy. J. Korean Soc. Mar. Environ. Saf. 2016, 22, 138–144. [Google Scholar] [CrossRef]
- Lu, G.; Zangari, G. Corrosion resistance of ternary Ni-p based alloys in sulfuric acid solutions. Electrochim. Acta 2002, 47, 2969–2979. [Google Scholar] [CrossRef]
- Moretti, G.; Guidi, F. Tryptophan as copper corrosion inhibitor in 0.5 m aerated sulfuric acid. Corros. Sci. 2002, 44, 1995–2011. [Google Scholar] [CrossRef]
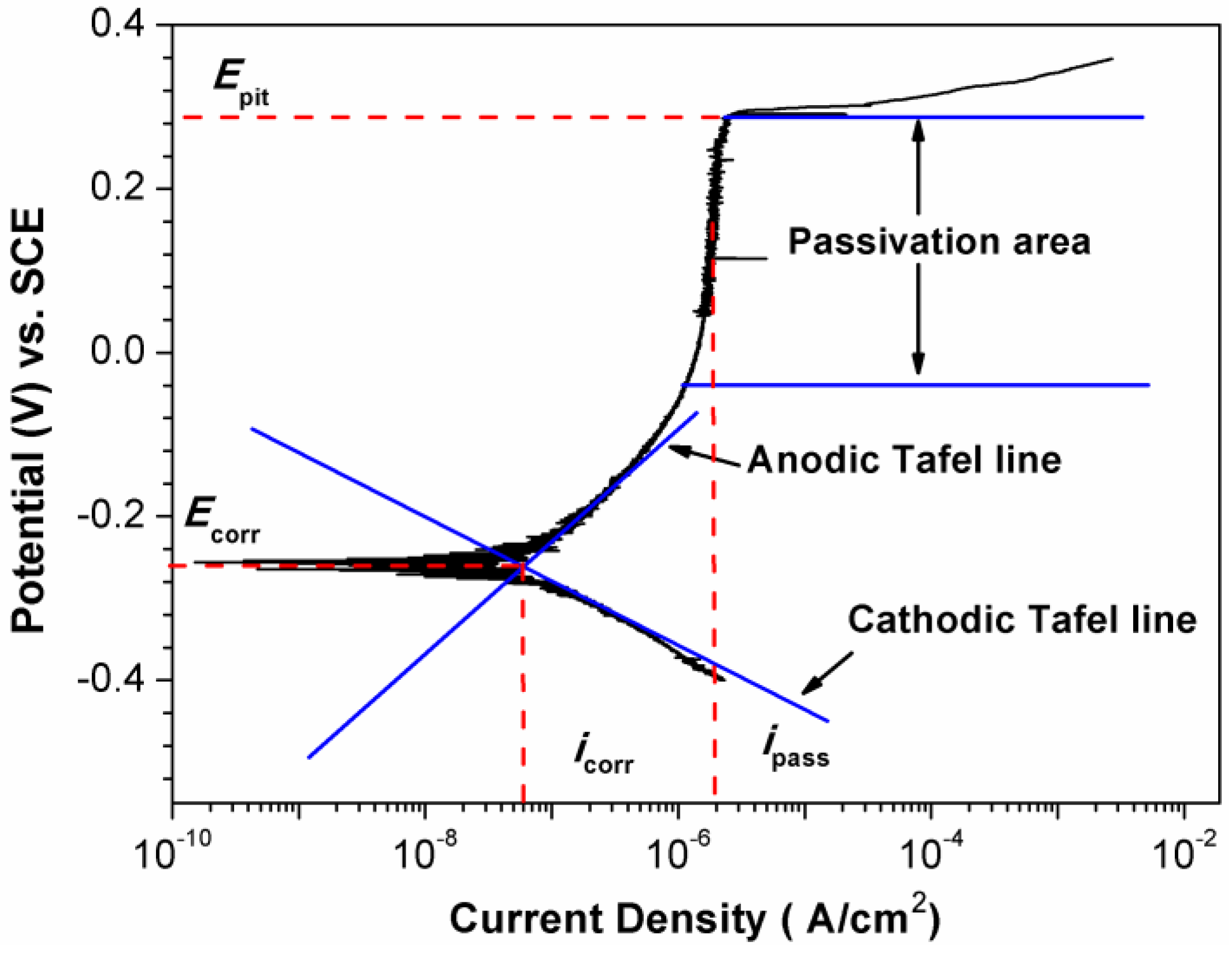
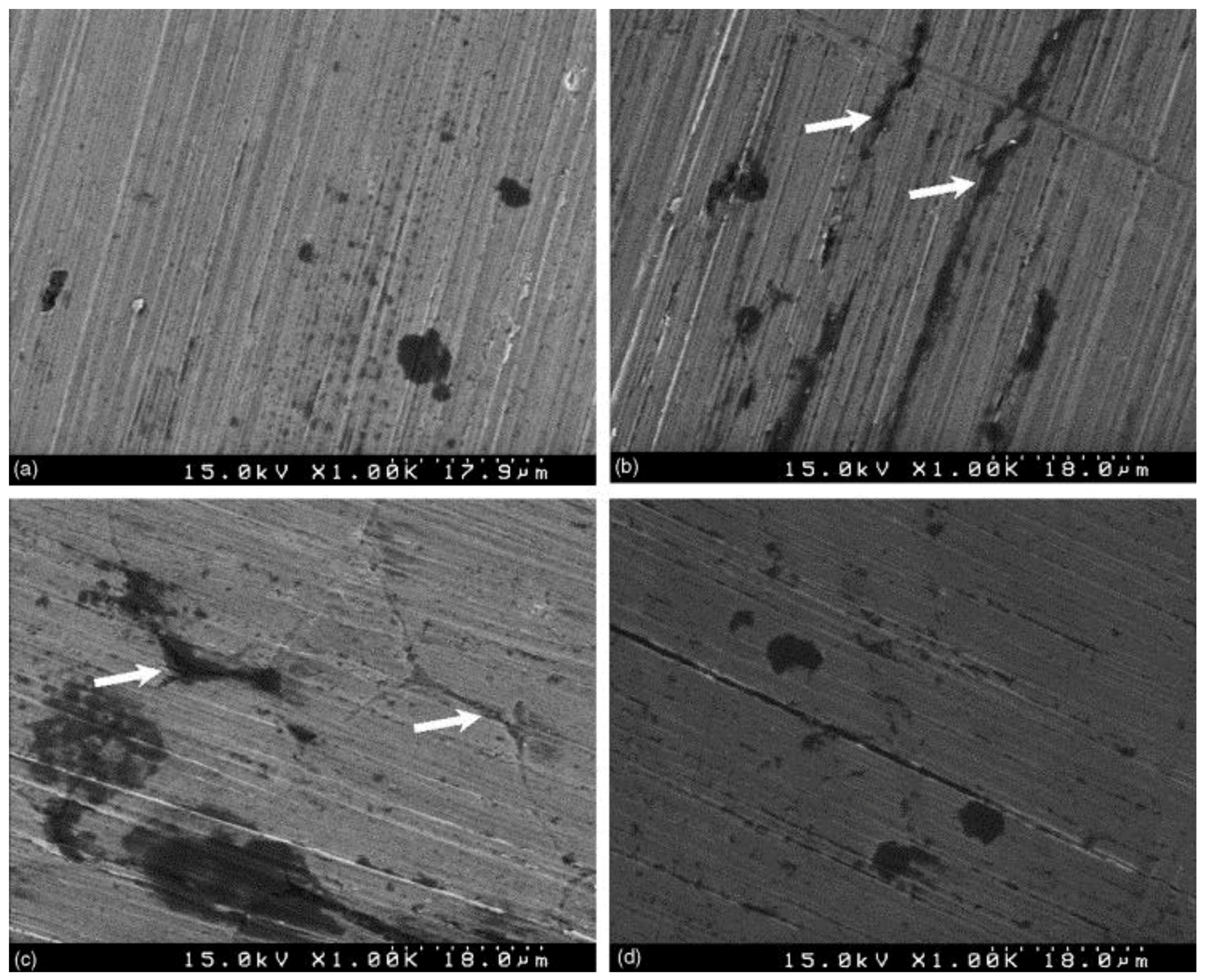
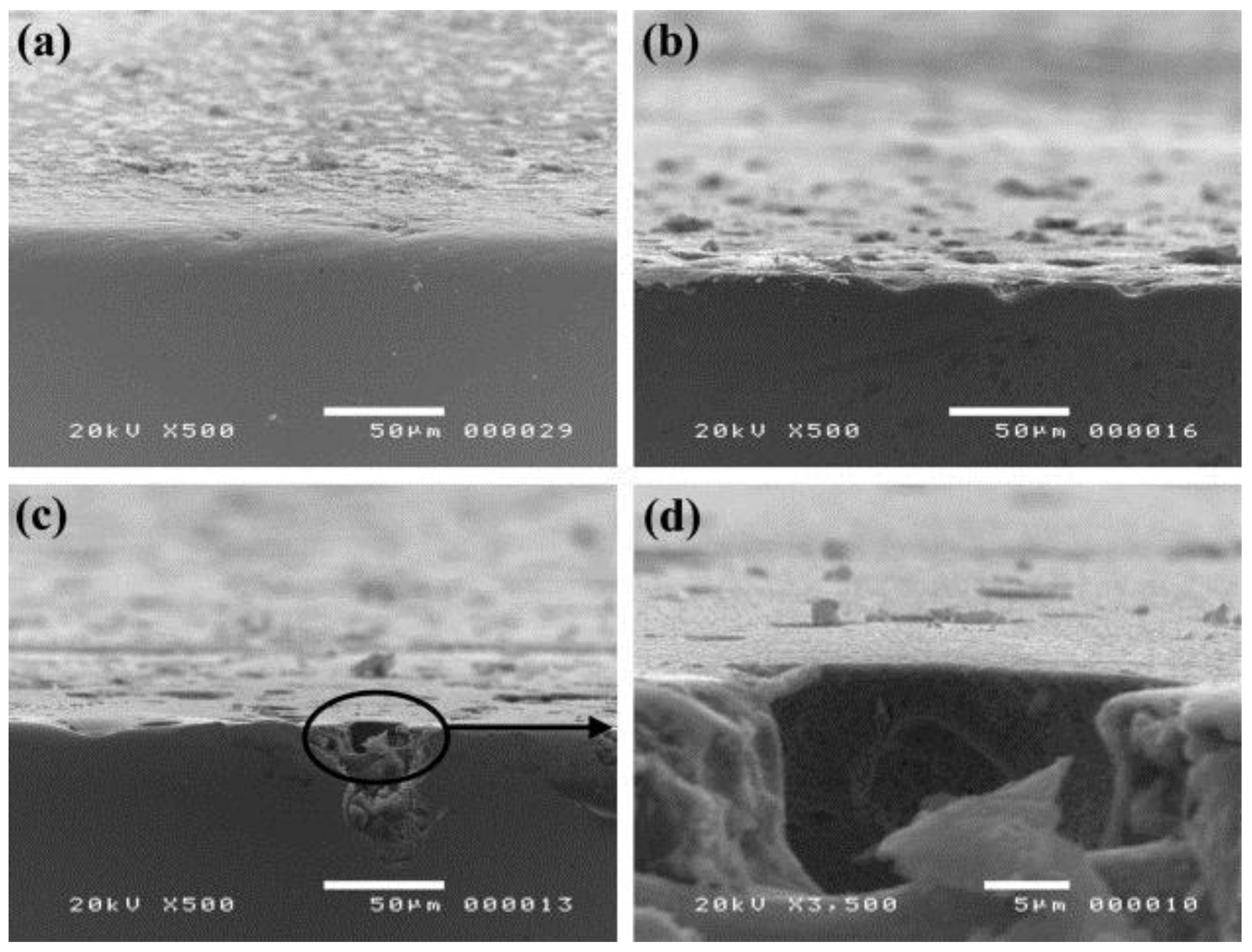
| Alloy | Solution | Ecorr 1 (VSHE 4) | icorr 2 (μA/cm2) | Epit 3 (VSHE) | Reference |
|---|---|---|---|---|---|
| CrFe1.5MnNi0.5 | 1 M NaCl | −0.39 | 0.45 | 0.019 | [41] |
| Al0.3CrFe1.5MnNi0.5 | 1 M NaCl | −0.40 | 0.69 | −0.15 | [41] |
| Al0.5CrFe1.5MnNi0.5 | 1 M NaCl | −0.50 | 1.02 | −0.12 | [41] |
| FeCoNiCr | 0.6 M NaCl | −0.46 | 0.035 | 0.31 | [44] |
| FeCoNiCrCu0.5 | 0.6 M NaCl | −0.49 | 0.72 | 0.09 | [44] |
| FeCoNiCrCu | 0.6 M NaCl | −0.53 | 1.23 | 0.08 | [44] |
| Co1.5CrFeNi1.5Ti0.5 | 1 M NaCl | −0.44 | 0.57 | 0.33 | [45] |
| Co1.5CrFeNi1.5Ti0.5Mo0.1 | 1 M NaCl | −0.38 | 0.13 | 1.21 | [45] |
| Co1.5CrFeNi1.5Ti0.5Mo0.5 | 1 M NaCl | −0.49 | 0.20 | 1.16 | [45] |
| Co1.5CrFeNi1.5Ti0.5Mo0.8 | 1 M NaCl | −0.55 | 0.41 | 1.18 | [45] |
| Alloy | Solution | Ecorr 1 (VSHE 4) | icorr 2 (μA/cm2) | ΔEb 3 (VSHE) | Reference |
|---|---|---|---|---|---|
| CrFe1.5MnNi0.5 | 0.5 M H2SO4 | −0.23 | 31.4 | 1.227 | [41] |
| Al0.3CrFe1.5MnNi0.5 | 0.5 M H2SO4 | −0.19 | 73.9 | 1.176 | [41] |
| Al0.5CrFe1.5MnNi0.5 | 0.5 M H2SO4 | −0.21 | 68.2 | 1.114 | [41] |
| CoCrFeNi | 0.5 M H2SO4 | −0.081 | 15.8 | 1.098 | [42] |
| Al0.25CoCrFeNi | 0.5 M H2SO4 | −0.095 | 16.7 | 1.092 | [42] |
| Al0.5CoCrFeNi | 0.5 M H2SO4 | −0.084 | 13.4 | 1.083 | [42] |
| AlCoCrFeNi | 0.5 M H2SO4 | −0.094 | 13.1 | 1.09 | [42] |
| Co1.5CrFeNi1.5Ti0.5 | 0.5 M H2SO4 | −0.092 | 30 | 1.13 | [45] |
| Co1.5CrFeNi1.5Ti0.5Mo0.1 | 0.5 M H2SO4 | −0.071 | 78 | 1.112 | [45] |
| Co1.5CrFeNi1.5Ti0.5Mo0.5 | 0.5 M H2SO4 | −0.064 | 62 | 1.109 | [45] |
| Co1.5CrFeNi1.5Ti0.5Mo0.8 | 0.5 M H2SO4 | −0.070 | 69 | 1.109 | [45] |
| Cu0.5CrFeNiMn | 1 M H2SO4 | −0.073 | 20.9 | 0.050 | [48] |
| CuCr0.5FeNi0.5Mn | 1 M H2SO4 | −0.090 | 40.2 | 0.035 | [48] |
| Al0.5CoCrCuFeNi | 1 M H2SO4 | −0.115 | 78.7 | 0.348 | [50] |
| Al0.5CoCrCuFeNiB0.2 | 1 M H2SO4 | −0.121 | 103 | 0.336 | [50] |
| Alloy | Solution | Ecorr 1 (VSCE 3) | icorr 2 (μA/cm2) | Reference |
|---|---|---|---|---|
| AlCoCrFeNi | 0.05 M HCl | −0.55 | 29.2 | [52] |
| Al1.3CoCrFeNi | 0.05 M HCl | −0.58 | 47.9 | [52] |
| Al1.5CoCrFeNi | 0.05 M HCl | −0.92 | 31.4 | [52] |
| Al1.8CoCrFeNi | 0.05 M HCl | −0.67 | 7.6 | [52] |
| Al2CoCrFeNi | 0.05 M HCl | −0.66 | 3.7 | [52] |
| Al2CrFeNiCoCu | 0.5 M HNO3 | −0.18 | 38 | [53] |
| Al2CrFeNiCoCuTi0.5 | 0.5 M HNO3 | −0.30 | 22 | [53] |
| Al2CrFeNiCoCuTi1 | 0.5 M HNO3 | −0.33 | 7.3 | [53] |
| Al2CrFeNiCoCuTi1.5 | 0.5 M HNO3 | −0.30 | 4.4 | [53] |
| Al2CrFeNiCoCuTi2 | 0.5 M HNO3 | −0.15 | 2.7 | [53] |
| Al2CrFeCoCuTi | 0.6 M NaCl | −0.51 | 68 | [54] |
| Al2CrFeCoCuTiNi0.5 | 0.6 M NaCl | −0.43 | 32 | [54] |
| Al2CrFeCoCuTiNi1 | 0.6 M NaCl | −0.22 | 13 | [54] |
| Al2CrFeCoCuTiNi1.5 | 0.6 M NaCl | −0.48 | 64 | [54] |
| Al2CrFeCoCuTiNi2 | 0.6 M NaCl | −0.50 | 67 | [54] |
| CoCrCuFeNi | 0.5 M H2SO4 | −0.32 | 21.9 | [55] |
| AlCoCrFeNi | 0.6 M NaCl | −0.17 | 0.073 | [38] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43. https://doi.org/10.3390/met7020043
Shi Y, Yang B, Liaw PK. Corrosion-Resistant High-Entropy Alloys: A Review. Metals. 2017; 7(2):43. https://doi.org/10.3390/met7020043
Chicago/Turabian StyleShi, Yunzhu, Bin Yang, and Peter K. Liaw. 2017. "Corrosion-Resistant High-Entropy Alloys: A Review" Metals 7, no. 2: 43. https://doi.org/10.3390/met7020043







