Effect of Batch Annealing Temperature on Microstructure and Resistance to Fish Scaling of Ultra-Low Carbon Enamel Steel
Abstract
:1. Introduction
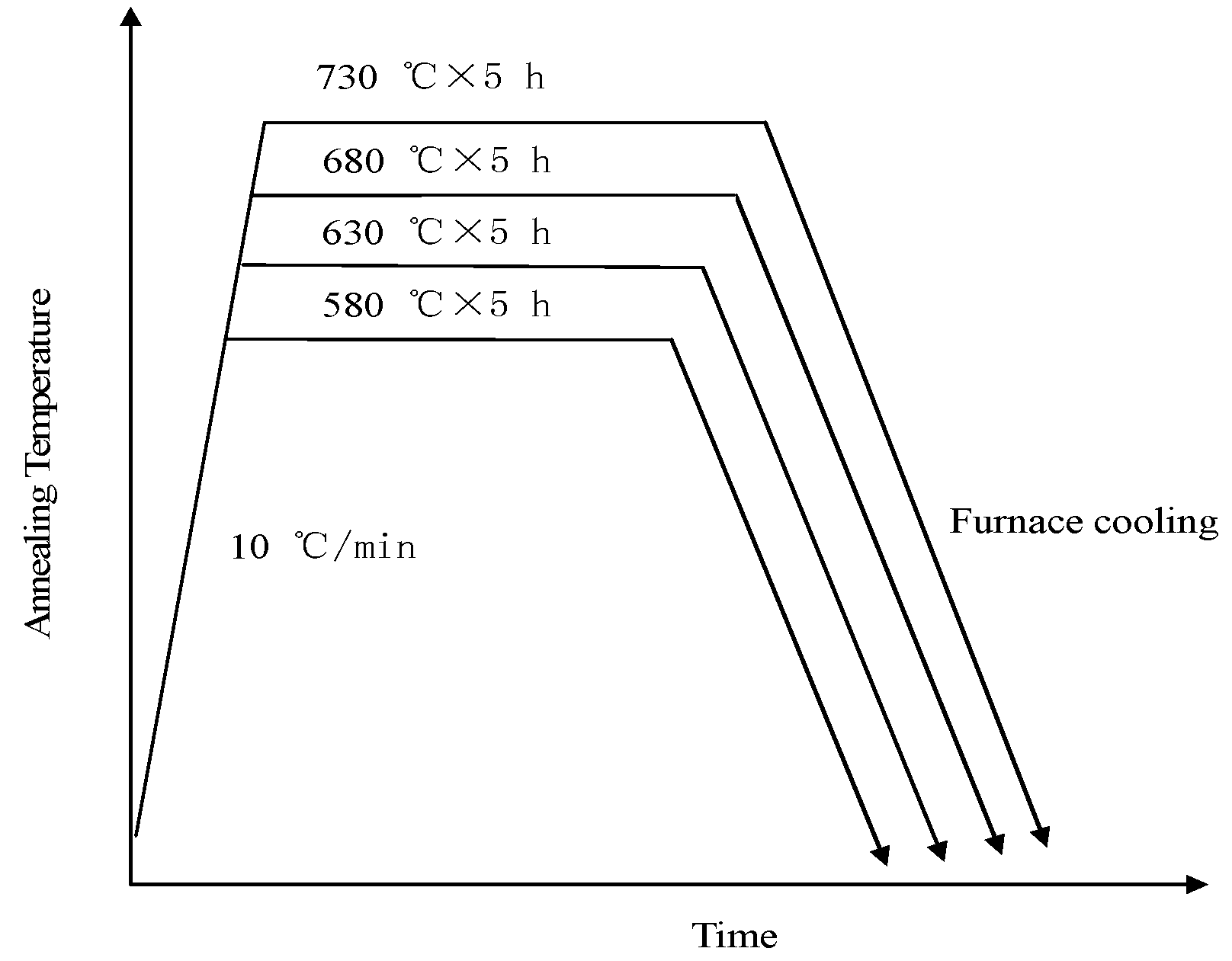
2. Materials and Methods
3. Results and Discussion
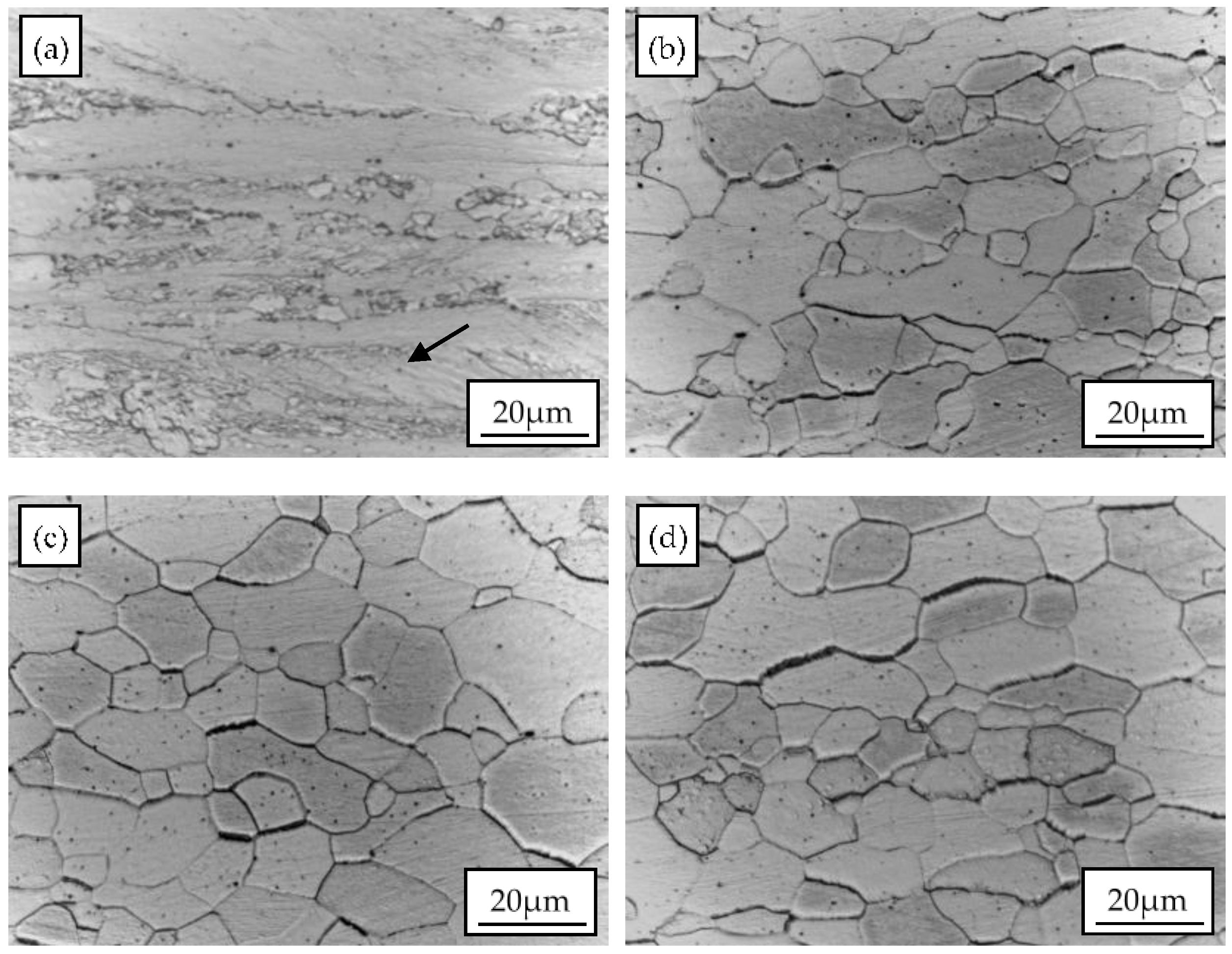
3.1. Microstructure
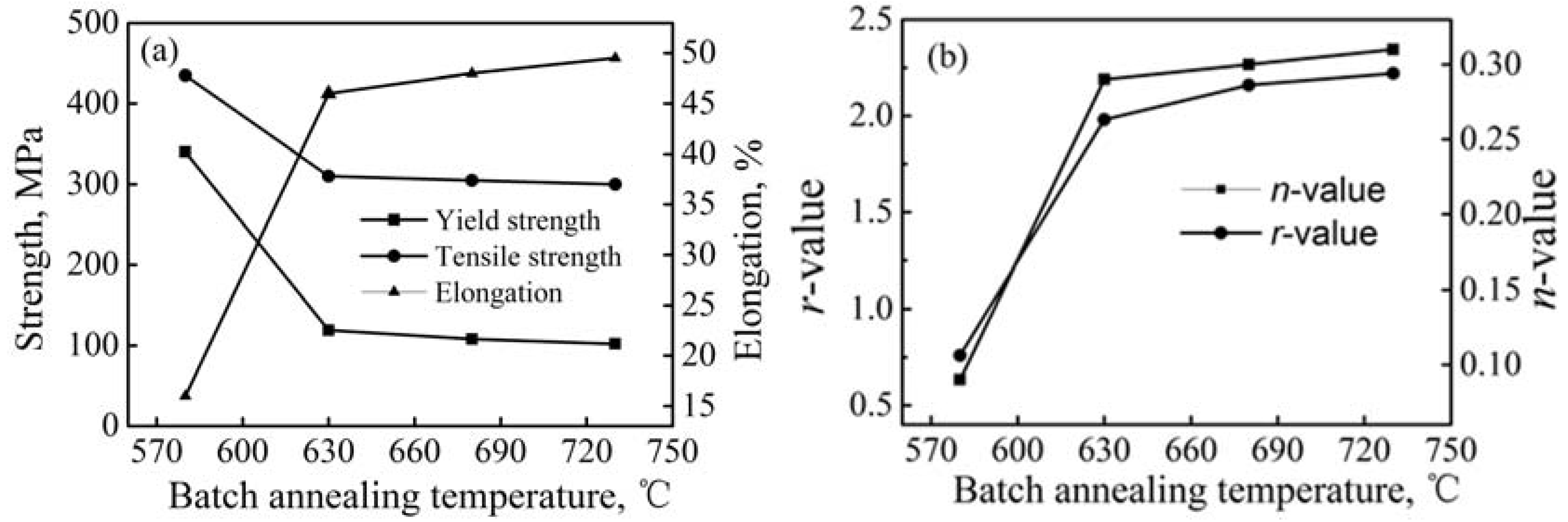
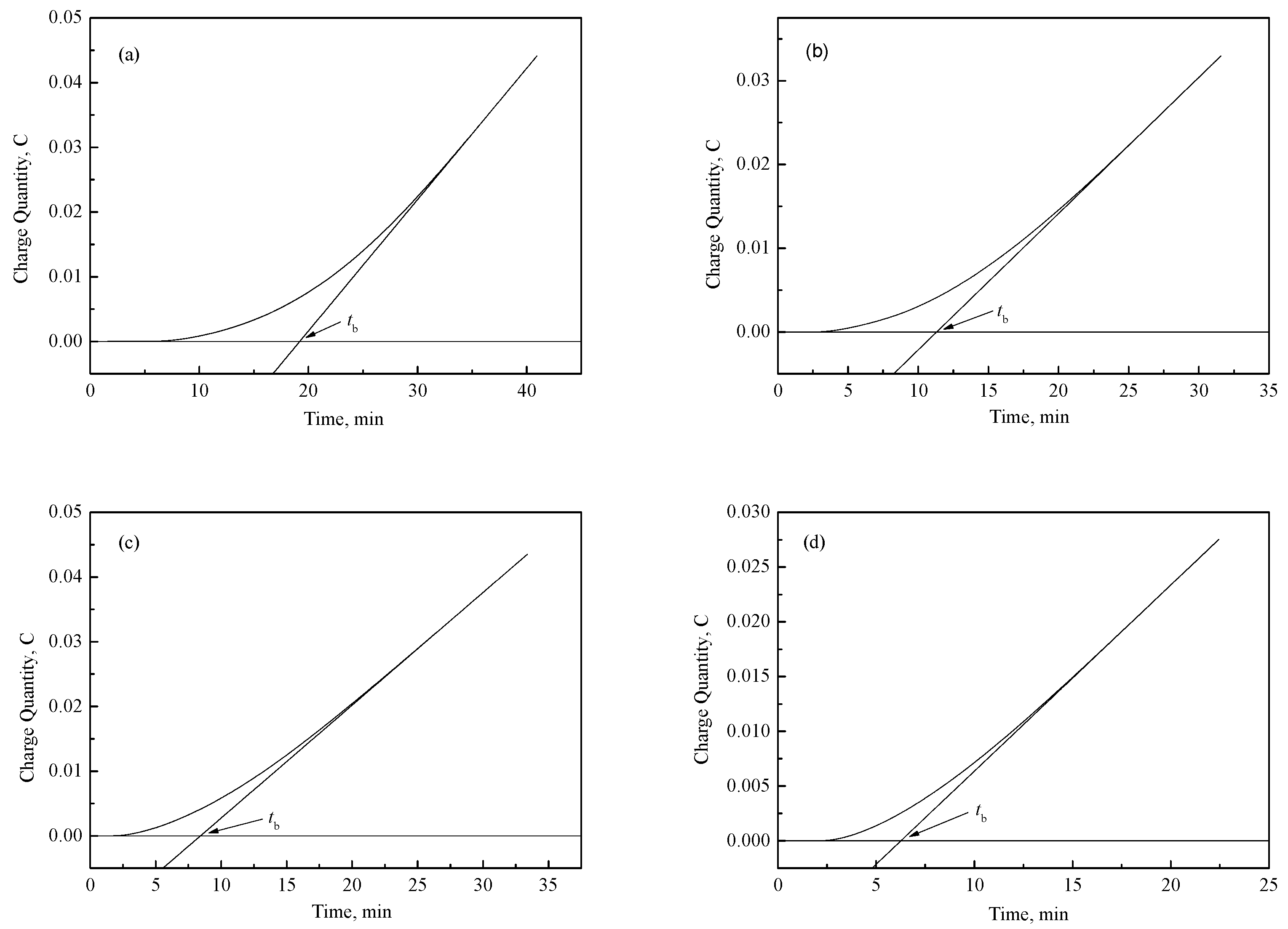
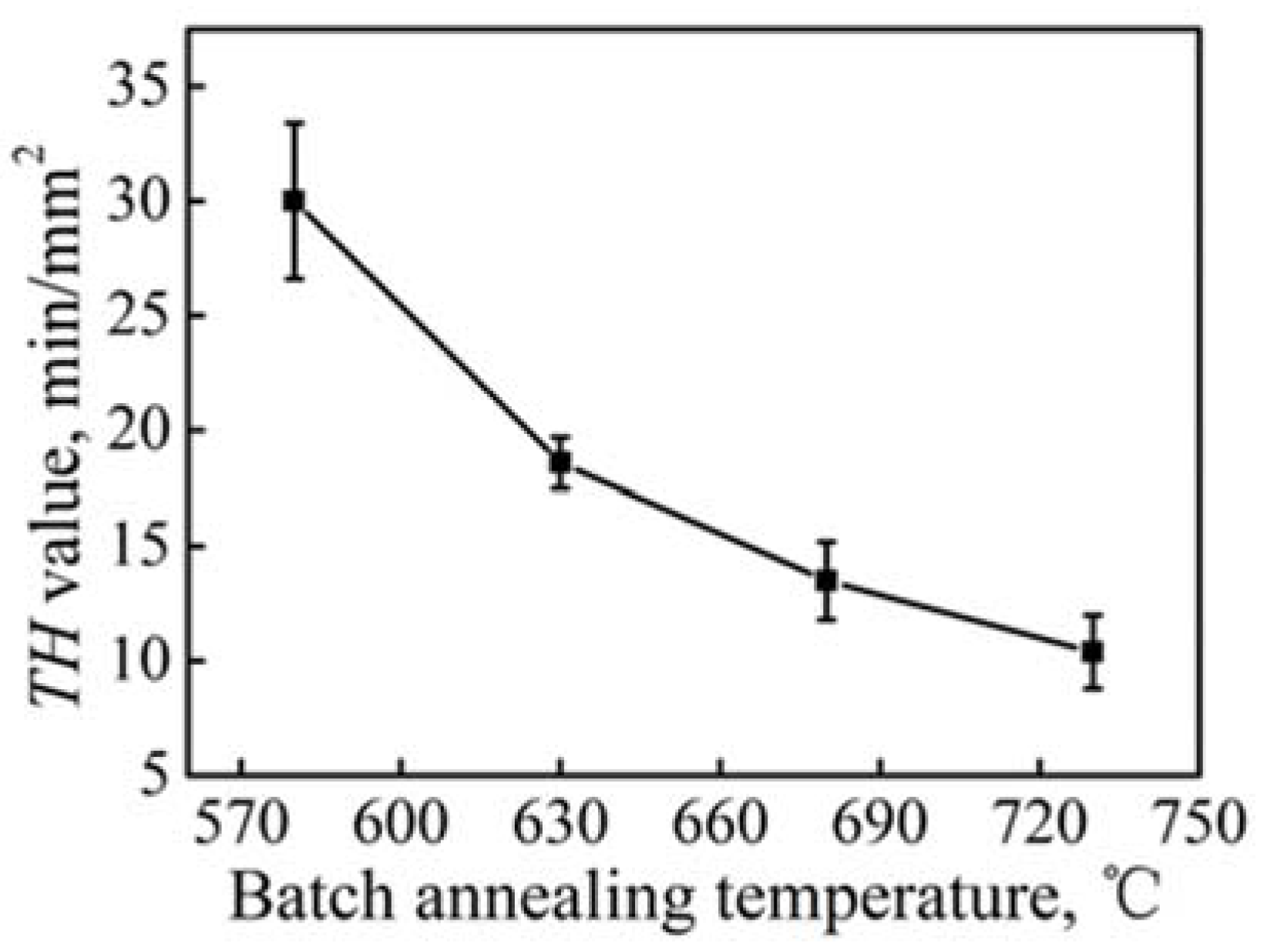
3.2. Mechanical Properties and Resistance to Fish Scaling
4. Conclusions
- (1)
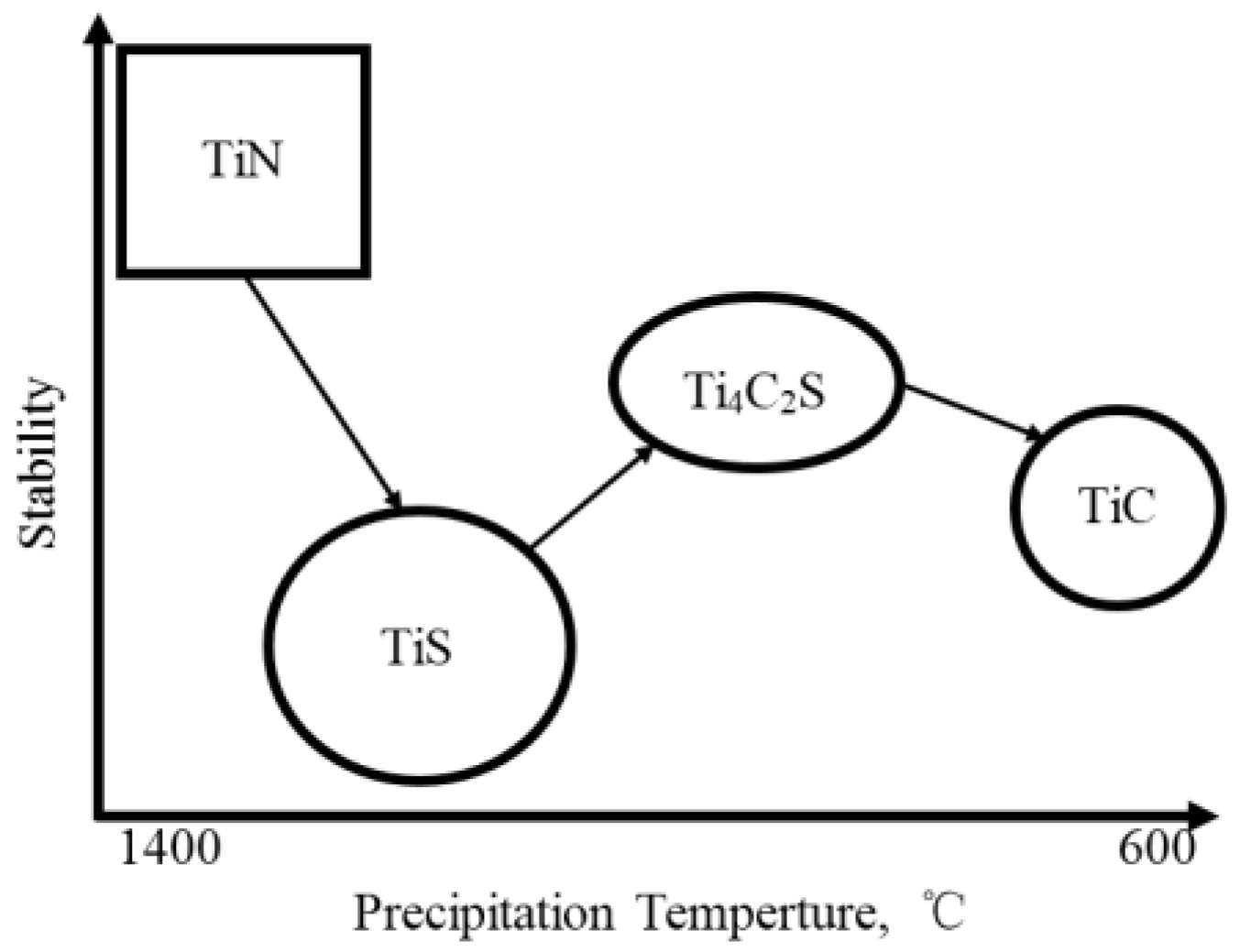
- The main irreversible hydrogen traps of ultra-low carbon enamel steel are fine TiC and coarse Ti4C2S2 particles. The reversible hydrogen traps are grain boundaries and dislocations. The mean sizes of TiC and Ti4C2S2 particles increase with increasing the batch annealing temperature.
- (2)
- Both reversible and irreversible traps will influence the resistance to fish scaling. The resistance to fish scaling can be enhanced with increasing the number of reversible and irreversible traps. The resistance to fish scaling decreases with increasing the batch annealing temperature, which is caused by the growth of ferrite grain and the coarsening of TiC and Ti4C2S2 particles.
Author Contributions
Conflicts of Interest
References
- Dong, F.T.; Du, L.X.; Liu, X.H.; Hu, J.; Xue, F. Effect of Ti(C, N) precipitation on texture evolution and fish-scale resistance of ultra-low carbon Ti bearing enamel steel. J. Iron Steel Res. Int. 2013, 20, 39–45. [Google Scholar] [CrossRef]
- Yuan, X. Precipitates and hydrogen permeation behavior in ultra-low carbon steel. Mater. Sci. Eng. A 2007, 452, 116–120. [Google Scholar] [CrossRef]
- Son, Y.K.; Lee, C.J.; Lee, J.M.; Kim, B.M. Deformation prediction of porcelain-enameled steels with strain history by press forming and high-temperature behavior of coating layer. Trans. Nonferr. Met. Soc. China 2012, 22, s838–s844. [Google Scholar] [CrossRef]
- Okuyamas, T.; Nishimoto, A.; Kurokawa, T. New type cold rolled steel sheet for enamelling produced by the continuous casting method. Vitreous Enamel. 1990, 41, 49–60. [Google Scholar]
- Mohtadi-Bonab, M.A.; Szpunar, J.A.; Collins, L.; Stankievech, R. Evaluation of hydrogen induced cracking behavior of API X70 pipeline steel at different heat treatments. Int. J. Hydrog. Energy 2014, 39, 6076–6088. [Google Scholar] [CrossRef]
- Krom, A.H.; Bakker, A. Hydrogen trapping models in steel. Metall. Mater. Trans. B 2000, 31, 1475–1482. [Google Scholar] [CrossRef]
- Liu, Q.; Atrens, A. Reversible hydrogen trapping in a 3.5NiCrMoV medium strength steel. Corros. Sci. 2015, 96, 112–120. [Google Scholar] [CrossRef]
- Liu, Q.L.; Venezuela, J.; Zhang, M.X.; Zhou, Q.J.; Atrens, A. Hydrogen trapping in some advanced high strength steels. Corros. Sci. 2016, 111, 770–785. [Google Scholar] [CrossRef]
- Hua, M.; Garcia, C.I.; Eloot, K.; DeArdo, A.J. Identification of Ti-S-C-containing multi-phase precipitates in ultra-low carbon steels by analytical electron microscopy. ISIJ Int. 1997, 37, 1129–1132. [Google Scholar] [CrossRef]
- Funakawa, Y.; Shiozaki, T.; Tomita, K.; Yamamoto, T.; Maeda, E. Development of high strength hot-rolled sheet steel consisting of ferrite and nanometer-sized carbides. ISIJ Int. 2004, 44, 1945–1951. [Google Scholar] [CrossRef]
- Huo, X.; Li, L.; Peng, Z.; Chen, S. Effect of TMCP schedule on precipitation, microstructure and properties of Ti-microalloyed high strength steel. J. Iron Steel Res. Int. 2016, 23, 593–601. [Google Scholar] [CrossRef]
- Kim, Y.W.; Song, S.W.; Seo, S.J.; Hong, S.; Lee, C.S. Development of Ti and Mo micro-alloyed hot-rolled high strength sheet steel by controlling thermomechanical controlled processing schedule. Mater. Sci. Eng. A 2013, 565, 430–438. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Sun, M.; Yi, H.; Liu, Z. The blocking effects of interphase precipitation on dislocations’ movement in Ti-bearing micro-alloyed steels. Mater. Lett. 2015, 139, 177–181. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X. Comparison of precipitate behaviors in ultra-low carbon, titanium-stabilized interstitial free steel sheets under different annealing processes. J. Mater. Eng. Perform. 1999, 8, 641–648. [Google Scholar] [CrossRef]
- Ghosh, P.; Ghosh, C.; Ray, R.K.; Bhattcharjee, D. Precipitation behavior and texture formation at different stages of processing in an interstitial free high strength steel. Scr. Mater. 2008, 59, 276–278. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The mechanism of hydrogen evolution on iron in acid solutions by determination of permeation rates. J. Electrochem. Soc. 1964, 111, 619–623. [Google Scholar] [CrossRef]
- International Organization for Standardization. Method of Measure of Hydrogen Permeation and Determination of Hydrogen Uptake and Transport in Metals by an Electrochemical Technique; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- Mohtadi-Bonab, M.A.; Szpunar, J.A.; Razavi-Tousi, S.S. A comparative study of hydrogen induced cracking behavior in API 5L X60 and X70 pipeline steels. Eng. Fail. Anal. 2013, 33, 163–175. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Karimdadashi, R.; Eskandari, M.; Szpunar, J.A. Hydrogen-induced cracking assessment in pipeline steels through permeation and crystallographic texture measurements. J. Mater. Eng. Perform. 2016, 25, 1781–1793. [Google Scholar] [CrossRef]
- Haq, A.J.; Muzaka, K.; Dunne, D.P.; Calka, A.; Pereloma, E.V. Effect of microstructure and composition on hydrogen permeation in X70 pipeline steels. Int. J. Hydroen Energy 2013, 38, 2544–2556. [Google Scholar] [CrossRef]
- Winzer, N.; Rott, O.; Thiessen, R.; Thomas, I.; Mraczek, K.; Höche, T.; Wright, L.; Mrovec, M. Hydrogen diffusion and trapping in Ti-modified advanced high strength steels. Mater. Des. 2016, 92, 450–461. [Google Scholar] [CrossRef]
- Yoshinaga, N.; Ushioda, K.; Akamatsu, S.; Akisue, O. Precipitation behavior of sulfides in Ti-added ultra low-carbon steels in austenite. ISIJ Int. 1994, 34, 24–32. [Google Scholar] [CrossRef]
- Ghosh, P.; Ghosh, C.; Ray, R.K. Thermodynamics of precipitation and textural development in batch-annealed interstitial-free high-strength steels. Acta Mater. 2010, 58, 3842–3850. [Google Scholar] [CrossRef]
- Hua, M.; Garcia, C.I.; DeArdo, A.J. Multi-phase precipitates in interstitial-free steels. Scr. Metall. Mater. 1993, 28, 973–978. [Google Scholar] [CrossRef]
- Carabajar, S.; Merlin, J.; Massardier, V.; Chabanet, S. Precipitation evolution during the annealing of an interstitial-free steel. Mater. Sci. Eng. A 2000, 281, 132–142. [Google Scholar] [CrossRef]
- Ono, K.; Meshii, M. Hydrogen detrapping from grain boundaries and dislocations in high purity iron. Acta Metall. Mater. 1992, 40, 1357–1364. [Google Scholar] [CrossRef]
- Choo, W.Y.; Lee, J.Y. Thermal analysis of trapped hydrogen in pure iron. Metall. Trans. A 1982, 13, 135–140. [Google Scholar] [CrossRef]
- Valentini, R.; Solina, A.; Matera, S.; de Gregorio, P. Influence of titanium and carbon contents on the hydrogen trapping of microalloyed steels. Metall. Mater. Trans. A 1996, 27, 3773–3780. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y.; Tarui, T. The first direct observation of hydrogen trapping sites in TiC precipitation-hardening steel through atom probe tomography. Scr. Mater. 2010, 63, 261–264. [Google Scholar] [CrossRef]
- Wei, F.G.; Tsuzaki, K. Quantitative analysis on hydrogen trapping of TiC particles in steel. Metall. Mater. Trans. A 2006, 37, 331–353. [Google Scholar] [CrossRef]
- Pressouyre, G.M.; Bernstein, I.M. A quantitative analysis of hydrogen trapping. Metall. Trans. A 1978, 9, 1571–1580. [Google Scholar] [CrossRef]
- Jebaraj, J.J.M.; Morrison, D.J.; Suni, I.I. Hydrogen diffusion coefficients through Inconel 718 in different metallurgical conditions. Corros. Sci. 2014, 80, 517–522. [Google Scholar] [CrossRef]



| C | Si | Mn | P | S | Alt | Ti |
|---|---|---|---|---|---|---|
| ≤0.005 | ≤0.05 | 0.12–0.25 | ≤0.02 | ≤0.05 | ≤0.05 | 0.05–0.12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Kang, Y.; Zhang, Z.; Shao, X. Effect of Batch Annealing Temperature on Microstructure and Resistance to Fish Scaling of Ultra-Low Carbon Enamel Steel. Metals 2017, 7, 51. https://doi.org/10.3390/met7020051
Liu Z, Kang Y, Zhang Z, Shao X. Effect of Batch Annealing Temperature on Microstructure and Resistance to Fish Scaling of Ultra-Low Carbon Enamel Steel. Metals. 2017; 7(2):51. https://doi.org/10.3390/met7020051
Chicago/Turabian StyleLiu, Zaiwang, Yonglin Kang, Zhimin Zhang, and Xiaojing Shao. 2017. "Effect of Batch Annealing Temperature on Microstructure and Resistance to Fish Scaling of Ultra-Low Carbon Enamel Steel" Metals 7, no. 2: 51. https://doi.org/10.3390/met7020051





