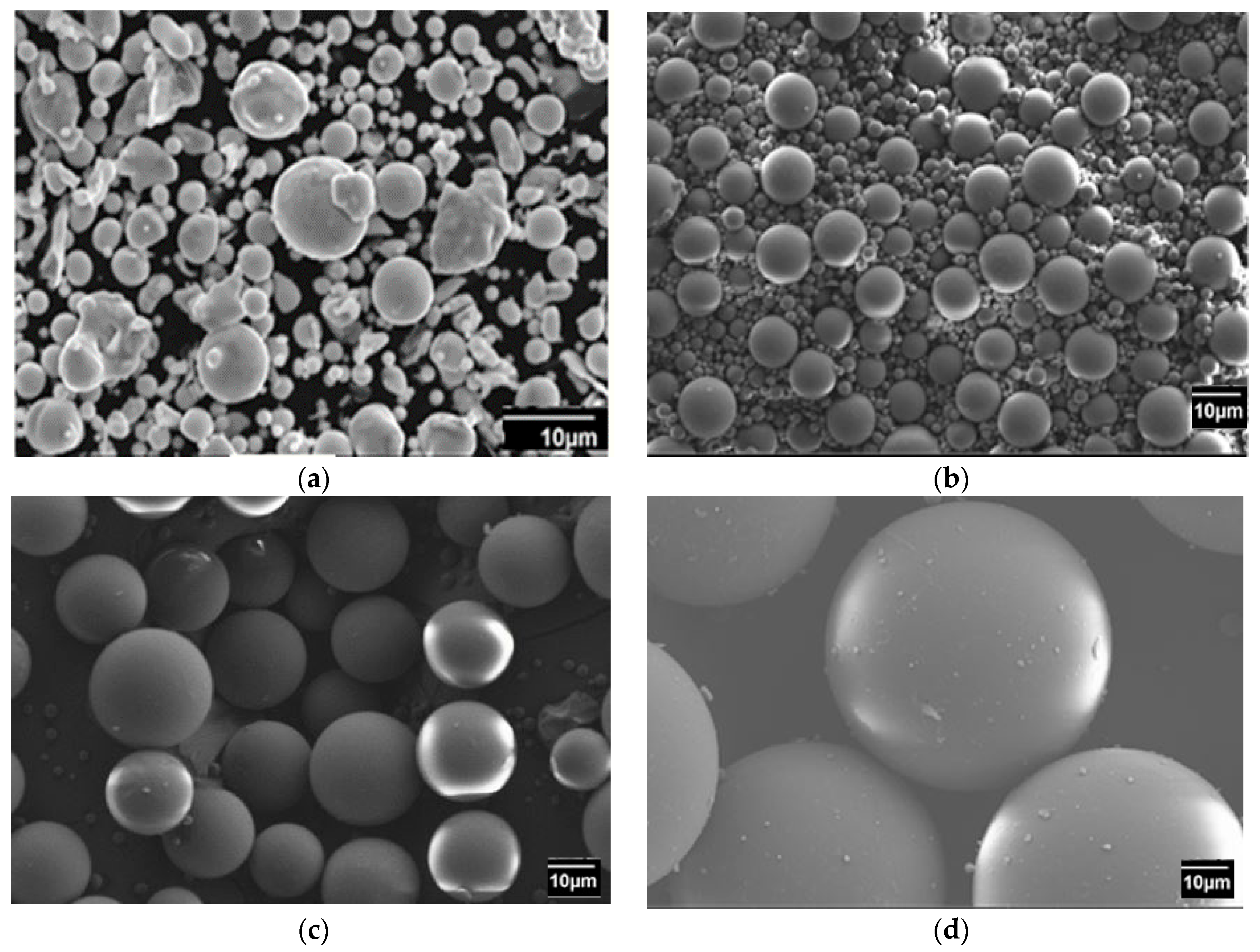
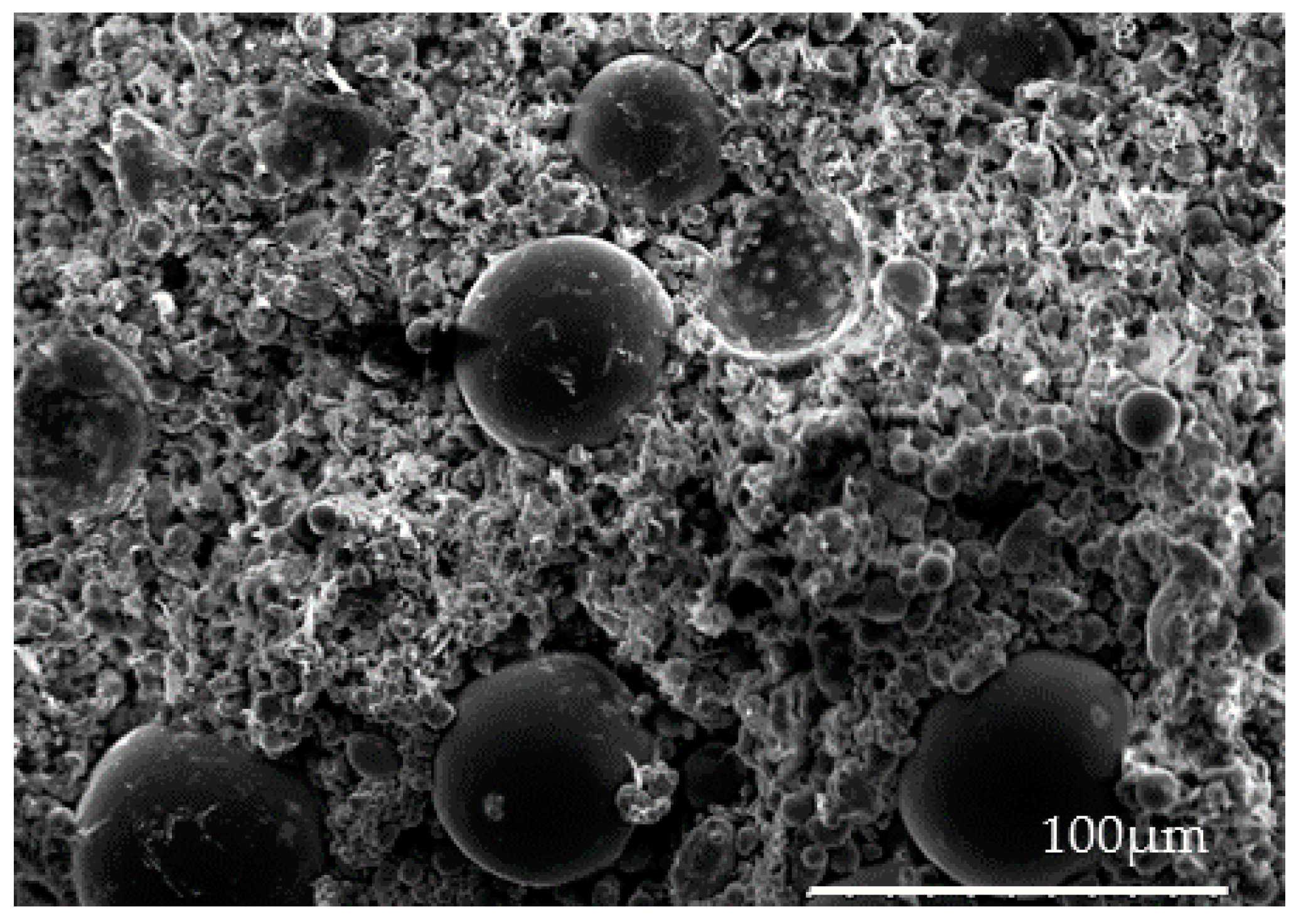
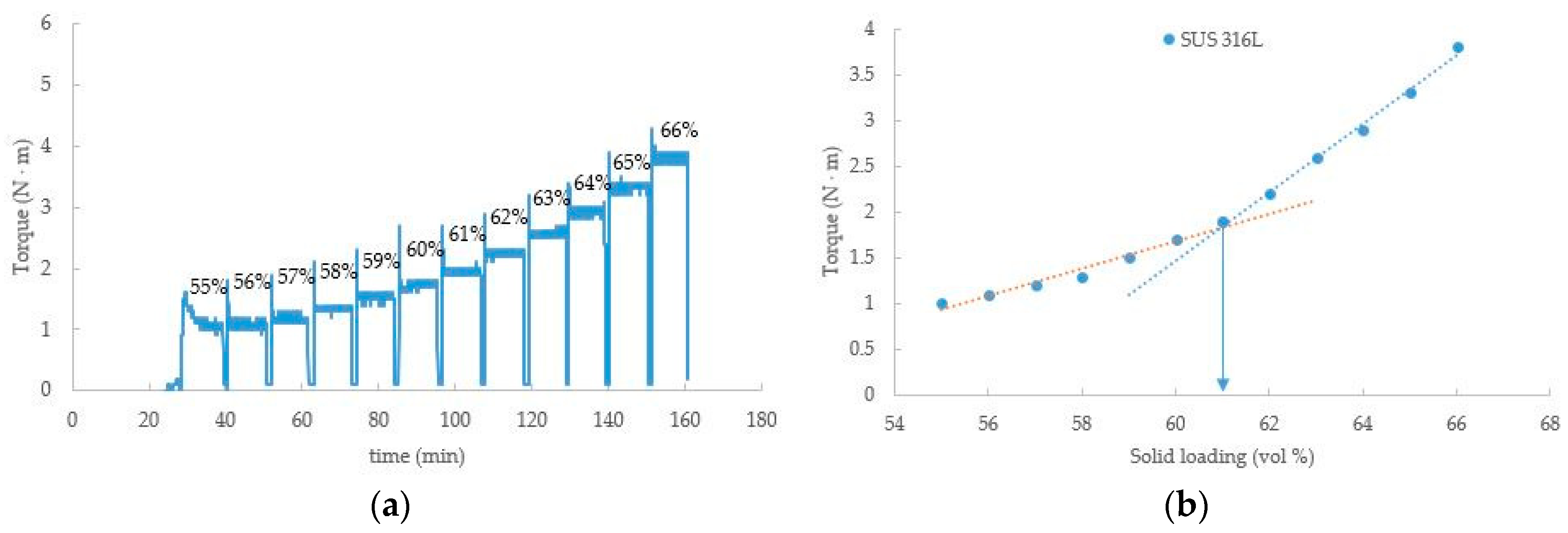
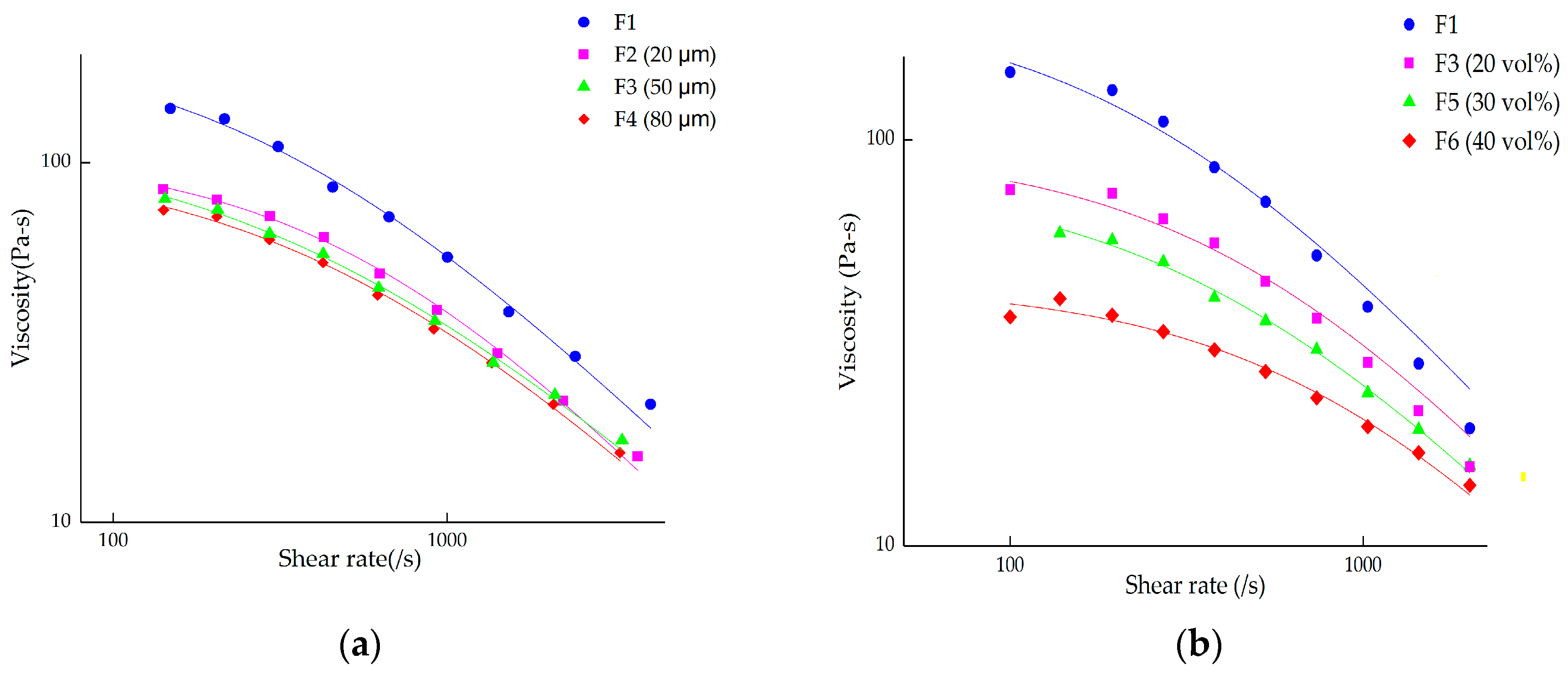
4.2. Debinding and Sintering Process
The debinding process is generally a process of removing the binder from the green part. In our study, space holders have also been removed from the debinding process and are one of the key technologies in the overall process. PMMA also can be used as a binder, but since it is used as a space holder in this study, it should keep its shape in the binder during the mixing and injection molding process as mentioned earlier. In order to remove the binder and PMMA space holder, two sequential debinding processes were performed, solvent and thermal debinding. In this study, solvent debinding was performed using a hexane solution and the appropriate debinding time was determined by examining the weight reduction rate of the product after a certain period of time as follows [
16]:
is the initial weight of the product, and
is the weight after the solvent debinding process. The following graph shows the experimental results of the weight loss ratio of the part over time in hexane at a constant temperature of 50 °C. The appropriate time for solvent debinding can be deduced from
Figure 9 that the weight reduction rate during the solvent debinding increases for the first 8 h, but then converges afterwards.
Binders with low molecular weights, such as SA or PW, are weak to heat and may decompose and crack before sufficient channels to the surface are formed. Therefore, it is necessary to form a channel during the solvent debinding. 5.68 wt. % of SA and PW (total 5.75 wt. %) are removed after the solvent debinding process and the remaining SA and PW are removed completely at the thermal debinding stage as shown in
Table 8. The thermal debinding process conditions can be obtained from the thermal properties of the constituents of the binder and space holder. Therefore, mass change with time was observed using a thermogravimetric analyzer (Mettler Toledo TGA/DSC 1, Columbus, OH, USA) to obtain the decomposition temperature of the binder and PMMA. The TGA measurement was carried out at 30–600 °C at a heating rate of 10 °C/min in a hydrogen atmosphere, much like the debinding process atmosphere (
Figure 10).
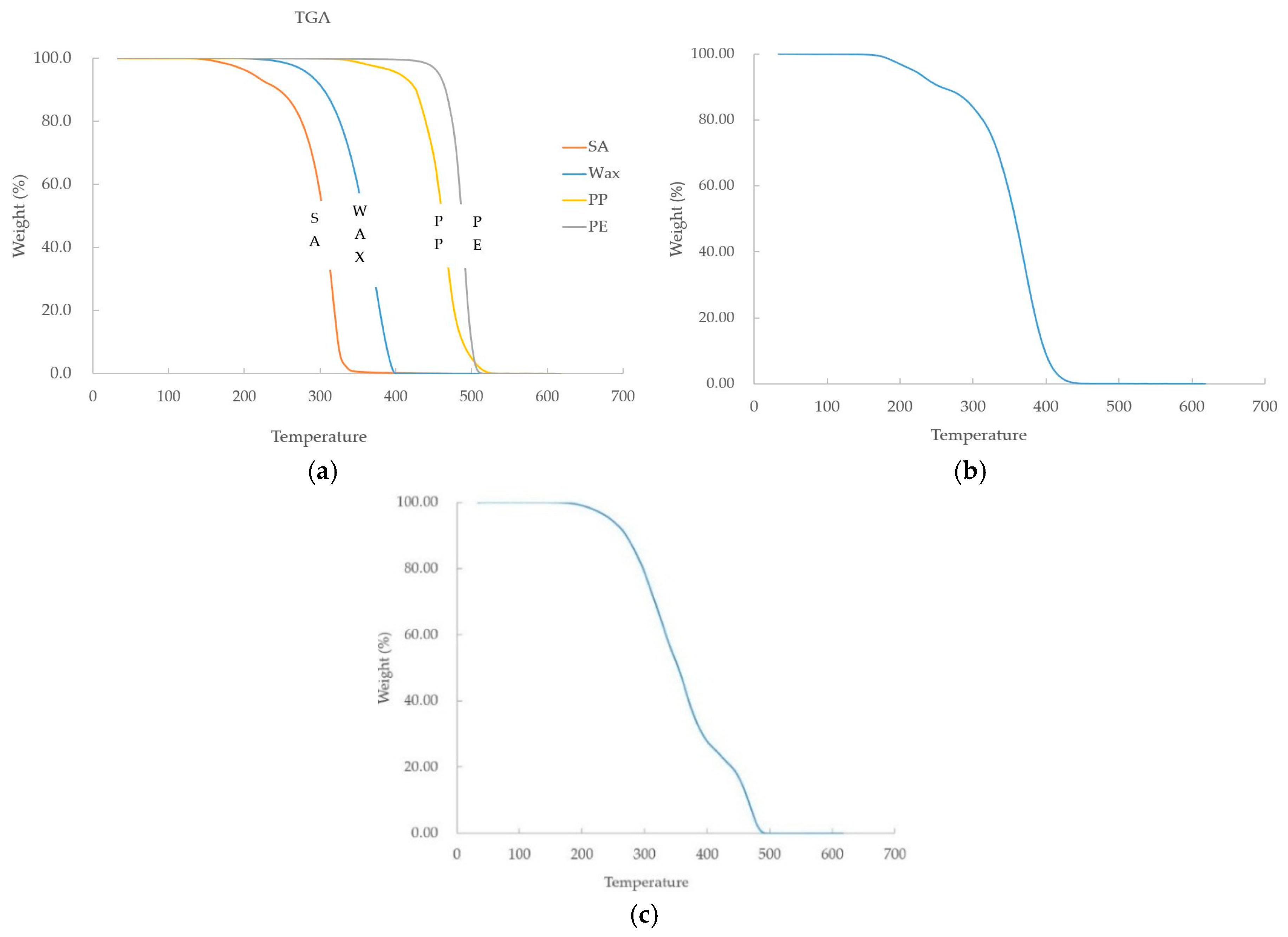
In this study, a binder composed of PW, SA, PP, and PE was used. The TGA results show that SA and PW start to decompose between 180 °C and 200 °C, and decompose completely between 380 °C and 400 °C. Then, decomposition of PP and PE start at 380–400 °C, where SA and PW were completely decomposed, and decomposed completely at about 500 °C. In the case of PMMA, decomposition starts at 180 °C to 200 °C, and decomposes completely at 440 °C, similar to decomposition start temperature of PW and SA. The TGA results of the feedstock show that the mass started to decrease at 180 °C to 200 °C as the result of the binder and PMMA. After that, SA, PW, and PMMA decreased rapidly at 200 °C and 400 °C, respectively.
Debinding conditions were set through the decomposition temperature obtained from TGA results.
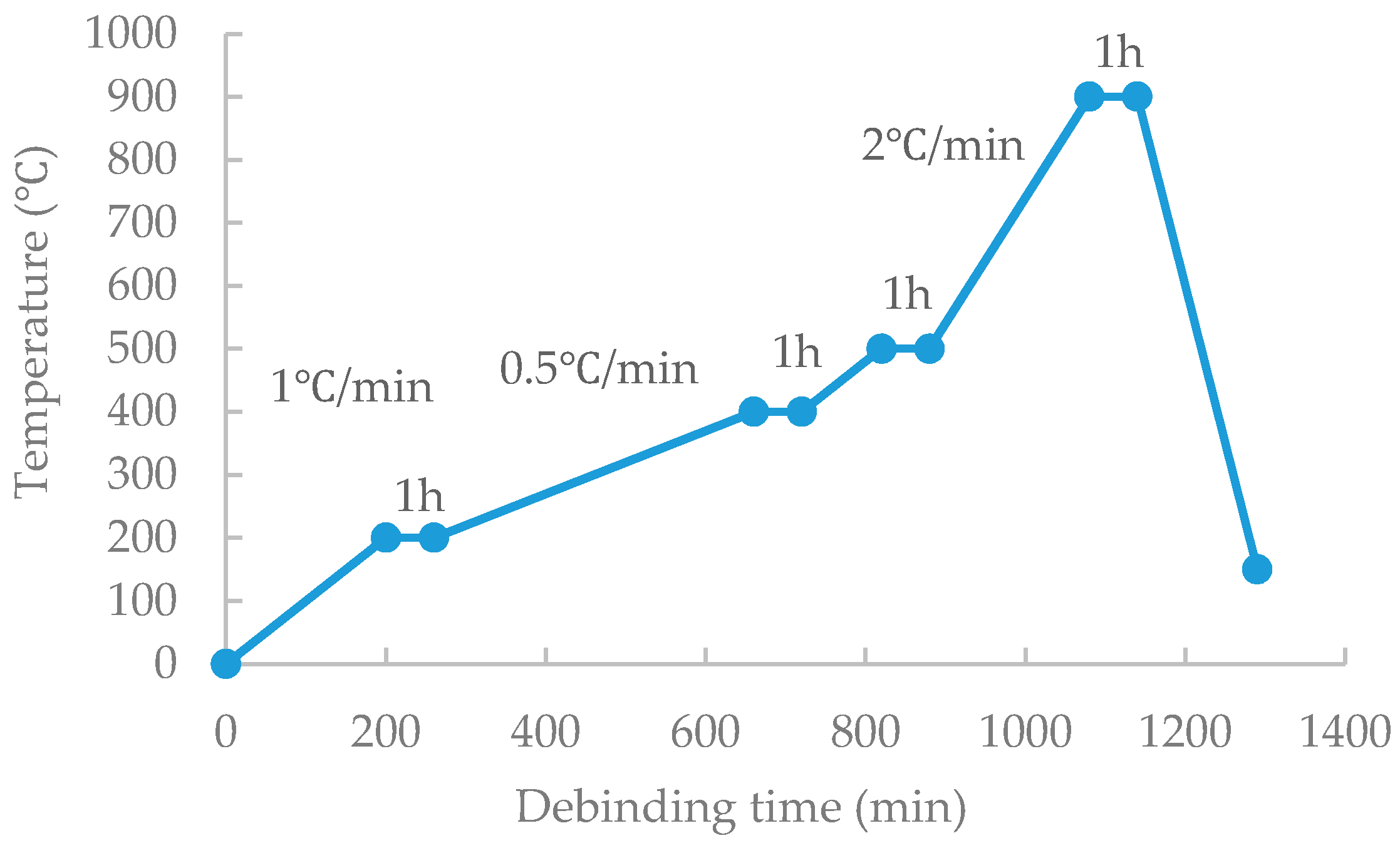
Stage 1. Decomposition of SA and PW (Up to 200 °C by 1 °C/min for channel formation and then held for 1 h).
Stage 2. Complete decomposition of SA and PW, and complete decomposition of space holder (up to 400 °C by 1 °C/min then holding for 1 h).
Stage 3. Complete decomposition of PP and PE (up to 500 ° C by 1 °C/min and holding for 1 h).
During the debinding process, it was maintained at 200 °C, 400 °C, and 500 °C for 1 h so that sufficient channels for the binder’s flows can be formed. In the binder removal process, hydrogen gas was continuously supplied to remove the gas generated by decomposition of the binder.
Moreover, in order to prevent the formation of oxides on the surface of the green part, the debinding process was carried out in a hydrogen atmosphere. Through the derived debinding profile, at the first attempt, a crack was found in the debinded body.
Considering that at the first debinding stage, the weight loss at 250–400 °C originated by not only SA and PW, but also the space holder. The mixture is composed of 20 vol % of the space holder, so more time is needed than a conventional debinding. The heating rate in the range of 200–400 °C was changed from 1 °C/min to 0.5 °C/min so the space holder could be decomposed with a sufficient time. After this, the remaining binder was removed to prevent breakage in the brown part by heating up to 900 °C for pre-sintering. Finally, a brown part without cracks could be produced. The optimized debinding and pre-sintering processes are shown in
Figure 11.
The mass change during solvent debinding and thermal debinding was calculated using the density and fraction of powder, binder, and the space holder by using the following equations [
1]:
where,
is the density of the green part and
,
,
is the density of the powder, binder, and the space holder.
,
,
is the volume fraction of powder, binder, and space holder. The sum of the volume fraction of powder, binder and space holder in the green part is constant.
In this study, the volume fraction of binder is constant at 0.4, and the value of
,
,
are 7.95
, 0.9058
, and 1.19
. Using these values, the mass of the green part,
, and the volume of the green part,
, can be calculated. Then, the mass of binder,
, and space holder,
, can be calculated by the following equations and the values are listed in
Table 8:
After the solvent debinding process, the weight of the green part was reduced by 5.68 wt %; mainly, the reduced weight was the PW and SA. Since the total wt. % of PW and SA is 5.75 wt. %, the remaining PW and SA will be further removed in the following thermal debinding process. After the thermal debinding process, the final brown part weighs 16.14 g so it can be assured that the remained binders are completely removed. The sintering process was performed under the following conditions as listed in
Table 9. Since the binder and space holder were removed during the debinding process, the sintering process was carried out in the same way as the conventional SUS 316L sintering process.
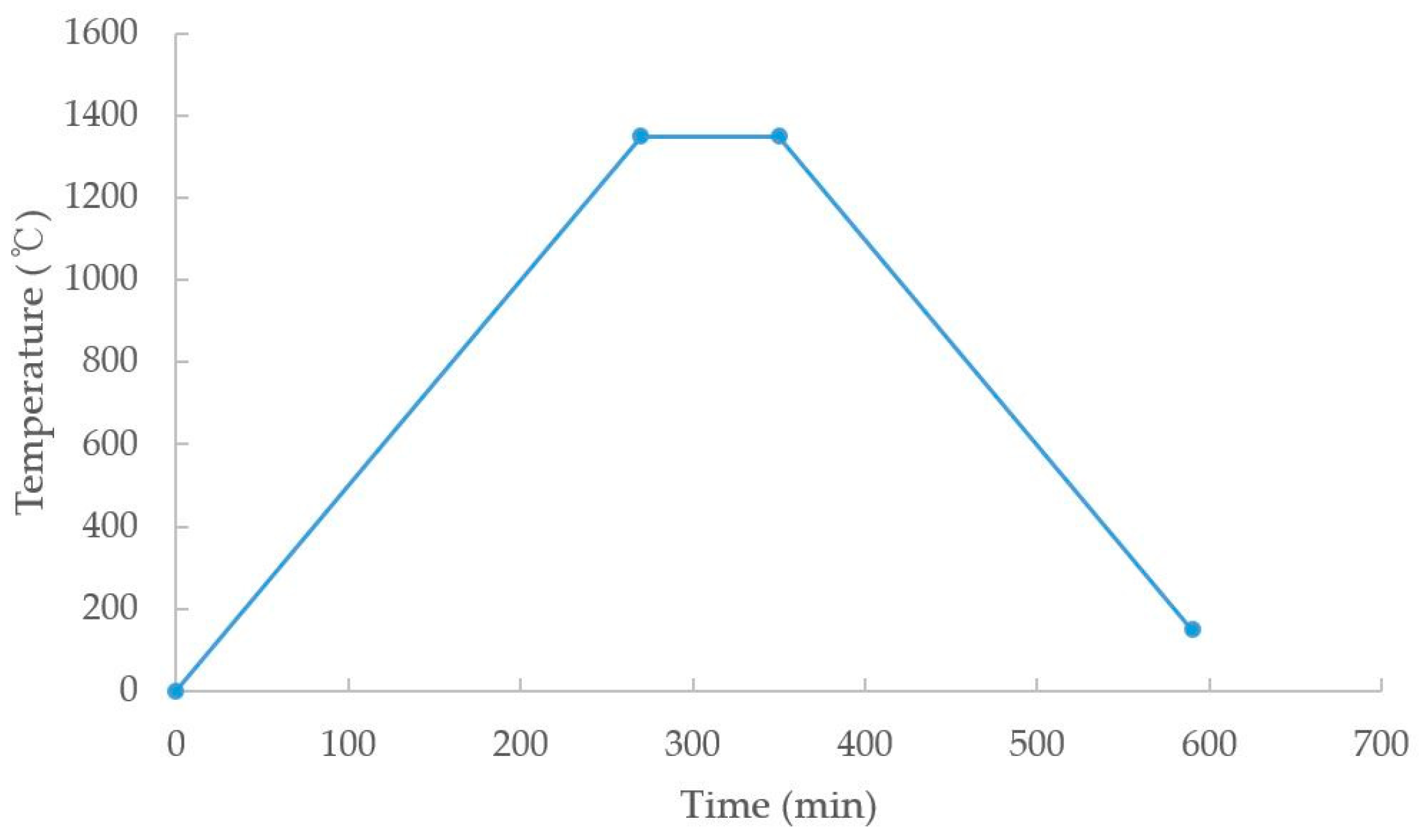
In general, the residual binder can be removed by maintaining the temperature around 450 °C to 500 °C, but since the binder was removed through pre-sintering during the debinding process in this study, it was omitted (
Figure 12).
The sintering process was performed in a hydrogen atmosphere to prevent the formation of oxides due to air, and the sintering was completed by maintaining the temperature at 1350 °C for 80 min. As a result, the tensile specimen without deformation was obtained.