Transferring Nanoscale Bainite Concept to Lower C Contents: A Perspective
Abstract
:1. Structural Refinement of Bainitic Steels: General Considerations
2. Nanostructured Bainite
3. Transferring Nanostructured Bainite Concept
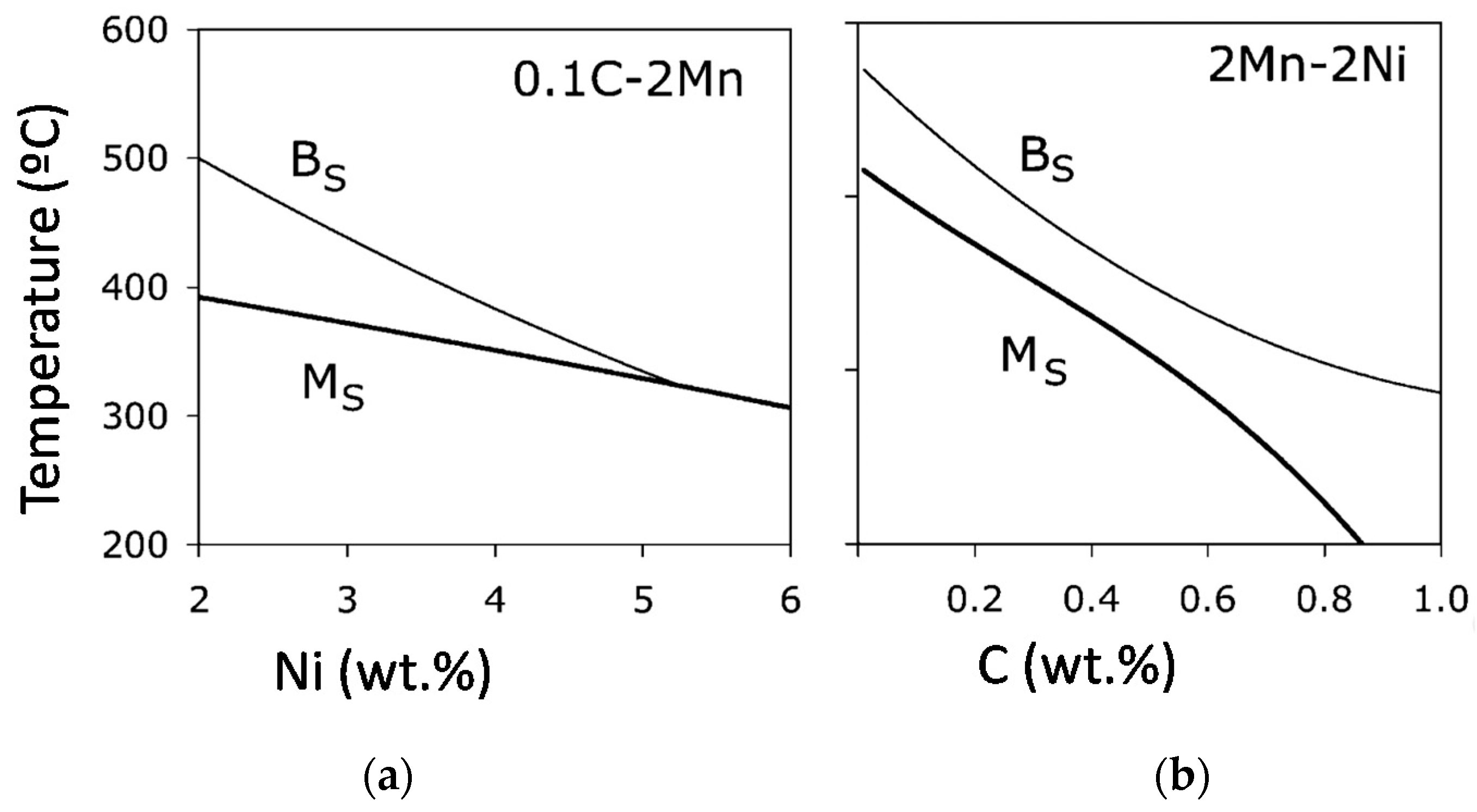
3.1. Chemical Composition Modification
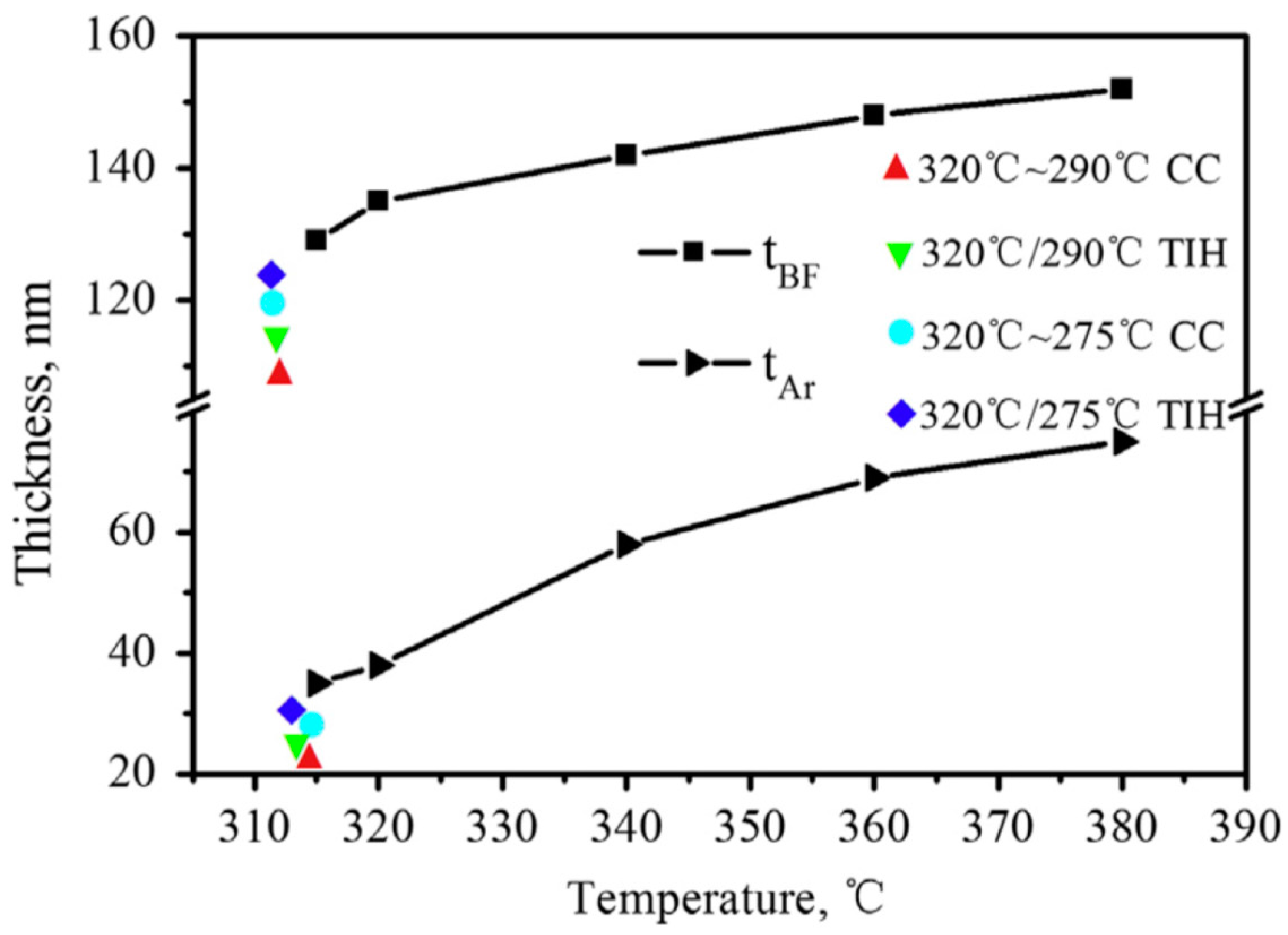
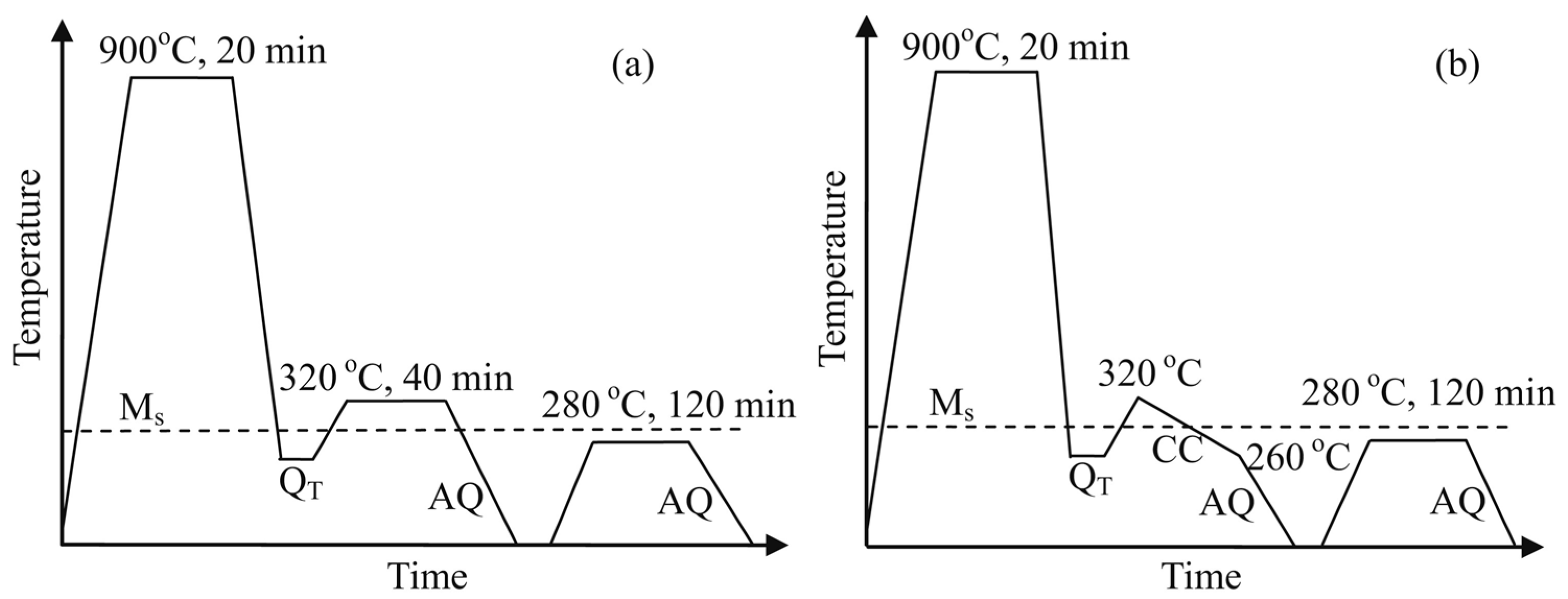
3.2. Heat Treatment Variations
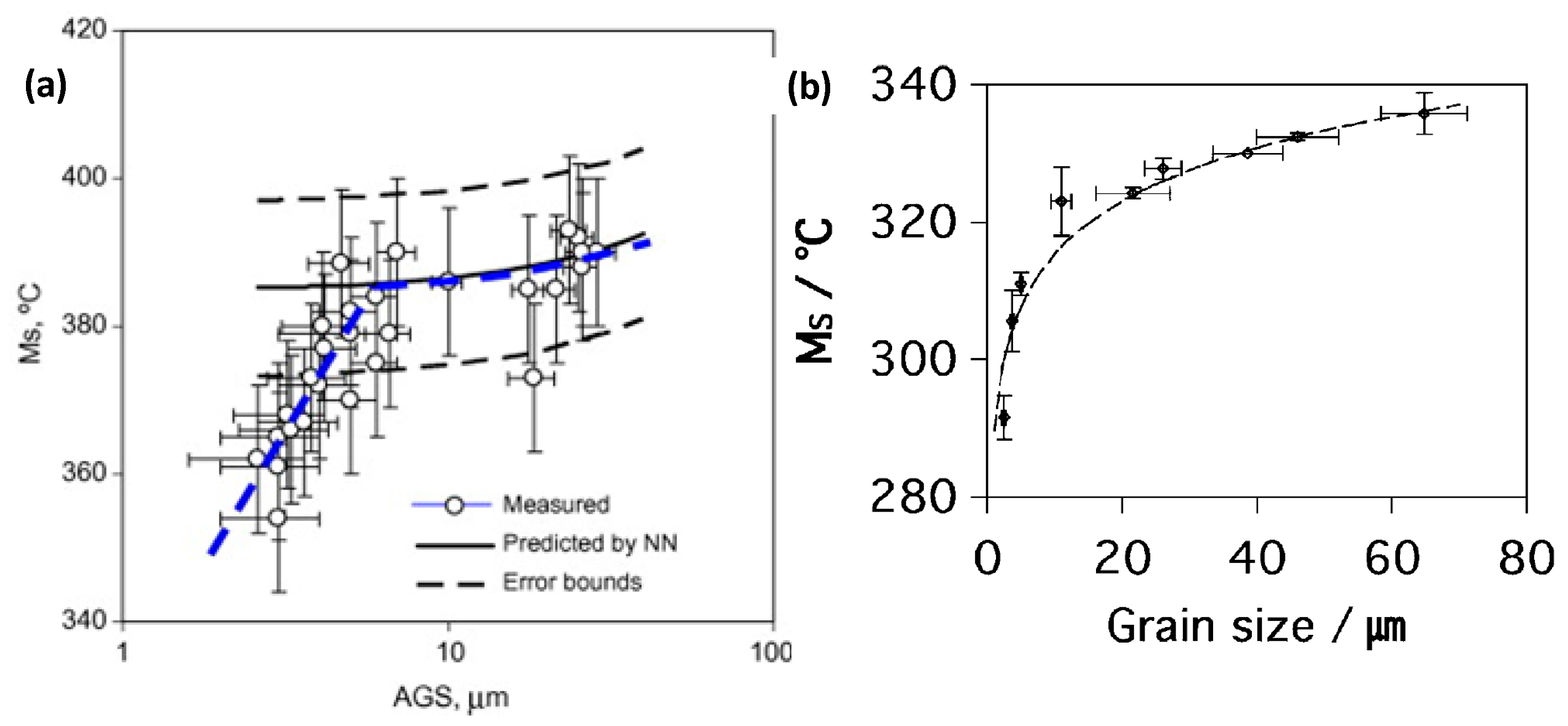
3.3. Prior Autenite Grain Size Control (No Deformation)
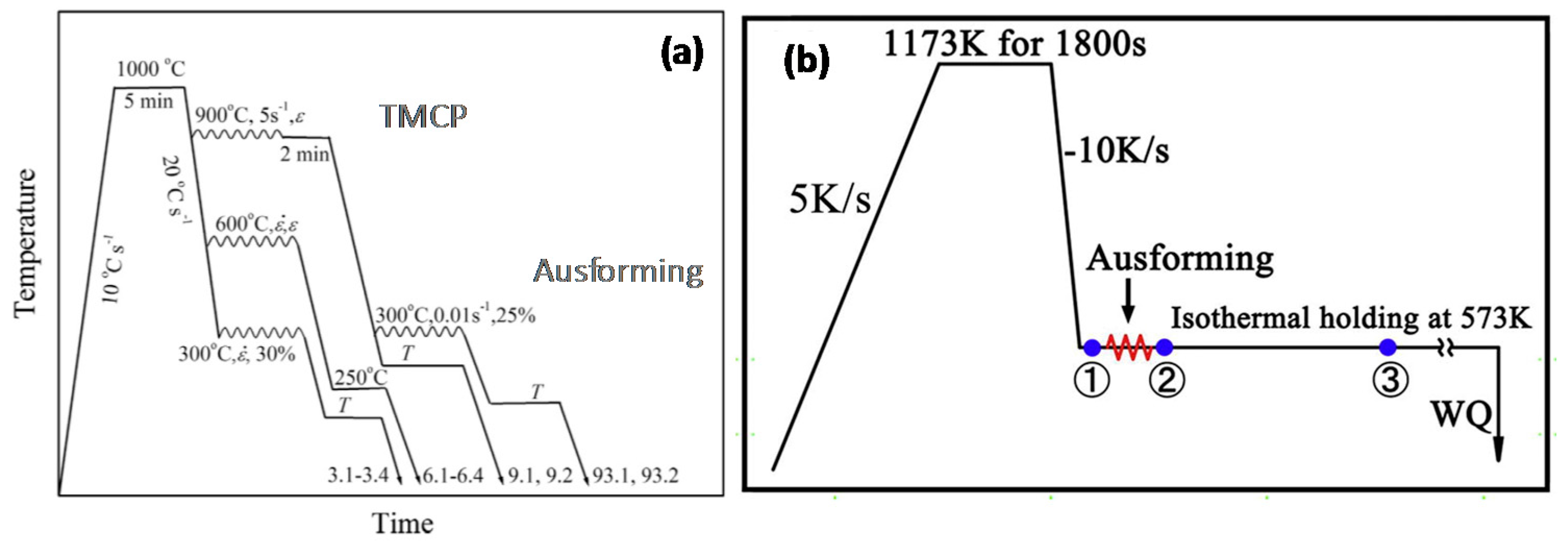
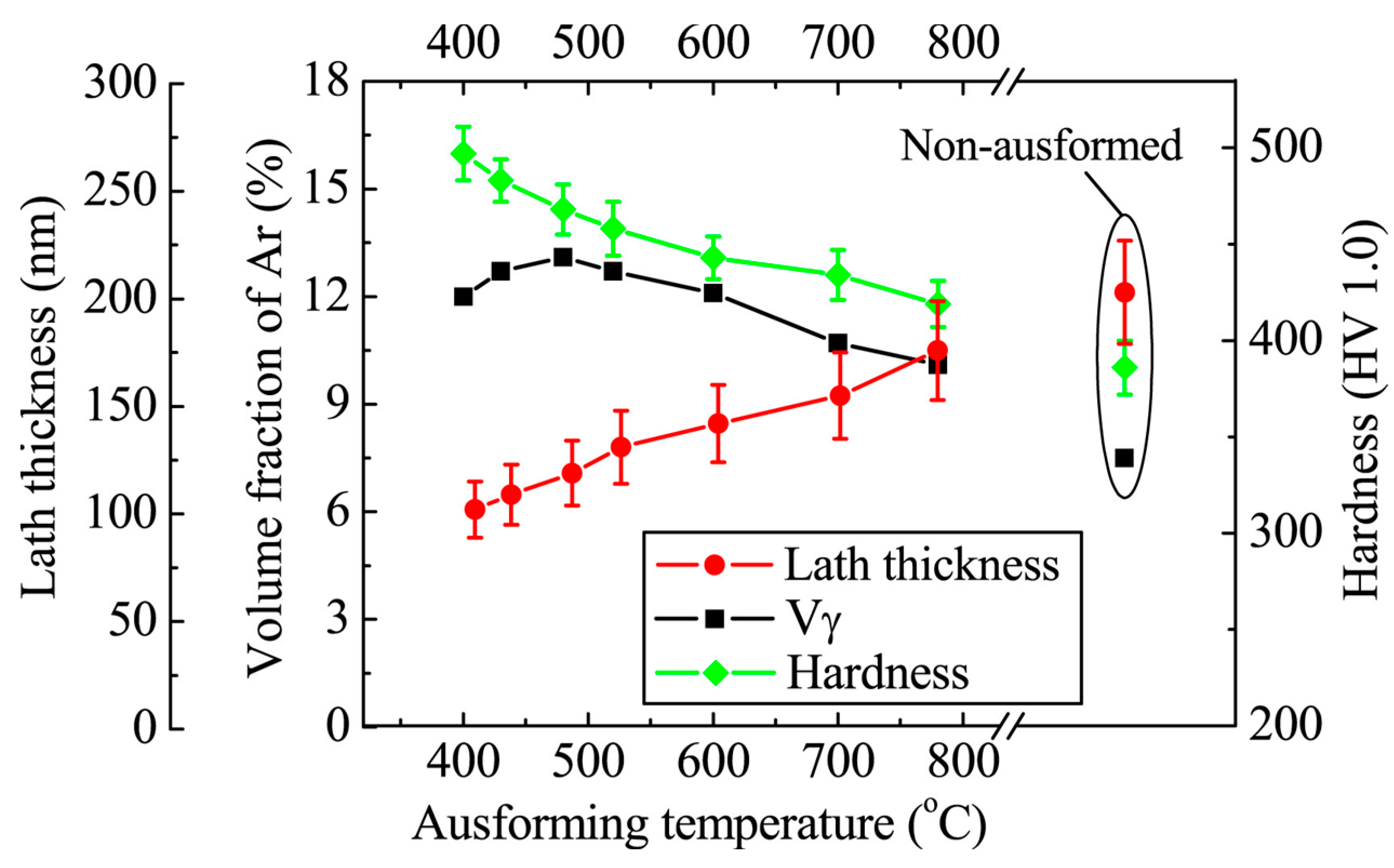
3.4. Deformation of Austenite (AUSFORMING)
3.5. Considerations
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Singh, S.B.; Bhadeshia, H.K.D.H. Estimation of bainite plate-thickness in low-alloy steels. Mater. Sci. Eng. A 1998, 245, 72–79. [Google Scholar] [CrossRef]
- Cornide, J.; Garcia-Mateo, C.; Capdevila, C.; Caballero, F.G. An assessment of the contributing factors to the nanoscale structural refinement of advanced bainitic steels. J. Alloys Compd. 2013, 577, S43–S47. [Google Scholar] [CrossRef]
- Caballero, F.G.; Garcia-Mateo, C.; Sourmail, T. Bainitic steel: Nanostructured. In Encyclopedia of Iron, Steel, and Their Alloys; Taylor & Francis Inc.: Abingdon, UK, 2016; pp. 271–290. [Google Scholar]
- Garcia-Mateo, C.; Caballero, F.G.; Sourmail, T.; Smanio, V.; Garcia de Andres, C. Industrialised nanocrystalline bainitic steels. Design approach. Int. J. Mater. Res. 2014, 105, 725–734. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Sourmail, T.; Caballero, F.G.; Smanio, V.; Kuntz, M.; Ziegler, C.; Leiro, A.; Vuorinen, E.; Elvira, R.; Teeri, T. Nanostructured steel industrialisation: Plausible reality. Mater. Sci. Technol. 2014, 30, 1071–1078. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Very strong low temperature bainite. Mater. Sci. Technol. 2002, 18, 279–284. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G. Ultra-high-strength bainitic steels. ISIJ Int. 2005, 45, 1736–1740. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Sourmail, T.; Kuntz, M.; Cornide, J.; Smanio, V.; Elvira, R. Tensile behaviour of a nanocrystalline bainitic steel containing 3 wt % silicon. Mater. Sci. Eng. A 2012, 549, 185–192. [Google Scholar] [CrossRef]
- Sourmail, T.; Smanio, V.; Ziegler, C.; Heuer, V.; Kuntz, M.; Caballero, F.G.; Garcia-Mateo, C.; Cornide, J.; Elvira, R.; Leiro, A.; et al. Novel Nanostructured Bainitic Steel Grades to Answer the Need for High-Performance Steel Components (Nanobain); Rfsr-ct-2008-00022; European Commission: Luxembourg, 2013; p. 129. [Google Scholar]
- Leiro, A.; Vuorinen, E.; Sundin, K.G.; Prakash, B.; Sourmail, T.; Smanio, V.; Caballero, F.G.; Garcia-Mateo, C.; Elvira, R. Wear of nano-structured carbide-free bainitic steels under dry rolling-sliding conditions. Wear 2013, 298, 42–47. [Google Scholar] [CrossRef]
- Yang, H.S.; Bhadeshia, H.K.D.H. Designing low carbon, low temperature bainite. Mater. Sci. Technol. 2008, 24, 335–342. [Google Scholar] [CrossRef]
- Ohmori, Y.; Ohtsubo, H.; Jung, Y.C.; Okaguchi, S.; Ohtani, H. Morphology of bainite and widmanstätten ferrite. Metall. Mater. Trans. A 1994, 25, 1981–1989. [Google Scholar] [CrossRef]
- Pak, J.; Suh, D.W.; Bhadeshia, H.K.D.H. Promoting the coalescence of bainite platelets. Scr. Mater. 2012, 66, 951–953. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Keehan, E.; Karlsson, L.; Andrén, H.O. Coalesced bainite. Trans. Indian Inst. Met. 2006, 59, 689–694. [Google Scholar]
- Keehan, E.; Karlsson, L.; Andren, H.O. Influence of carbon, manganese and nickel on microstructure and properties of strong steel weld metals: Part 1—Effect of nickel content. Sci. Technol. Weld. Join. 2006, 11, 1–8. [Google Scholar] [CrossRef]
- Pak, J.H.; Bhadeshia, H.K.D.H.; Karlsson, L.; Keehan, E. Coalesced bainite by isothermal transformation of reheated weld metal. Sci. Technol. Weld. Join. 2008, 13, 593–597. [Google Scholar] [CrossRef]
- Caballero, F.G.; Chao, J.; Cornide, J.; Garcia-Mateo, C.; Santofimia, M.J.; Capdevila, C. Toughness deterioration in advanced high strength bainitic steels. Mater. Sci. Eng. A 2009, 525, 87–95. [Google Scholar] [CrossRef]
- Soliman, M.; Mostafa, H.; El-Sabbagh, A.S.; Palkowski, H. Low temperature bainite in steel with 0.26 wt % C. Mater. Sci. Eng. A 2010, 527, 7706–7713. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.; Bhadeshia, H. Acceleration of low-temperature bainite. ISIJ Int. 2003, 43, 1821–1825. [Google Scholar] [CrossRef]
- Soliman, M.; Palkowski, H. Ultra-fine bainite structure in hypo-eutectoid steels. ISIJ Int. 2007, 47, 1703–1710. [Google Scholar] [CrossRef]
- Qian, L.; Zhou, Q.; Zhang, F.; Meng, J.; Zhang, M.; Tian, Y. Microstructure and mechanical properties of a low carbon carbide-free bainitic steel co-alloyed with Al and Si. Mater. Des. 2012, 39, 264–268. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, F.C.; Wang, T.S. A novel bainitic steel comparable to maraging steel in mechanical properties. Scr. Mater. 2013, 68, 763–766. [Google Scholar] [CrossRef]
- Long, X.Y.; Kang, J.; Lv, B.; Zhang, F.C. Carbide-free bainite in medium carbon steel. Mater. Des. 2014, 64, 237–245. [Google Scholar] [CrossRef]
- Wang, X.L.; Wu, K.M.; Hu, F.; Yu, L.; Wan, X.L. Multi-step isothermal bainitic transformation in medium-carbon steel. Scr. Mater. 2014, 74, 56–59. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Huang, W. Microstructure and mechanical properties of a medium-carbon bainitic steel by a novel quenching and dynamic partitioning (Q-DP) process. Mater. Sci. Eng. A 2016, 662, 129–135. [Google Scholar] [CrossRef]
- Zhao, L.; Qian, L.; Meng, J.; Zhou, Q.; Zhang, F. Below-Ms austempering to obtain refined bainitic structure and enhanced mechanical properties in low-C high-Si/Al steels. Scr. Mater. 2016, 112, 96–100. [Google Scholar] [CrossRef]
- Da Silva, E.P.; De Knijf, D.; Xu, W.; Föjer, C.; Houbaert, Y.; Sietsma, J.; Petrov, R. Isothermal transformations in advanced high strength steels below martensite start temperature. Mater. Sci. Technol. 2015, 31, 808–816. [Google Scholar] [CrossRef]
- Hofer, C.; Leitner, H.; Winkelhofer, F.; Clemens, H.; Primig, S. Structural characterization of “carbide-free” bainite in a Fe–0.2C–1.5Si–2.5Mn steel. Mater. Charact. 2015, 102, 85–91. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Kobayashi, J.; Sugimoto, K.-I. Notch-fatigue properties of advanced trip-aided bainitic ferrite steels. Metall. Mater. Trans. A 2012, 43, 4129–4136. [Google Scholar] [CrossRef]
- Sugimoto, K.I. Fracture strength and toughness of ultra high strength trip aided steels. Mater. Sci. Technol. 2009, 25, 1108–1117. [Google Scholar] [CrossRef]
- Kolmskog, P.; Borgenstam, A.; Hillert, M.; Hedström, P.; Babu, S.S.; Terasaki, H.; Komizo, Y.I. Direct observation that bainite can grow below Ms. Metall. Mater. Trans. A 2012, 43, 4984–4988. [Google Scholar] [CrossRef]
- van Bohemen, S.M.C.; Santofimia, M.J.; Sietsma, J. Experimental evidence for bainite formation below Ms in Fe-0.66C. Scr. Mater. 2008, 58, 488–491. [Google Scholar] [CrossRef]
- Samanta, S.; Biswas, P.; Giri, S.; Singh, S.B.; Kundu, S. Formation of bainite below the Ms temperature: Kinetics and crystallography. Acta Mater. 2016, 105, 390–403. [Google Scholar] [CrossRef]
- Smanio, V.; Sourmail, T. Effect of partial martensite transformation on bainite reaction kinetics in different 1%C steels. Solid State Phenom. 2011, 172–174, 821–826. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. Bainite in silicon steels: New composition-property approach. Part 2. Met. Sci. 1983, 17, 420–425. [Google Scholar] [CrossRef]
- Kim, K.W.; Il Kim, K.; Lee, C.H.; Kang, J.Y.; Lee, T.H.; Cho, K.M.; Oh, K.H. Control of retained austenite morphology through double bainitic transformation. Mater. Sci. Eng. A 2016, 673, 557–561. [Google Scholar] [CrossRef]
- Hase, K.; Garcia-Mateo, C.; Bhadeshia, H.K.D.H. Bimodal size-distribution of bainite plates. Mater. Sci. Eng. A 2006, 438, 145–148. [Google Scholar] [CrossRef]
- Long, X.Y.; Zhang, F.C.; Kang, J.; Lv, B.; Shi, X.B. Low-temperature bainite in low-carbon steel. Mater. Sci. Eng. A 2014, 594, 344–351. [Google Scholar] [CrossRef]
- García-Junceda, A.; Capdevila, C.; Caballero, F.G.; de Andrés, C.G. Dependence of martensite start temperature on fine austenite grain size. Scr. Mater. 2008, 58, 134–137. [Google Scholar] [CrossRef]
- Yang, H.-S.; Bhadeshia, H.K.D.H. Austenite grain size and the martensite-start temperature. Scr. Mater. 2009, 60, 493–495. [Google Scholar] [CrossRef]
- Lee, S.-J.; Park, K.-S. Prediction of martensite start temperature in alloy steels with different grain sizes. Metall. Mater. Trans. A 2013, 44, 3423–3427. [Google Scholar] [CrossRef]
- Yang, H.S.; Suh, D.W.; Bhadeshia, H.K.D.H. More complete theory for the calculation of the martensite-start temperature in steels. ISIJ Int. 2012, 52, 164–166. [Google Scholar] [CrossRef]
- Maki, T. Current state and future prospect of microstructure control in steels. Tetsu To Hagane-J. ISIJ 1995, 81, N547–N555. [Google Scholar] [CrossRef]
- Krauss, G. Steels: Heat Treatment and Processing Principles; ASM International: Materials Park, OH, USA, 1989. [Google Scholar]
- Calcagnotto, M.; Ponge, D.; Raabe, D. Effect of grain refinement to 1 μm on strength and toughness of dual-phase steels. Mater. Sci. Eng. A 2010, 527, 7832–7840. [Google Scholar] [CrossRef]
- Calcagnotto, M.; Adachi, Y.; Ponge, D.; Raabe, D. Deformation and fracture mechanisms in fine- and ultrafine-grained ferrite/martensite dual-phase steels and the effect of aging. Acta Mater. 2011, 59, 658–670. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.H.; Zheng, C.L.; Zhang, F.C.; Wang, T.S. Effects of ausforming on isothermal bainite transformation behaviour and microstructural refinement in medium-carbon Si-Al-rich alloy steel. Mater. Des. 2014, 62, 168–174. [Google Scholar] [CrossRef]
- Gong, W.; Tomota, Y.; Adachi, Y.; Paradowska, A.M.; Kelleher, J.F.; Zhang, S.Y. Effects of ausforming temperature on bainite transformation, microstructure and variant selection in nanobainite steel. Acta Mater. 2013, 61, 4142–4154. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.S.; Wang, Y.H.; Yang, J.; Zhang, F.C. Preparation of nanostructured bainite in medium-carbon alloy steel. Mater. Sci. Eng. A 2013, 568, 123–126. [Google Scholar] [CrossRef]
- Gong, W.; Tomota, Y.; Koo, M.S.; Adachi, Y. Effect of ausforming on nanobainite steel. Scr. Mater. 2010, 63, 819–822. [Google Scholar] [CrossRef]
- Chakraborty, J.; Bhattacharjee, D.; Manna, I. Development of ultrafine bainite + martensite duplex microstructure in sae 52100 bearing steel by prior cold deformation. Scr. Mater. 2009, 61, 604–607. [Google Scholar] [CrossRef]
- Chakraborty, J.; Manna, I. Development of ultrafine ferritic sheaves/plates in sae 52100 steel for enhancement of strength by controlled thermomechanical processing. Mater. Sci. Eng. A 2012, 548, 33–42. [Google Scholar] [CrossRef]
- Lonardelli, I.; Bortolotti, M.; Van Beek, W.; Girardini, L.; Zadra, M.; Bhadeshia, H.K.D.H. Powder metallurgical nanostructured medium carbon bainitic steel: Kinetics, structure, and in situ thermal stability studies. Mater. Sci. Eng. A 2012, 555, 139–147. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.H.; Zheng, C.L.; Zhang, F.C.; Wang, T.S. Austenite deformation behavior and the effect of ausforming process on martensite starting temperature and ausformed martensite microstructure in medium-carbon Si-Al-rich alloy steel. Mater. Sci. Eng. A 2014, 596, 9–14. [Google Scholar] [CrossRef]
- He, J.; Zhao, A.; Huang, Y.; Zhi, C.; Zhao, F. Acceleration of Bainite Transformation at Low Temperature by Warm Rolling Process. Mater. Today Proc. 2015, 2, S289–S294. [Google Scholar] [CrossRef]
- Timokhina, I.; Beladi, H.; Xiong, X.; Hodgson, P. Effect of composition and processing parameters on the formation of nano-bainite in advanced high strength steels. J. Iron Steel Res. Int. 2011, 18, 238–241. [Google Scholar]
- Golchin, S.; Avishan, B.; Yazdani, S. Effect of 10% ausforming on impact toughness of nano bainite austempered at 300 °C. Mater. Sci. Eng. A 2016, 656, 94–101. [Google Scholar] [CrossRef]
- He, J.; Zhao, A.; Zhi, C.; Fan, H. Acceleration of nanobainite transformation by multi-step ausforming process. Scr. Mater. 2015, 107, 71–74. [Google Scholar] [CrossRef]
- Kabirmohammadi, M.; Avishan, B.; Yazdani, S. Transformation kinetics and microstructural features in low-temperature bainite after ausforming process. Mater. Chem. Phys. 2016, 184, 306–317. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, G.; Wang, L.; Hu, H. Combined effect of the prior deformation and applied stress on the bainite transformation. Met. Mater. Int. 2016, 22, 956–961. [Google Scholar] [CrossRef]
- Zhi, C.; Zhao, A.; He, J.; Yang, H.; Qi, L. Effects of the multi-step ausforming process on the microstructure evolution of nanobainite steel. In Proceedings of the International Conference on Advanced Materials, Structures and Mechanical Engineering, Incheon, Korea, 29–31 May 2015; Kaloop, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 399–403. [Google Scholar]
- Zhou, M.; Xu, G.; Zhang, Y.; Xue, Z. The effects of external compressive stress on the kinetics of low temperature bainitic transformation and microstructure in a superbainite steel. Int. J. Mater. Res. 2015, 106, 1040–1045. [Google Scholar] [CrossRef]
- Hu, H.; Xu, G.; Wang, L.; Zhou, M. Effects of strain and deformation temperature on bainitic transformation in a Fe-C-Mn-Si alloy. Steel Res. Int. 2016, 88. [Google Scholar] [CrossRef]
- Zhao, L.; Qian, L.; Liu, S.; Zhou, Q.; Meng, J.; Zheng, C.; Zhang, F. Producing superfine low-carbon bainitic structure through a new combined thermo-mechanical process. J. Alloys Compd. 2016, 685, 300–303. [Google Scholar] [CrossRef]
- Tsuzaki, K.; Fukasaku, S.-I.; Tomota, Y.; Maki, T. Effect of prior deformation of austenite on the γ → ε martensitic transformation in Fe-Mn alloys. Mater. Trans. JIM 1991, 32, 222–228. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. The bainite transformation: Unresolved issues. Mater. Sci. Eng. A 1999, 273–275, 58–66. [Google Scholar] [CrossRef]
- Shipway, P.H.; Bhadeshia, H.K.D.H. The mechanical stabilisation of widmanstatten ferrite. Mater. Sci. Eng. A 1997, 223, 179–185. [Google Scholar] [CrossRef]
- Christian, J.W.; Mahajan, S. Deformation twinning. Prog. Mater. Sci. 1995, 39, 1–157. [Google Scholar] [CrossRef]
- Chatterjee, S.; Wang, H.S.; Yang, J.R.; Bhadeshia, H.K.D.H. Mechanical stabilisation of austenite. Mater. Sci. Technol. 2006, 22, 641–644. [Google Scholar] [CrossRef]
- Shipway, P.H.; Bhadeshia, H.K.D.H. Mechanical stabilisation of bainite. Mater. Sci. Technol. 1995, 11, 1116–1128. [Google Scholar] [CrossRef]
- Maalekian, M.; Kozeschnik, E.; Chatterjee, S.; Bhadeshia, H.K.D.H. Mechanical stabilisation of eutectoid steel. Mater. Sci. Technol. 2007, 23, 610–612. [Google Scholar] [CrossRef]
- Yi, H.L.; Lee, K.Y.; Bhadeshia, H.K.D.H. Mechanical stabilisation of retained austenite in δ-trip steel. Mater. Sci. Eng. A 2011, 528, 5900–5903. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Mateo, C.; Paul, G.; Somani, M.C.; Porter, D.A.; Bracke, L.; Latz, A.; Garcia De Andres, C.; Caballero, F.G. Transferring Nanoscale Bainite Concept to Lower C Contents: A Perspective. Metals 2017, 7, 159. https://doi.org/10.3390/met7050159
Garcia-Mateo C, Paul G, Somani MC, Porter DA, Bracke L, Latz A, Garcia De Andres C, Caballero FG. Transferring Nanoscale Bainite Concept to Lower C Contents: A Perspective. Metals. 2017; 7(5):159. https://doi.org/10.3390/met7050159
Chicago/Turabian StyleGarcia-Mateo, Carlos, Georg Paul, Mahesh C. Somani, David A. Porter, Lieven Bracke, Andreas Latz, Carlos Garcia De Andres, and Francisca G. Caballero. 2017. "Transferring Nanoscale Bainite Concept to Lower C Contents: A Perspective" Metals 7, no. 5: 159. https://doi.org/10.3390/met7050159







