TiO2 Nanotubes on Ti Dental Implant. Part 1: Formation and Aging in Hank’s Solution
Abstract
:1. Introduction
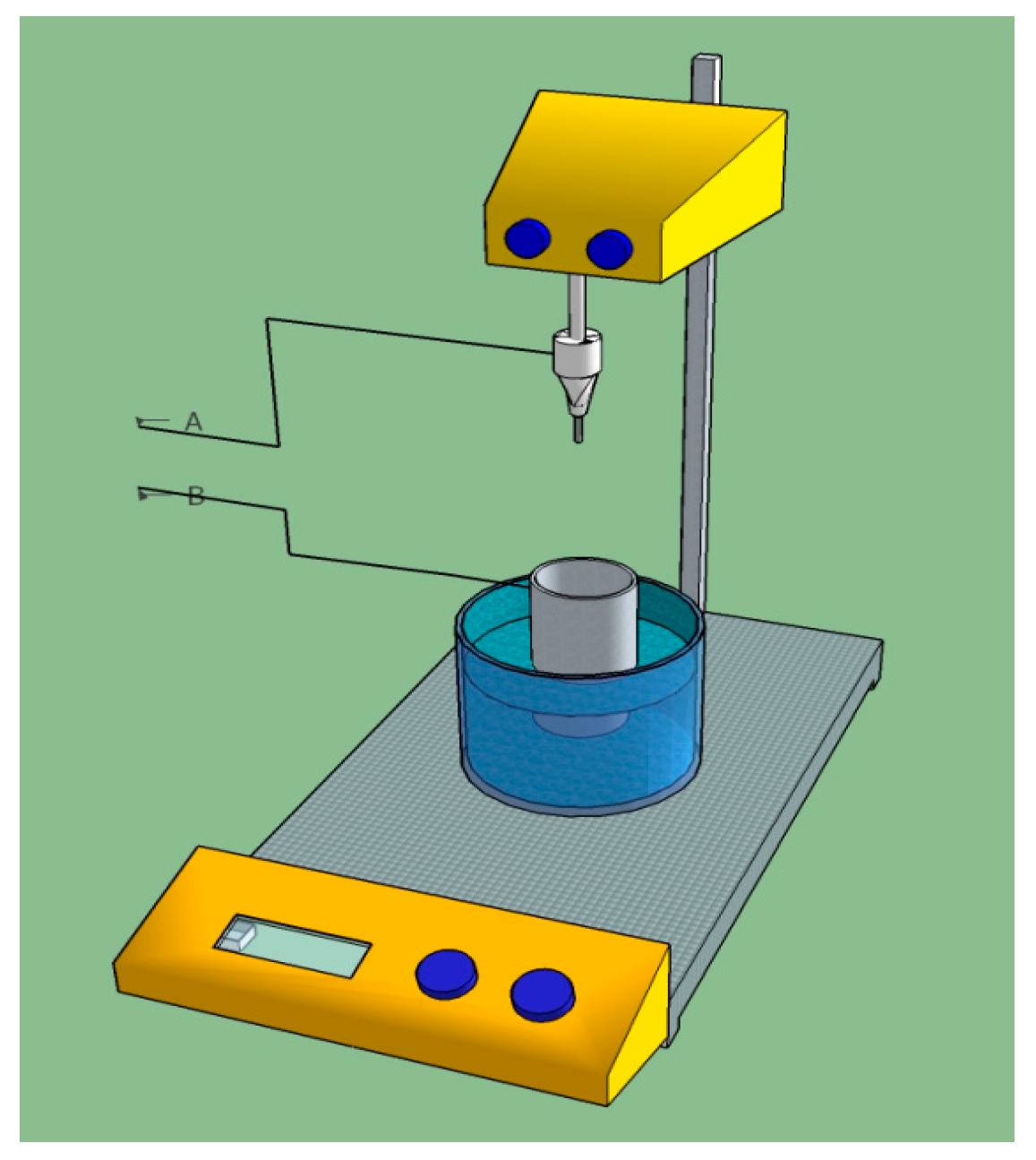
2. Materials and Methods
3. Results and Discussion
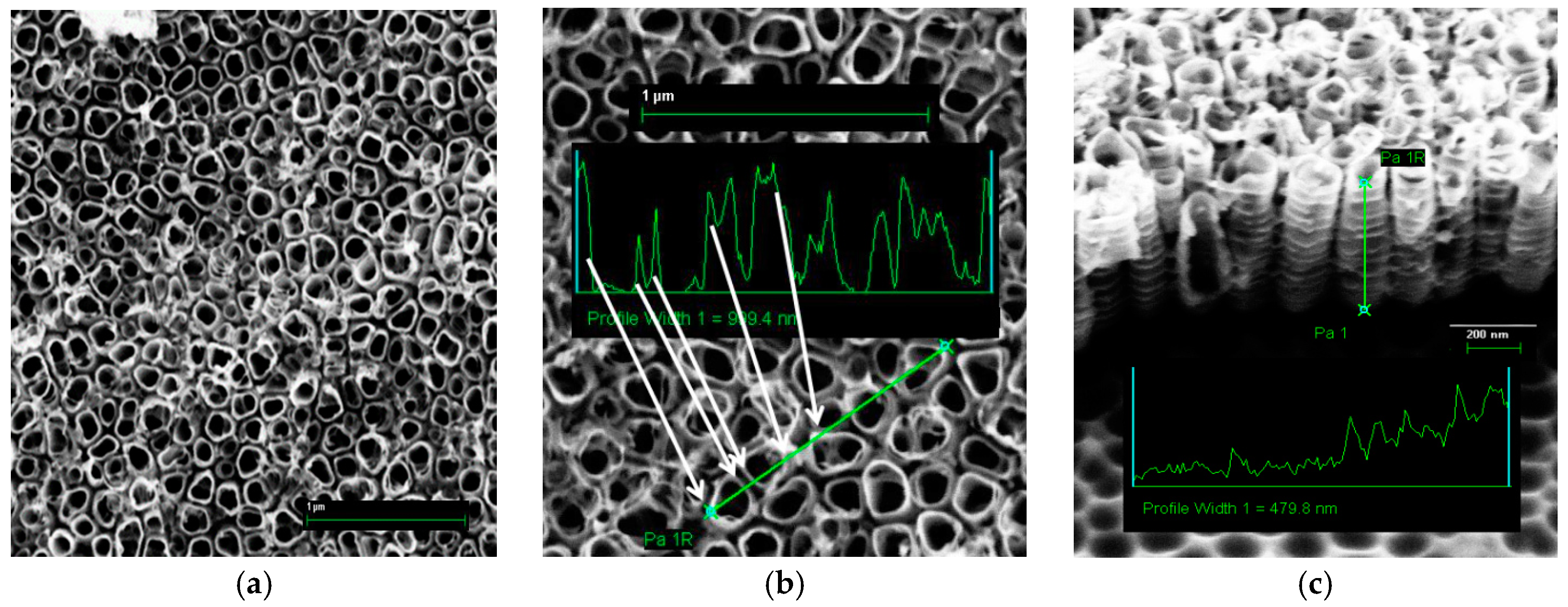
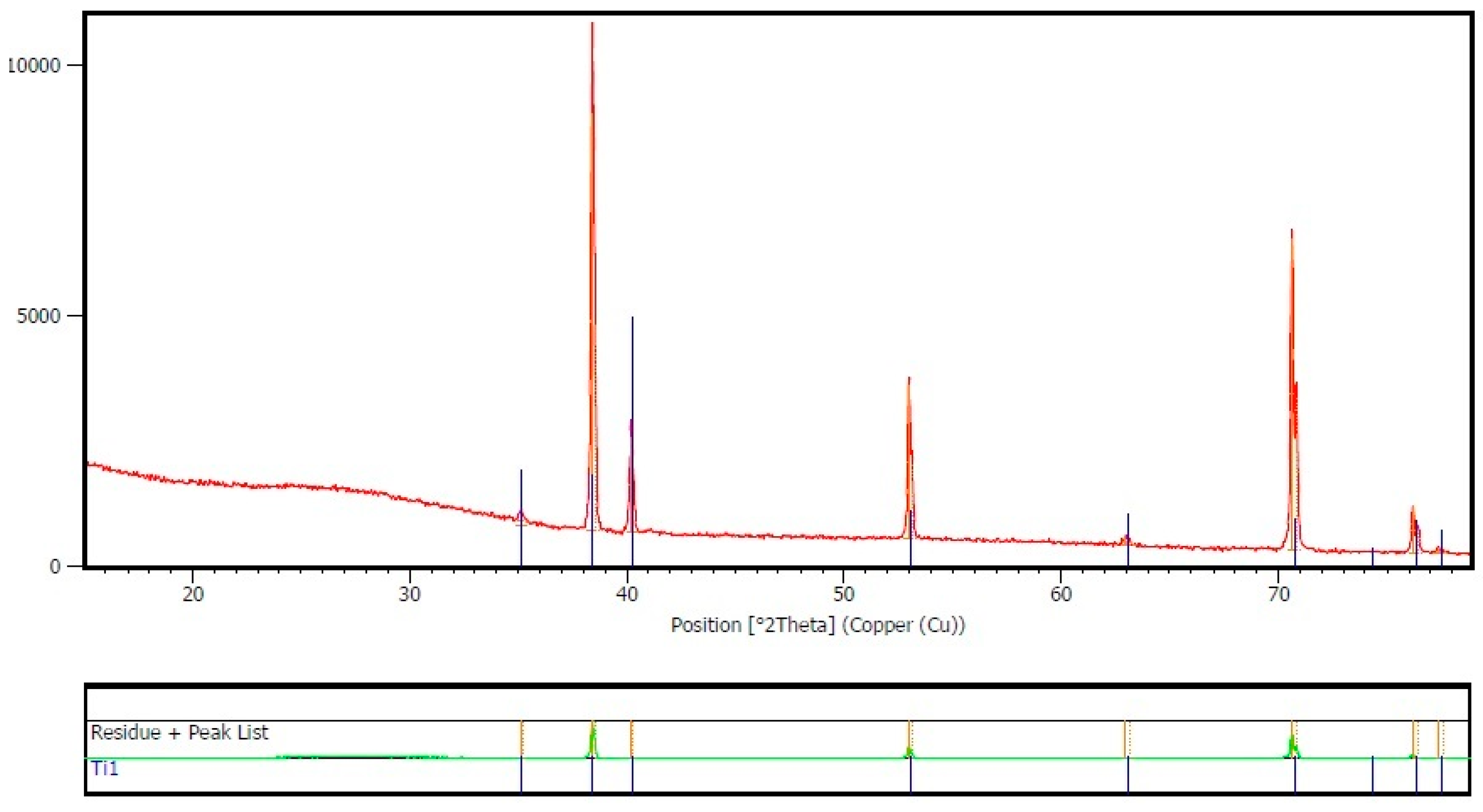
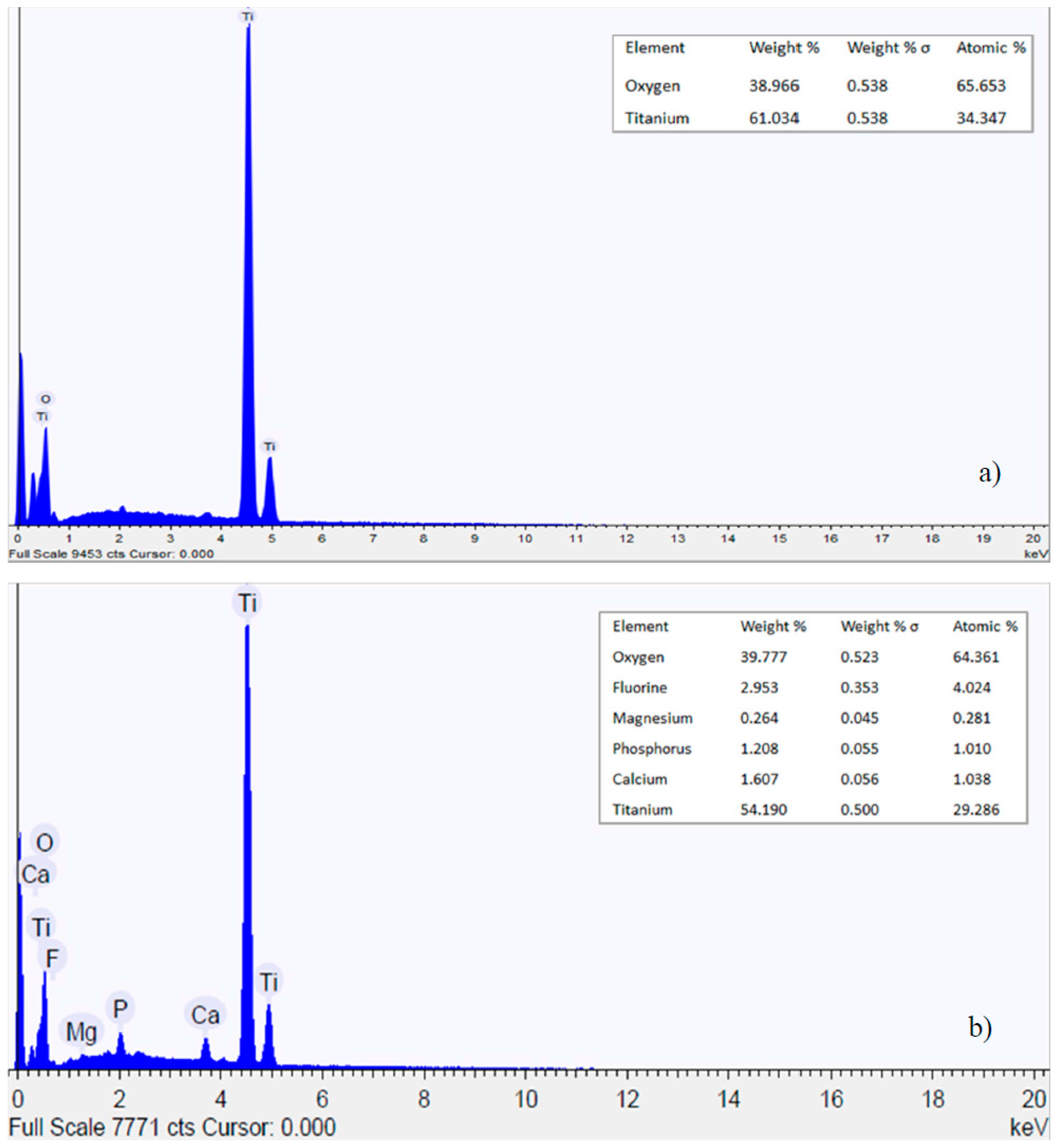
3.1. Nanotubes Formation and Structure
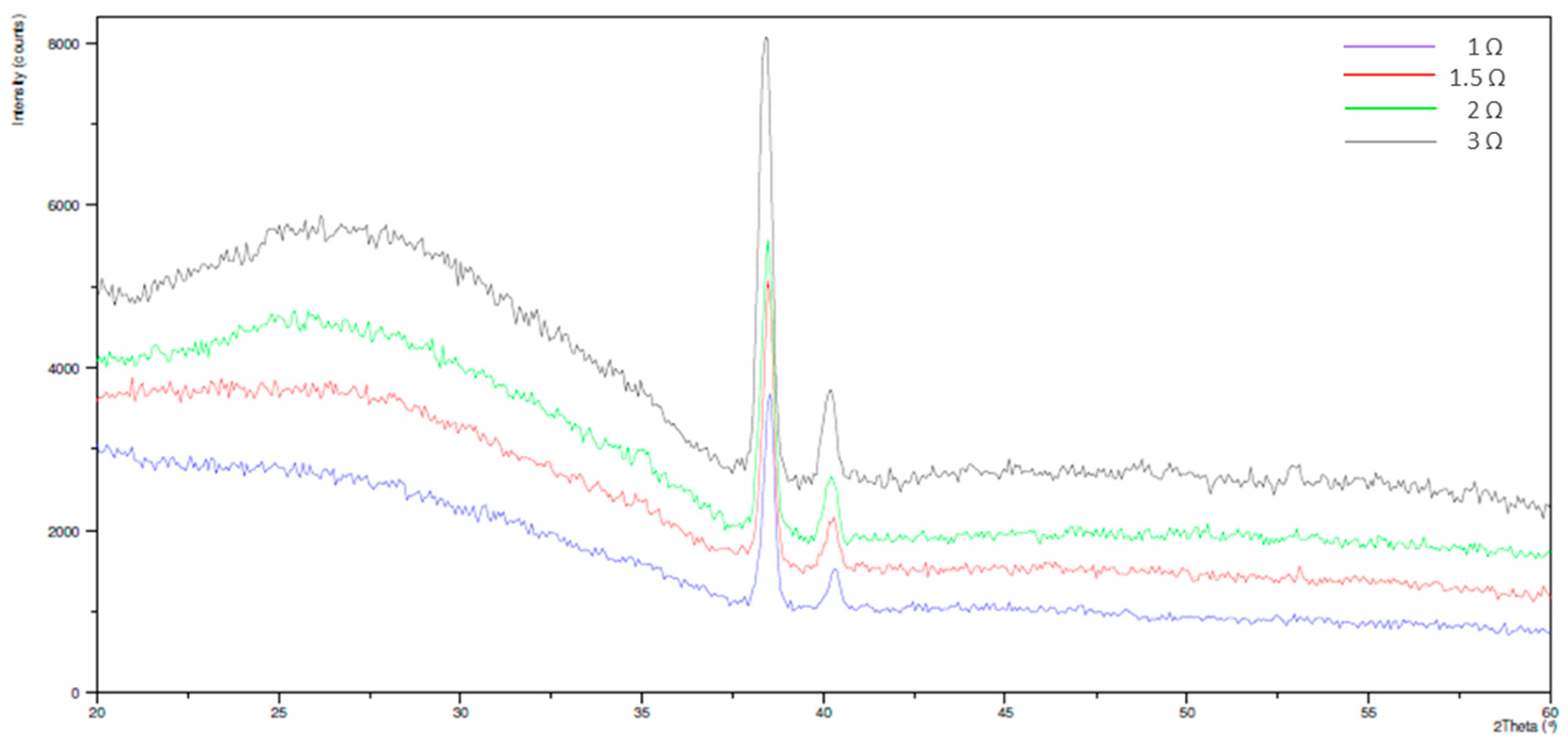
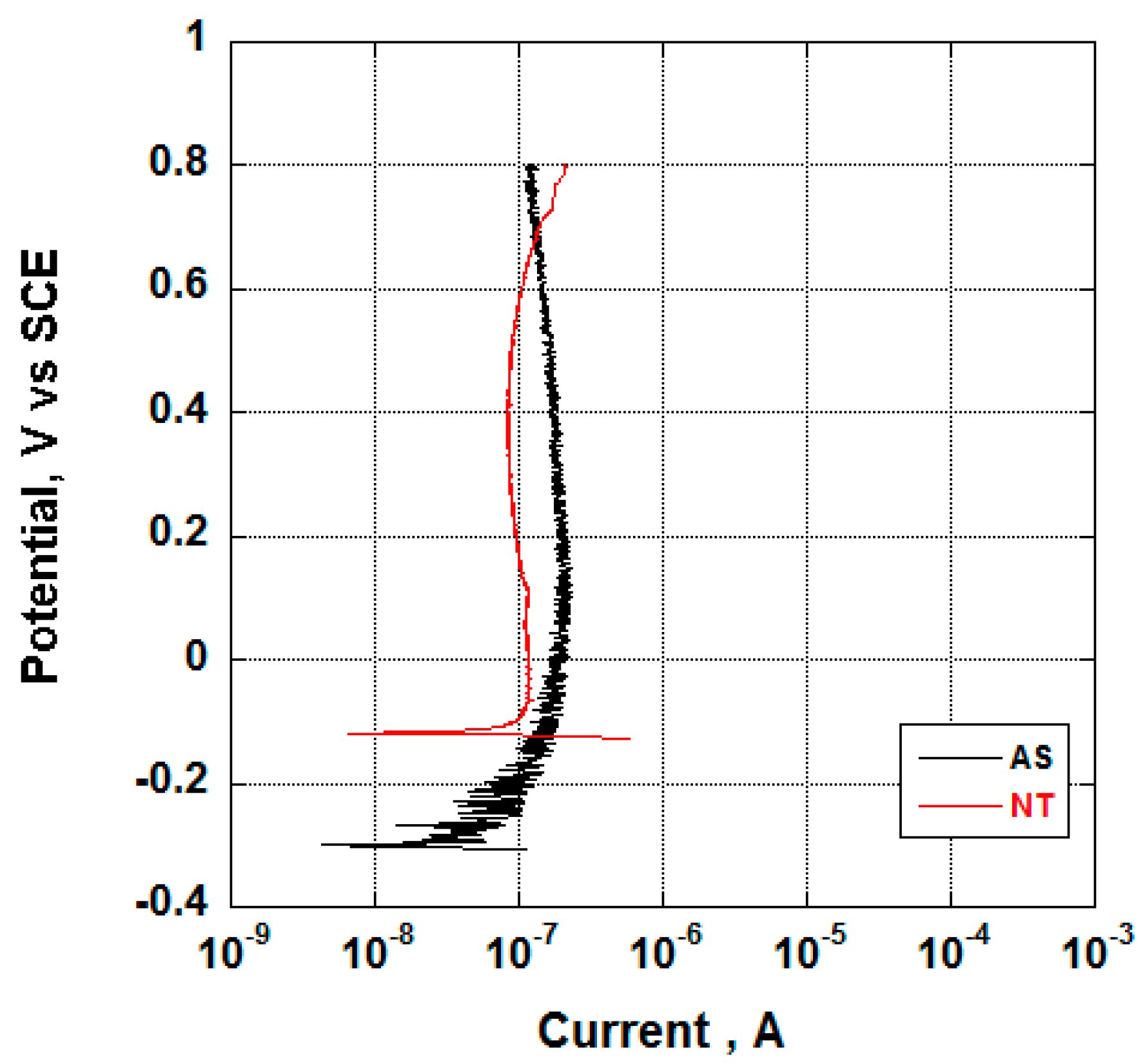
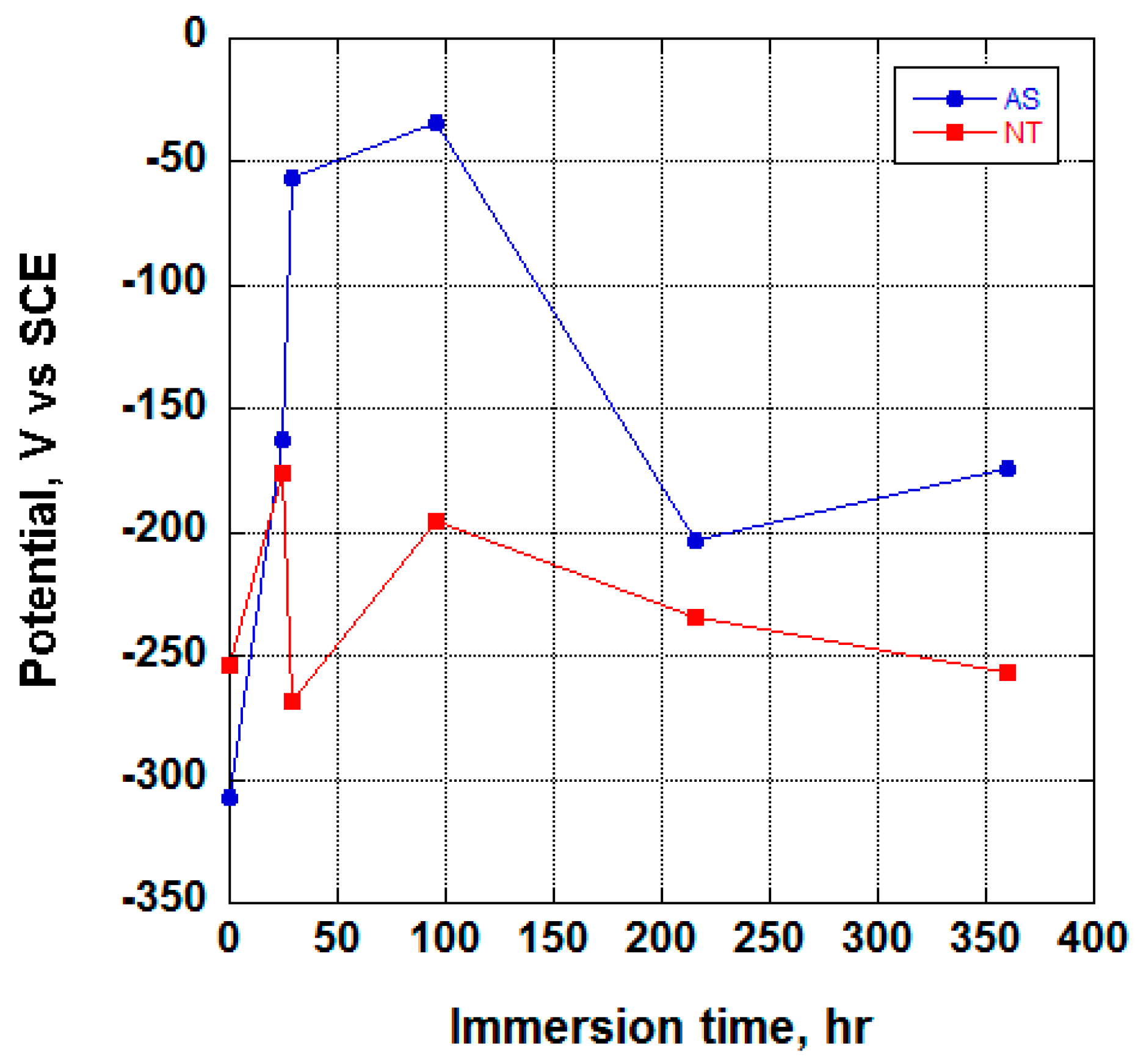
3.2. Electrochemical Behavior and Aging in Hank’s Solution
4. Conclusions
- (1)
- Nanotubes on titanium dental screw implant were grown by using a simple set up.
- (2)
- Nanotubes covering the implant possess an amorphous TiO2 structure, exhibiting better corrosion resistance as compared to the non-anodized Ti screw;
- (3)
- Upon aging in Hank’s solution for 15 days the anodized Ti screw shows compound containing Ca and P;
- (4)
- The nanotubes catalyzed the formation of compound containing Ca and P.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bavyki, D.V.; Walsh, F.C. Titanate and Titania Nanotubes: Synthesis, Properties and Applications; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Ge, M.Z.; Cao, C.Y.; Huang, J.Y.; Li, S.H.; Zhang, S.N.; Deng, S.; Li, Q.S.; Zhang, K.Q.; Lai, Y.K. Synthesis, modification, and photo/photoelectrocatalytic degradation applications of TiO2 nanotube arrays: A review. Nano Rev. 2016, 5, 75–112. [Google Scholar] [CrossRef]
- Lan, Z.; Wu, W.; Zhang, S.; Que, L.; Wu, J. An efficient method to prepare high-performance dye-sensitized photoelectrodes using ordered TiO2 nanotube arrays and TiO2 quantum dot blocking layers. J. Solid State Electrochem. 2016, 20, 2643–2650. [Google Scholar] [CrossRef]
- Comini, E.; Galstyan, V.; Faglia, G.; Bontempi, E.; Sberveglieri, G. Highly conductive titanium oxide nanotubes chemical sensors. Microporous Mesoporous Mater. 2015, 208, 165–170. [Google Scholar] [CrossRef]
- Smith, Y.R.; Ray, R.S.; Carlson, K.K. Self-ordered titanium dioxide nanotube arrays: Anodic synthesis and their photo/electro-catalytic applications. Materials 2013, 6, 2892–2957. [Google Scholar] [CrossRef]
- De Santo, I.; Sanguigno, L.; Causa, F.; Monetta, T.; Netti, P.A. Exploring doxorubicin localization in eluting TiO2 nanotube arrays through fluorescence correlation spectroscopy analysis. Analyst 2012, 137, 5076–5081. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R. Review of titania nanotubes: Fabrication and cellular response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Moreno-Ulloa, A.; Valdez-Salas, B.; Velasquillo, C.; Carrillo, M.; Escamilla, A.; Valdez, E.; Villarreal, F. Improved osteoblast and chondrocyte adhesion and viability by surface-modified Ti6Al4V alloy with anodized TiO2 nanotubes using a super-oxidative solution. Materials 2015, 8, 867–883. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Yao, C.; Webster, T.L. Anodization: A promising nano-modification technique of titanium implants for orthopedic applications. J. Nanosci. Nanotechnal. 2006, 6, 2682–2692. [Google Scholar] [CrossRef]
- Makihira, S.; Mine, Y.; Nikawa, H.; Shuto, T.; Iwata, S.; Hosokawa, R.; Kamoi, K.; Okazaki, S.; Yamaguchi, Y. Titanium ion induces necrosis and sensitivity to lipopolysaccharide in gingival epithelial-like cells. Toxicol. In Vitro 2010, 24, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Vallés, G.; Gil-Garay, E.; Munuera, L.; Vilaboa, N. Modulation of the cross-talk between macrophages and osteoblasts by titanium-based particles. Biomaterials 2008, 29, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Franková, J.; Pivodová, V.; Růžička, F.; Tománková, K.; Šafářová, K.; Vrbková, J.; Ulrichová, J. Comparing biocompatibility of gingival fibroblasts and bacterial strains on a different modified titanium discs. J. Biomed. Mater. Res. Part A 2013, 101, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Monetta, T.; Acquesta, A.; Bellucci, F. Evaluation of roughness and electrochemical behavior of titanium in biological environment. Metall. Ital. 2014, 106, 13–21. [Google Scholar]
- Beltrán-Partida, E.; Valdez-Salas, B.; Curiel-Álvarez, M.; Castillo-Uribe, S.; Escamilla, A.; Nedev, N. Enhanced antifungal activity by disinfected titanium dioxide nanotubes via reduced nano-adhesion bonds. Mater. Sci. Eng. 2017, 76, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.J.; Urban, R.M.; Pourzal, R.; Turner, T.M.; Skipor, A.K.; Jacobs, J.J. Nanoscale surface modification by anodic oxidation increased bone ingrowth and reduced fibrous tissue in the porous coating of titanium–alloy femoral hip arthroplasty implants. J. Biomed. Mater. Res. Part B 2017, 105, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Monetta, T.; Marchesano, G.; Bellucci, F.; Lupo, G.; Itro, A. Assessing the roughness of titanium dental implants. Dent. Cadmos 2014, 82, 498–508. [Google Scholar] [CrossRef]
- Von Wilmowsky, C.; Bauer, S.; Lutz, R. In vivo evaluation of anodic tio2 nanotubes: An experimental study in the pig. J. Biomed. Mater. Res. Part B 2009, 89, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Monetta, T.; Scala, A.; Malmo, C.; Bellucci, F. Antibacterial activity of cold plasma-treated titanium alloy. Plasma Med. 2011, 1, 205–214. [Google Scholar] [CrossRef]
- Monetta, T.; Bellucci, F. Strong and durable antibacterial effect of titanium treated in Rf oxygen plasma: Preliminary results. Plasma Chem. Plasma Process 2014, 34, 1247–1256. [Google Scholar] [CrossRef]
- Bestetti, M.; Franz, S.; Cuzzolin, S. Structure of nanotubular titanium oxide templates prepared by electrochemical anodization in H2SO4/HF solutions. Thin Solid Films 2007, 515, 5253–5258. [Google Scholar] [CrossRef]
- Craig, A.; Grimes, F. Synthesis and application of highly ordered arrays of TiO2 nanotubes. J. Mater. Chem. 2007, 17, 1451–1457. [Google Scholar]
- Minagar, S.; Berndt, C.C.; Wang, J. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ji, S.; Liu, G.; Xu, G.; Ye, C. Understanding the growth behavior of titania nanotubes. Electrochem. Commun. 2011, 13, 454–457. [Google Scholar] [CrossRef]
- Sul, Y.T. Electrochemical growth behavior, surface properties, and enhanced in vivo bone response of TiO2 nanotubes on microstructured surfaces of blasted, screw-shaped titanium implants. Int. J. Nanomed. 2010, 5, 87–100. [Google Scholar] [CrossRef]
- Monetta, T.; Bellucci, F. Formation of a uniform distribution of tio2 nanotubes array on complex geometry samples. Adv. Sci. Focus 2013, 1, 6–8. [Google Scholar] [CrossRef]
- Wang, J.; Fan, H.; Zhang, H.; Chen, Q.; Liu, Y.; Ma, W. Anodizing process of titanium and formation mechanism of anodic TiO2 nanotubes. Prog. Chem. 2016, 28, 284–295. [Google Scholar]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ghicov, A.; Albu, S.P.; Schmuki, P. Bamboo-type TiO2 nanotubes: Improved conversion efficiency in dye-sensitized solar cells. J. Am. Chem. Soc. 2008, 130, 16454–16455. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Fang, H.; Cao, G.; Zhang, Y.; Zhang, X. Effect of Tin+ defects on electrochemical properties of highly-ordered titania nanotube arrays. Thin Solid Films 2010, 518, 7152–7155. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Müller, L.; Kunze, J.; Müller, F.; Greil, P.; Virtanen, S.; Schmuki, P. Hydroxyapatite growth on anodic TiO2 nanotubes. J. Biomed. Mater. Res. 2006, 77, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Tian, T.; Liu, R.; She, H. Influence of titania nanotube arrays on biomimetic deposition apatite on titanium by alkali treatment. Mater. Chem. Phys. 2007, 106, 27–32. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Shi, Q.; Liu, X.; Wan, L. Calcium ion modification of TiO2 nanotube arrays to enhance apatite formation. Mater. Technol. 2016, 31, 791–798. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wu, S.C.; Hsu, S.K.; Chang, Y.C.; Ho, W.F. Fabrication of nanotube arrays on commercially pure titanium and their apatite-forming ability in a simulated body fluid. Mater. Charact. 2015, 100, 170–177. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M.; Nakamura, T. Bioactive metals: Preparation and properties. J. Mater. Sci. Mater. Med. 2004, 15, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Healy, K.E.; Ducheyne, P. The mechanisms of passive dissolution of titanium in a model physiological environment. J. Biomed. Mater. Res. 1992, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dunn, M.F.; Xiao, T.D.; Tomsia, A.P.; Saiz, E. Nanostructured hydroxyapatite coatings for improved adhesion and corrosion resistance for medical implants. MRS Proc. 2001, 703, 291–296. [Google Scholar] [CrossRef]

| Ti | O | N | H | C | Fe |
|---|---|---|---|---|---|
| 99.7 | 0.250 | 0.030 | 0.015 | 0.100 | 0.150 |
| Inorganic Salts Component | g/L |
|---|---|
| CaCl2·2H2O | 0.185 |
| MgSO4 | 0.09767 |
| KCl | 0.4 |
| KH2PO4 | 0.06 |
| NaHCO3 | 0.35 |
| NaCl | 8.0 |
| Na2HPO4 | 0.04788 |
| d-Glucose | 1.0 |
| Phenol Red·Na | - |
| NaHCO3 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monetta, T.; Acquesta, A.; Carangelo, A.; Bellucci, F. TiO2 Nanotubes on Ti Dental Implant. Part 1: Formation and Aging in Hank’s Solution. Metals 2017, 7, 167. https://doi.org/10.3390/met7050167
Monetta T, Acquesta A, Carangelo A, Bellucci F. TiO2 Nanotubes on Ti Dental Implant. Part 1: Formation and Aging in Hank’s Solution. Metals. 2017; 7(5):167. https://doi.org/10.3390/met7050167
Chicago/Turabian StyleMonetta, Tullio, Annalisa Acquesta, Anna Carangelo, and Francesco Bellucci. 2017. "TiO2 Nanotubes on Ti Dental Implant. Part 1: Formation and Aging in Hank’s Solution" Metals 7, no. 5: 167. https://doi.org/10.3390/met7050167






