Effect of Calcium Oxide on the Crushing Strength, Reduction, and Smelting Performance of High-Chromium Vanadium–Titanium Magnetite Pellets
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Softening-Melting-Dripping Procedure
2.3. Characterization Methods
3. Results and Discussion
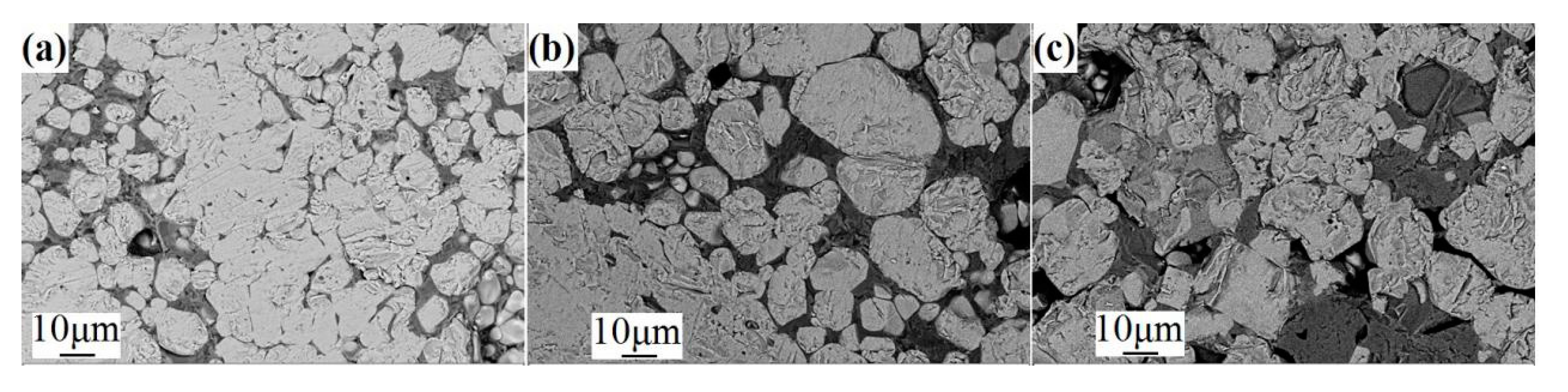
3.1. Effect of CaO on the Crushing Strength of HCVTMP
3.2. Effect of CaO on the Reduction and Smelting Performance of HCVTMP
3.2.1. Softening-Melting-Dripping Behavior
3.2.2. Chemical Analysis
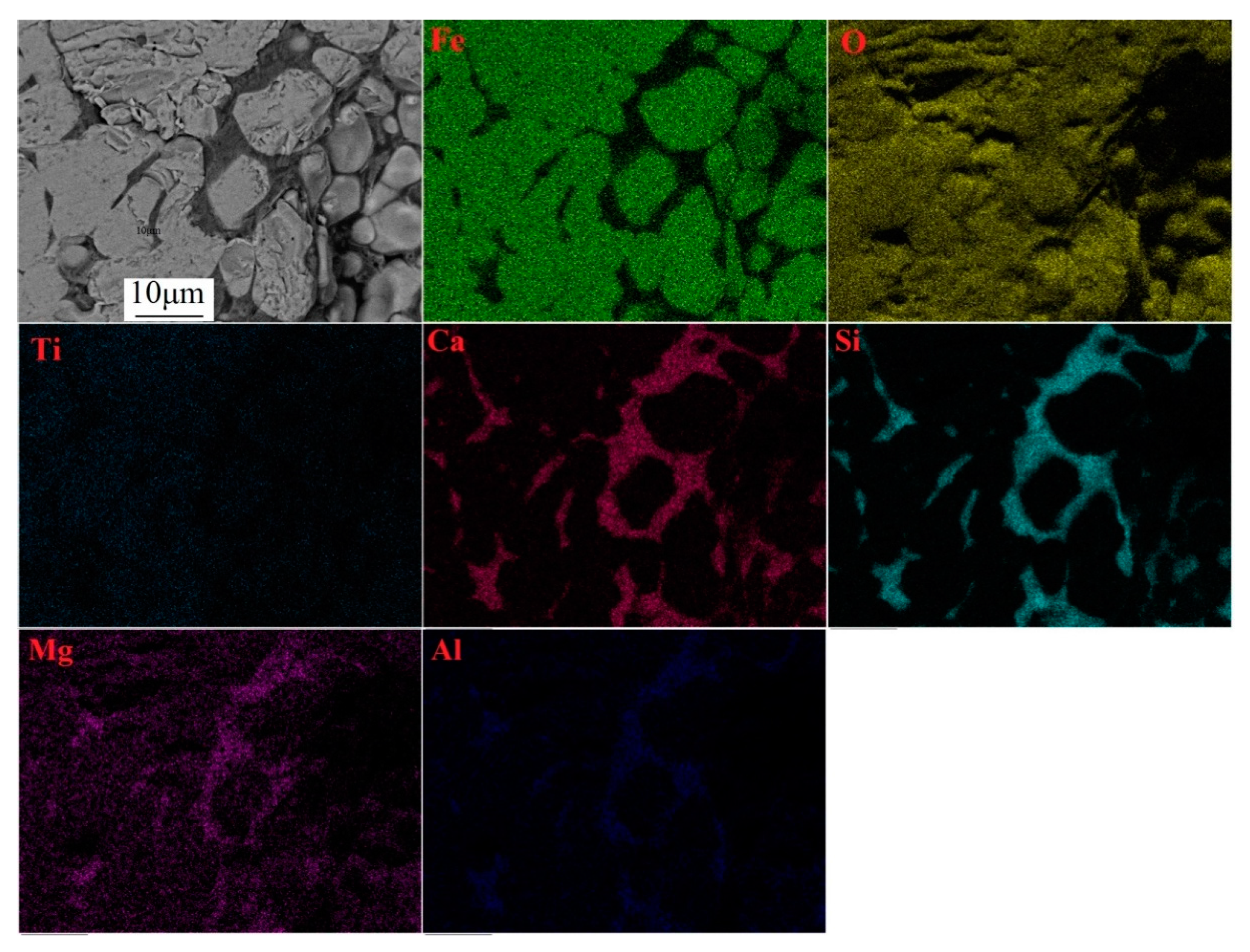
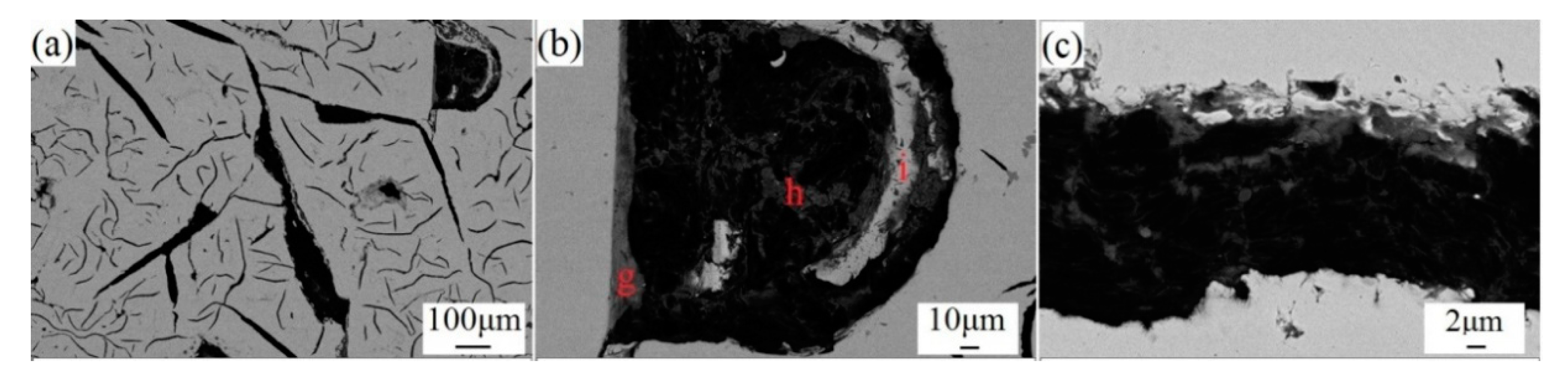
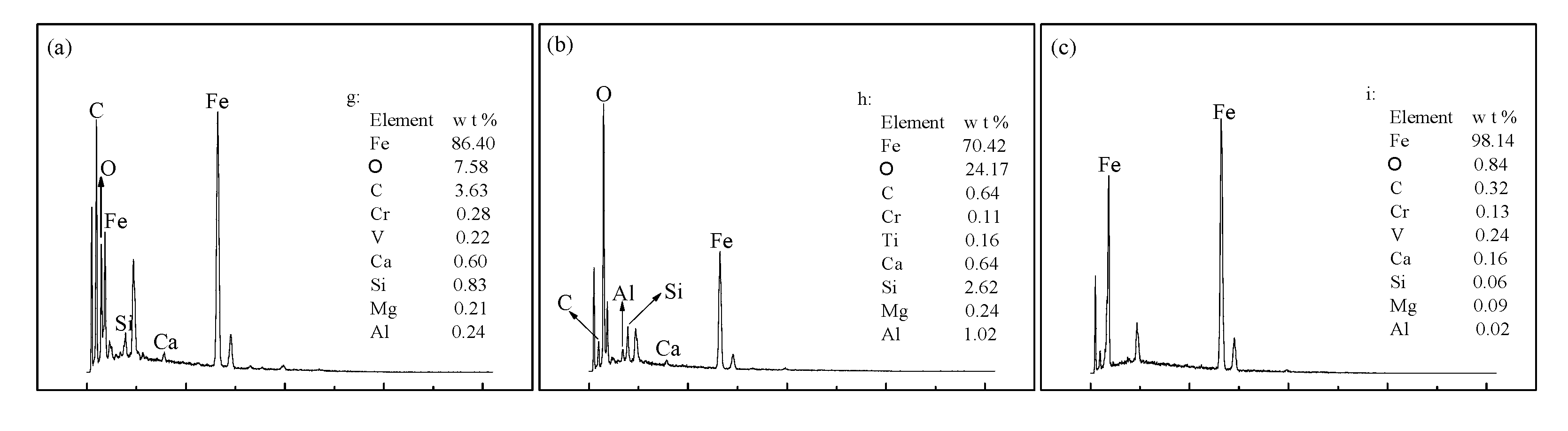
3.2.3. Microscopic Examination
4. Conclusions
- The mineral phases generated during oxidative baking, and the subsequently-formed pellet microstructures, affect the crushing strength owing to the CaO addition.
- Although HCVTMP showed the highest crushing strength with the CaO addition of ca. 2 wt %, more CaO addition may be needed to achieve a high permeability of the furnace burdens and good separation conditions of the slag and melted iron.
- With the predominant chemical composition analysis of ICP-AES and X-ray fluorescence (XRF), it can be determined that CaO could have a relationship with the transformation behavior of Cr, V, and Ti in the formation process of the slag and melted iron to some extent.
- With the microscopic examination, the restraining formation of Ti(C,N) and the promoting formation of CaTiO3 are in accordance with the improved melting-dripping index, including the decrease of the maximum external static load, gas permeability, and the increase of the melting-dripping zone and dripping difficulty.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.Q. Smelting of Vanadium-Titanium Magnetite in the Blast Furnace; Metallurgical Industry Press: Beijing, China, 1994. (In Chinese) [Google Scholar]
- Jena, B.C.; Dresler, W.; Reilly, I.G. Extraction of titanium, vanadium and iron from titanomagnetite deposits at pipestone lake, Manitoba, Canada. Miner. Eng. 1995, 8, 159–168. [Google Scholar] [CrossRef]
- Du, H.G. Principle of Smelting Vanadium-Titanium Magnetite in the Blast Furnace; Science Press: Beijing, China, 1996. (In Chinese) [Google Scholar]
- Chen, D.S.; Song, B.; Wang, L.N.; Qi, T.; Wang, Y.; Wang, W.J. Solid state reduction of Panzhihua titanomagnetite concentrates with pulverized coal. Miner. Eng. 2011, 24, 864–869. [Google Scholar] [CrossRef]
- Lv, X.W.; Lun, Z.G.; Yin, J.Q.; Bai, C.G. Carbothermic reduction of vanadium titanomagnetite by microwave irradiation and smelting behavior. ISIJ Int. 2013, 53, 1115–1119. [Google Scholar] [CrossRef]
- Cheng, G.J.; Liu, J.X.; Liu, Z.G.; Chu, M.S.; Xue, X.X. Non-isothermal reduction kinetics and mechanism of high chromium vanadium–titanium magnetite pellets. Ironmak. Steelmak. 2015, 42, 17–26. [Google Scholar] [CrossRef]
- Cheng, G.J.; Xue, X.X.; Jiang, T.; Duan, P.N. Effect of TiO2 on the crushing strength and smelting mechanism of high chromium vanadium–titanium magnetite pellets. Metall. Mater. Trans. B 2016, 47, 1713–1726. [Google Scholar] [CrossRef]
- Cheng, G.J.; Xue, X.X.; Gao, Z.X.; Jiang, T.; Yang, H.; Duan, P.N. Effect of Cr2O3 on the reduction and smelting mechanism of high-chromium vanadium-titanium magnetite pellets. ISIJ Int. 2016, 56, 1938–1947. [Google Scholar] [CrossRef]
- He, Z.W.; Liu, J.X.; Yang, S.T.; Yang, H.; Xue, X.X. Partition of valuable components between slag and metal in the blast furnace operating with high chromium, vanadium, titanium, magnetite ores. Metall. Res. Technol. 2016, 113, 607. [Google Scholar] [CrossRef]
- Biswas, A.K. Principles of Blast Furnace Ironmaking—Theory and Practice; Cootha Publishing House: Brisbane, Australia, 1981. [Google Scholar]
- Chun, T.J.; Zhu, D.Q.; Pan, J. Influence of sulfur content in raw materials on oxidized pellets. J. Cent. South Univ. Technol. 2011, 18, 1924–1929. [Google Scholar] [CrossRef]
- Gao, Q.J.; Shen, Y.S.; Wei, G.; Jiang, X.; Shen, F.M. Diffusion behavior and distribution regulation of MgO in MgO-bearing pellets. Int. J. Miner. Metall. Mater. 2016, 23, 1011–1018. [Google Scholar] [CrossRef]
- Ou, H.Z. Experimental Study on Reasonable Burden Structure of Blast Furnace for Smelting Imported High Chromium Vanadium-Titanium Magnetite. Master’s Thesis, Northeastern University, Shenyang, China, 2012. [Google Scholar]
- Fan, X.H.; Gan, M.; Jiang, T.; Yuan, L.S.; Chen, X.L. Influence of flux additives on iron ore oxidized pellets. J. Cent. South Univ. Technol. 2010, 17, 732–737. [Google Scholar] [CrossRef]
- Firth, A.R.; Garden, J.F.; Douglas, J.D. Phase equilibria and slag formation in the magnetite core of fluxed iron ore pellets. ISIJ Int. 2008, 48, 1485–1492. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ri, D.W.; Yi, S.H.; Sohn, I. Relationship between the reduction degree and strength of DRI pellets produced from iron and carbon bearing wastes using an RHF simulator. ISIJ Int. 2012, 52, 1454–1462. [Google Scholar] [CrossRef]
- Li, G.H.; Tang, Z.K.; Zhang, Y.B.; Cui, Z.X.; Jiang, T. Reduction swelling behavior of hematite/magnetite agglomerates with addition of MgO and CaO. Ironmak. Steelmak. 2010, 37, 393–397. [Google Scholar] [CrossRef]
- Wang, H.T.; Sohn, H.Y. Effect of CaO and SiO2 on swelling and iron whisker formation during reduction of iron oxide compact. Ironmak. Steelmak. 2011, 38, 447–452. [Google Scholar] [CrossRef]
- Wang, F.J.; Lv, Q.; Chen, S.J.; Liu, R.; Li, F.M. Research on influence of basicity on dropping performance of vanadium–titanium burden. Iron Steel Vanadium Titan. 2015, 36, 92–96. [Google Scholar]
- Chu, M.S.; Liu, Z.G.; Wang, Z.C.; Fu, L.; Li, Z.N. Effects of basicity on softening-dripping properties of carbon composite iron ore hot briquette. Iron Steel 2010, 45, 9–12. [Google Scholar]
- Wang, Z.C.; Chu, M.S.; Tang, J.; Xue, X.X. Effects of reducing atmosphere and gangue composition on reduction swelling of oxidized pellets. J. Northeast. Univ. Nat. Sci. 2012, 33, 94–97, 102. [Google Scholar]
- Liu, J.X.; Cheng, G.J.; Liu, Z.G.; Chu, M.S.; Xue, X.X. Reduction process of pellet containing high chromic vanadium–titanium magnetite in cohesive zone. Steel Res. Int. 2015, 86, 808–816. [Google Scholar] [CrossRef]
- Chu, M.S. Raw Fuels and Auxiliary Materials in Ferrous Metallurgy; Metallurgical Industry Press: Beijing, China, 2010. (In Chinese) [Google Scholar]
- Zhang, Y.M. Production Technology of Pellets; Metallurgical Industry Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Chen, Y.M.; Chen, R. Microstructure of Sinter and Pellet; Central South University Press: Changsha, China, 2011. (In Chinese) [Google Scholar]
- Yu, S.C.; Lee, J.S.; Tung, S.F.; Lan, C.L. Synthesis and structural features of a flux-grown hematite. J. Geol. Soc. China 1999, 42, 349–358. [Google Scholar]
- Jorgensen, J.D. Compression mechanisms in α-quartz structures—SiO2 and GeO2. J. Appl. Phys. 1978, 49, 5473–5478. [Google Scholar] [CrossRef]
- Novak, G.A.; Gibbs, G.V. The crystal chemistry of the silicate garnets. Am. Mineral. 1971, 56, 791–825. [Google Scholar]
- El-Geassy, A.A. Reduction of CaO and/or MgO-doped Fe2O3 compacts with carbon-monoxide at 1173–1473 K. ISIJ Int. 1996, 36, 1344–1353. [Google Scholar] [CrossRef]
- Gan, Q.; He, M.G.; He, Q. Effect of low Si and high basicity on the metallurgical properties of vanadium–titanium sinters. In Proceedings of the Ironmaking Production Technology Conference and Ironmaking Academic Convention Nationwide in 2010, Beijing, China, 26–28 May 2010; pp. 329–333, 338. [Google Scholar]
- Liu, Z.G.; Chu, M.S.; Wang, H.T.; Zhao, W.; Xue, X.X. Effect of MgO content in sinter on the softening-melting behavior of mixed burden made from chromium-bearing vanadium–titanium magnetite. Int. J. Miner. Metall. Mater. 2016, 23, 25–32. [Google Scholar] [CrossRef]









| Iron Ore | TFe | FeO | CaO | SiO2 | MgO | Al2O3 | TiO2 | V2O5 | Cr2O3 | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HCVTM | 62.45 | 27.29 | 0.21 | 2.69 | 0.71 | 3.20 | 5.05 | 1.032 | 0.58 | 0.16 | 0.02 |
| Magnetite a | 68.32 | 27.04 | 0.13 | 4.27 | 0.30 | 0.32 | - | - | - | 0.09 | 0.01 |
| Magnetite b | 65.02 | 23.90 | 0.17 | 8.35 | 0.19 | 0.05 | - | - | - | 0.01 | 0.02 |
| Temperature Range | 0–400 °C | 400–900 °C | 900–1020 °C | 1020 °C–Dripping Temperature |
|---|---|---|---|---|
| Furnace ramping rate | l0 °C/min | l0 °C/min | 3 °C/min | 5 °C/min |
| Gas composition | N2 3 L/min | N2 9 L/min CO 3.9 L/min CO2 2.1 L/min | N2 10.5 L/min CO 4.5 L/min | |
| CaO Additive/wt % | ΔPmax/Pa | TΔP/°C | ΔPd/Pa | S Value/Pa·°C |
|---|---|---|---|---|
| 0 | 17,722 | 1291 | 1448 | 1,232,343 |
| 2 | 16,243 | 1333 | 2049 | 1,011,039 |
| 4 | 17,344 | 1337 | 2036 | 1,033,882 |
| 6 | 10,564 | 1355 | 1654 | 842,193 |
| 8 | 9311 | 1369 | 1451 | 867,657 |
| CaO Additive/wt % | Slag | Melted Iron | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TFe | CaO | SiO2 | CaO/SiO2 | TiO2 | V | Cr2O3 | TFe | Ca | Ti | V | Cr | |
| 2 | 11.68 | 14.60 | 38.12 | 0.38 | 17.92 | 1.23 | 1.16 | 96.00 | 0.034 | 0.052 | 0.031 | 0.006 |
| 4 | 8.91 | 24.81 | 31.48 | 0.79 | 17.53 | 1.27 | 1.11 | 96.03 | 0.048 | 0.036 | 0.025 | 0.002 |
| 6 | 5.18 | 36.10 | 30.19 | 1.20 | 14.84 | 0.84 | 0.57 | 96.05 | 0.140 | 0.059 | 0.034 | 0.031 |
| 8 | 4.44 | 39.56 | 29.67 | 1.33 | 13.06 | 0.89 | 0.49 | 96.47 | 0.017 | 0.013 | 0.018 | 0.016 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, G.; Gao, Z.; Yang, H.; Xue, X. Effect of Calcium Oxide on the Crushing Strength, Reduction, and Smelting Performance of High-Chromium Vanadium–Titanium Magnetite Pellets. Metals 2017, 7, 181. https://doi.org/10.3390/met7050181
Cheng G, Gao Z, Yang H, Xue X. Effect of Calcium Oxide on the Crushing Strength, Reduction, and Smelting Performance of High-Chromium Vanadium–Titanium Magnetite Pellets. Metals. 2017; 7(5):181. https://doi.org/10.3390/met7050181
Chicago/Turabian StyleCheng, Gongjin, Zixian Gao, He Yang, and Xiangxin Xue. 2017. "Effect of Calcium Oxide on the Crushing Strength, Reduction, and Smelting Performance of High-Chromium Vanadium–Titanium Magnetite Pellets" Metals 7, no. 5: 181. https://doi.org/10.3390/met7050181





