High Temperature Oxidation and Wear Behaviors of Ti–V–Cr Fireproof Titanium Alloy
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials Preparation
2.2. Oxidation Testing
2.3. Wear Testing
2.4. Microstructural Characterization
3. Results and Discussion
4. Conclusions
- (1)
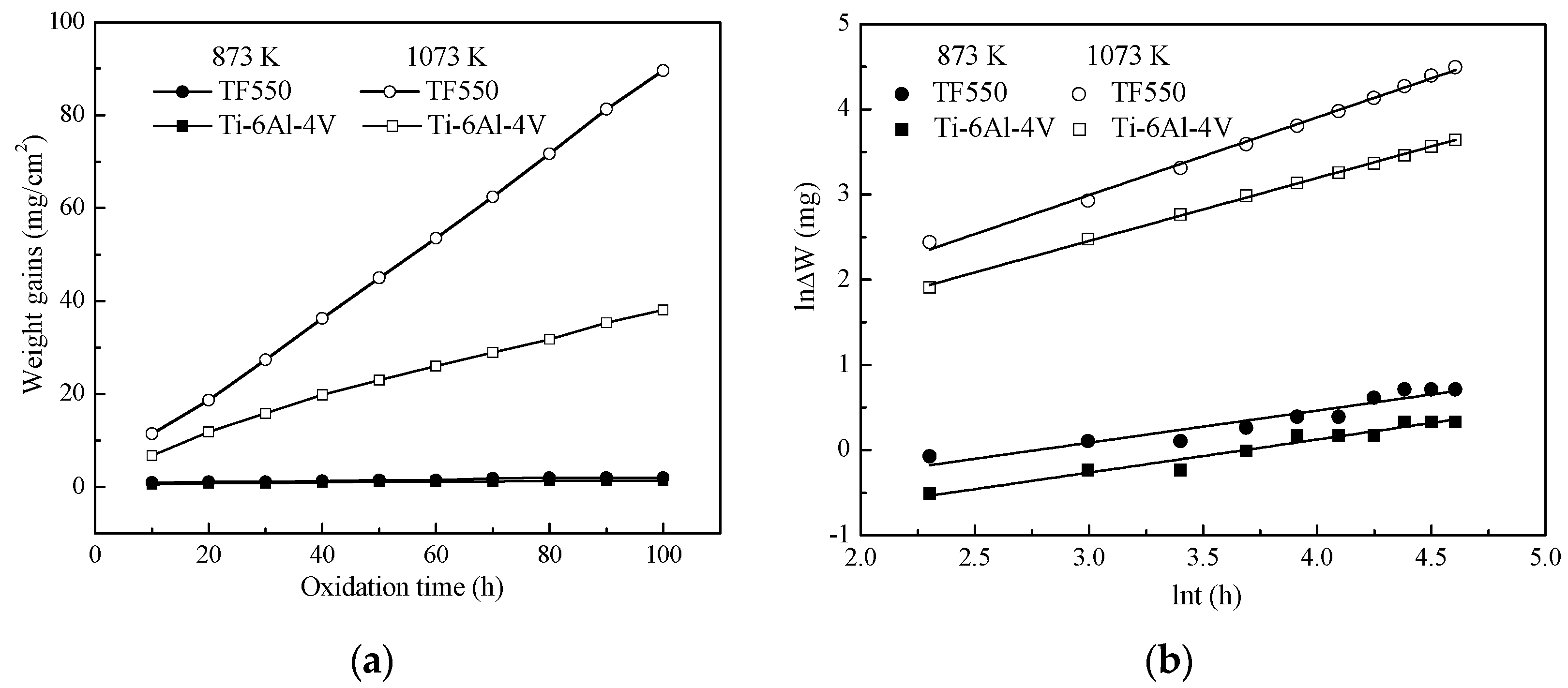
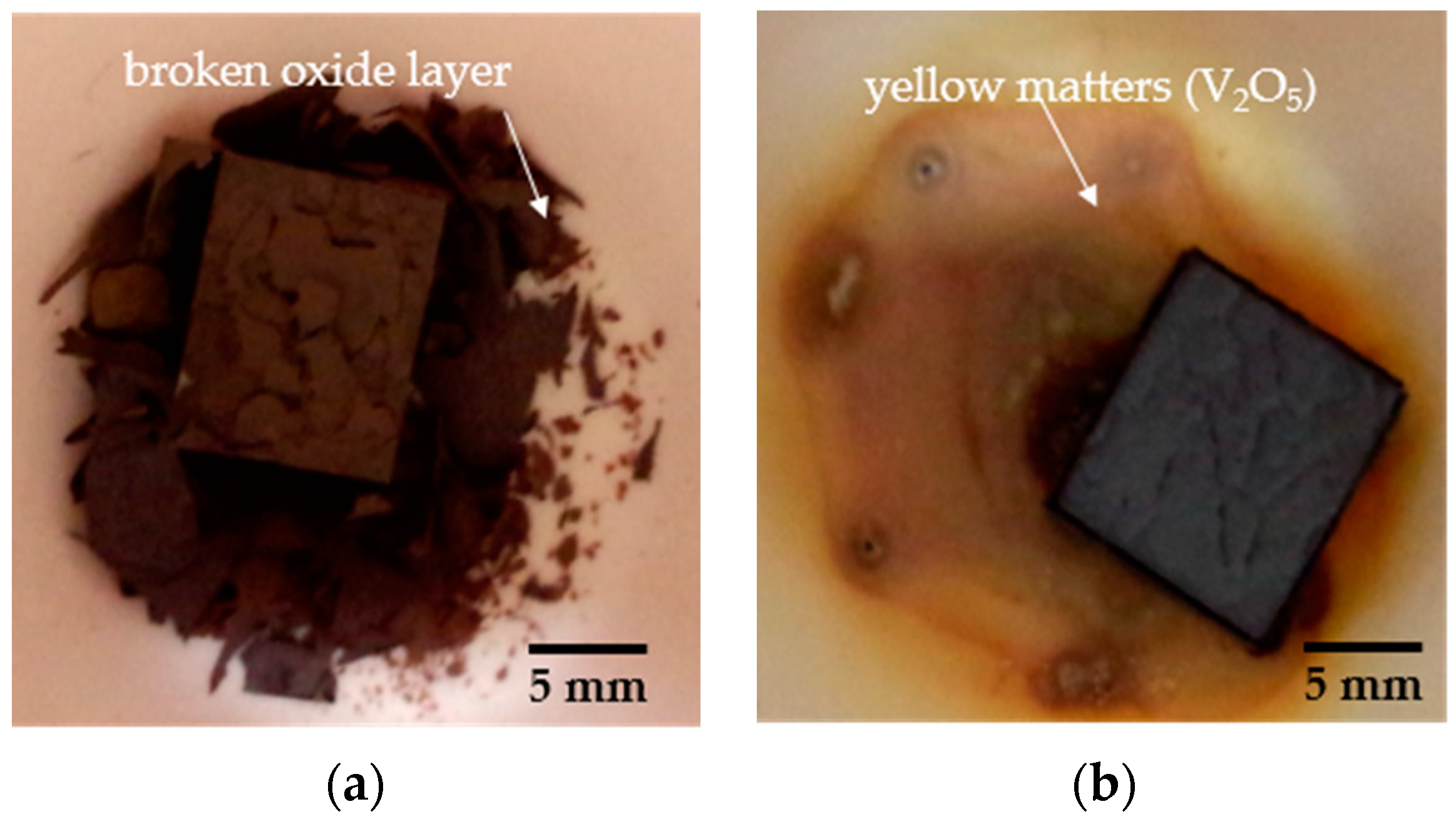
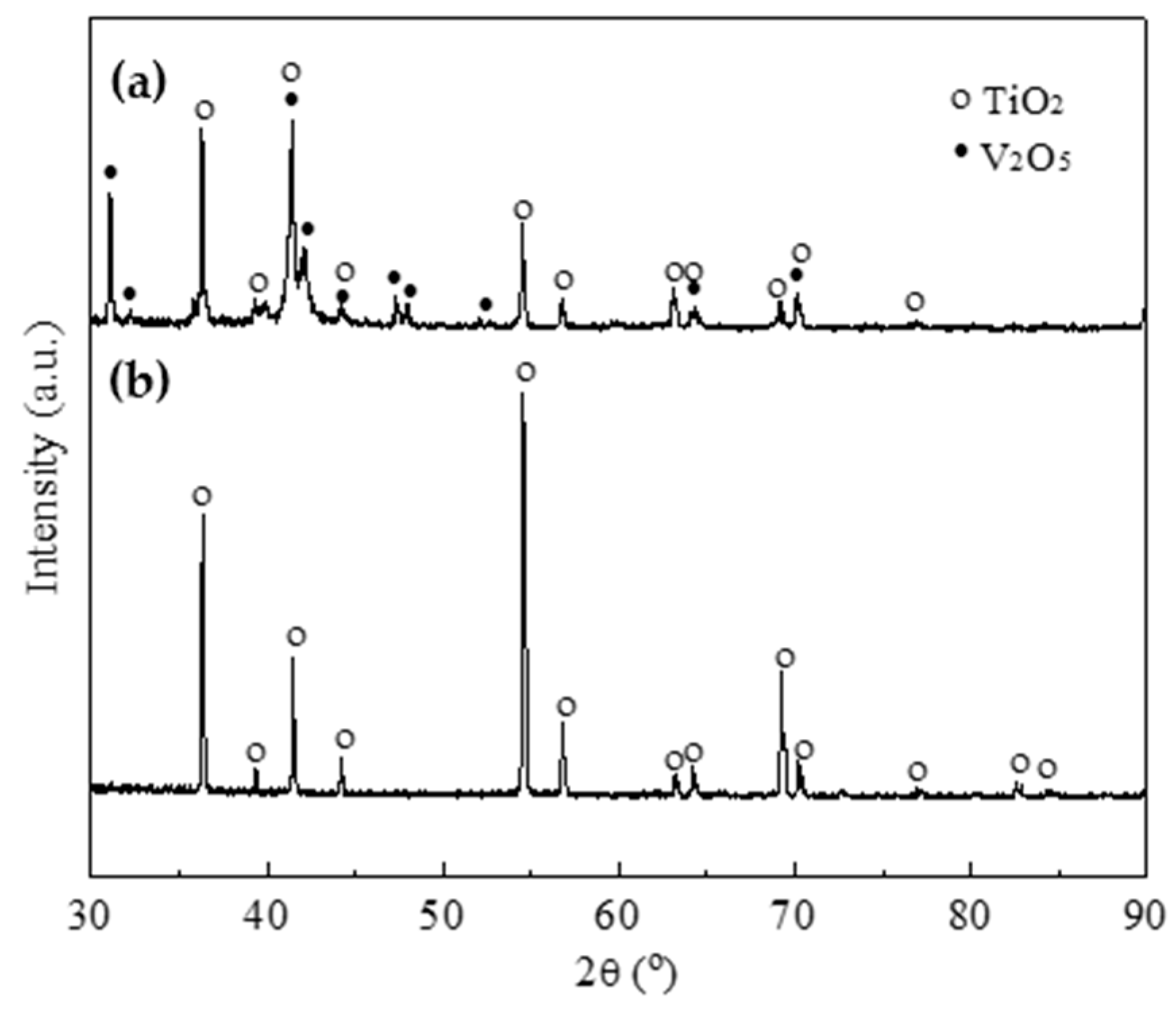
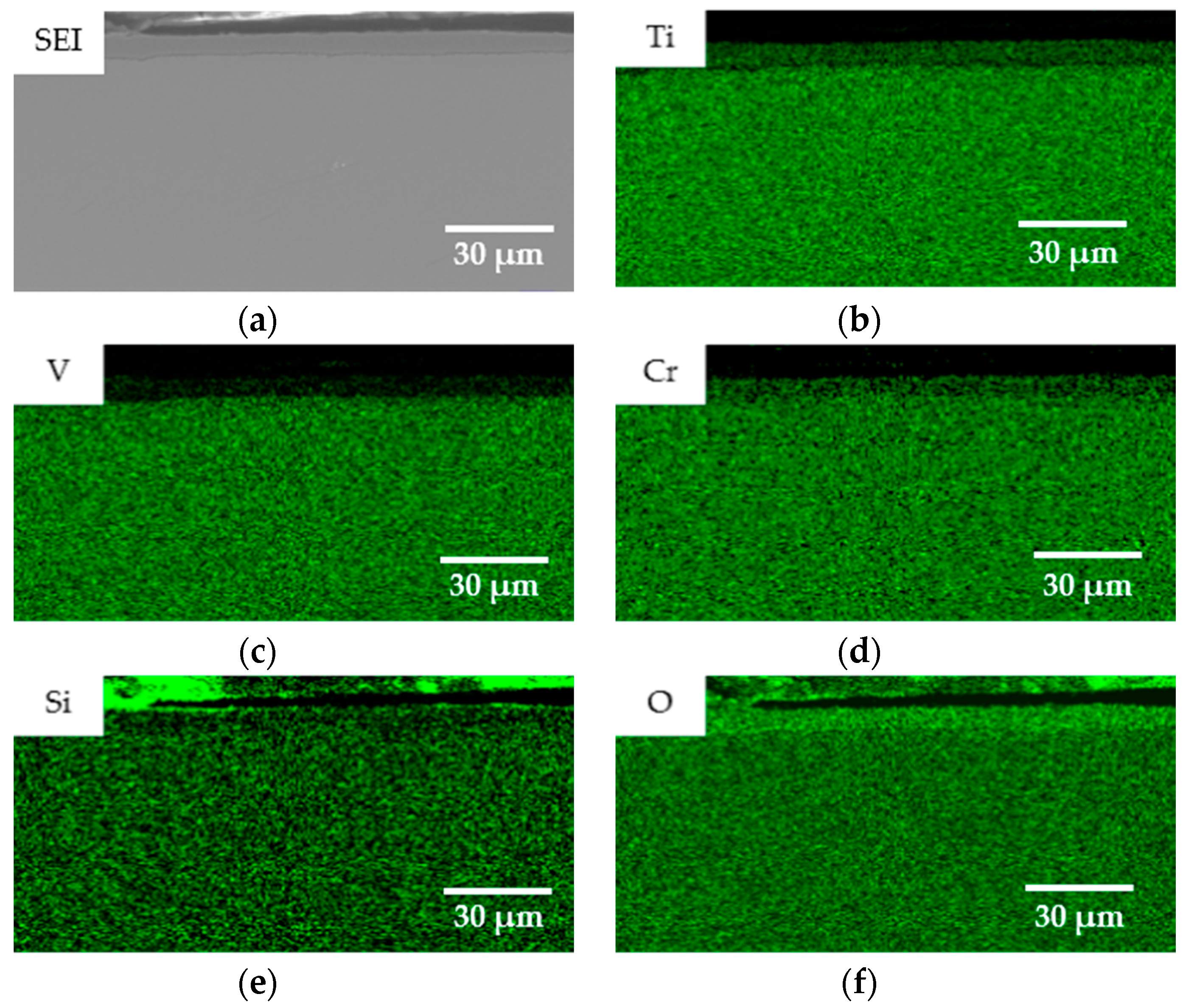
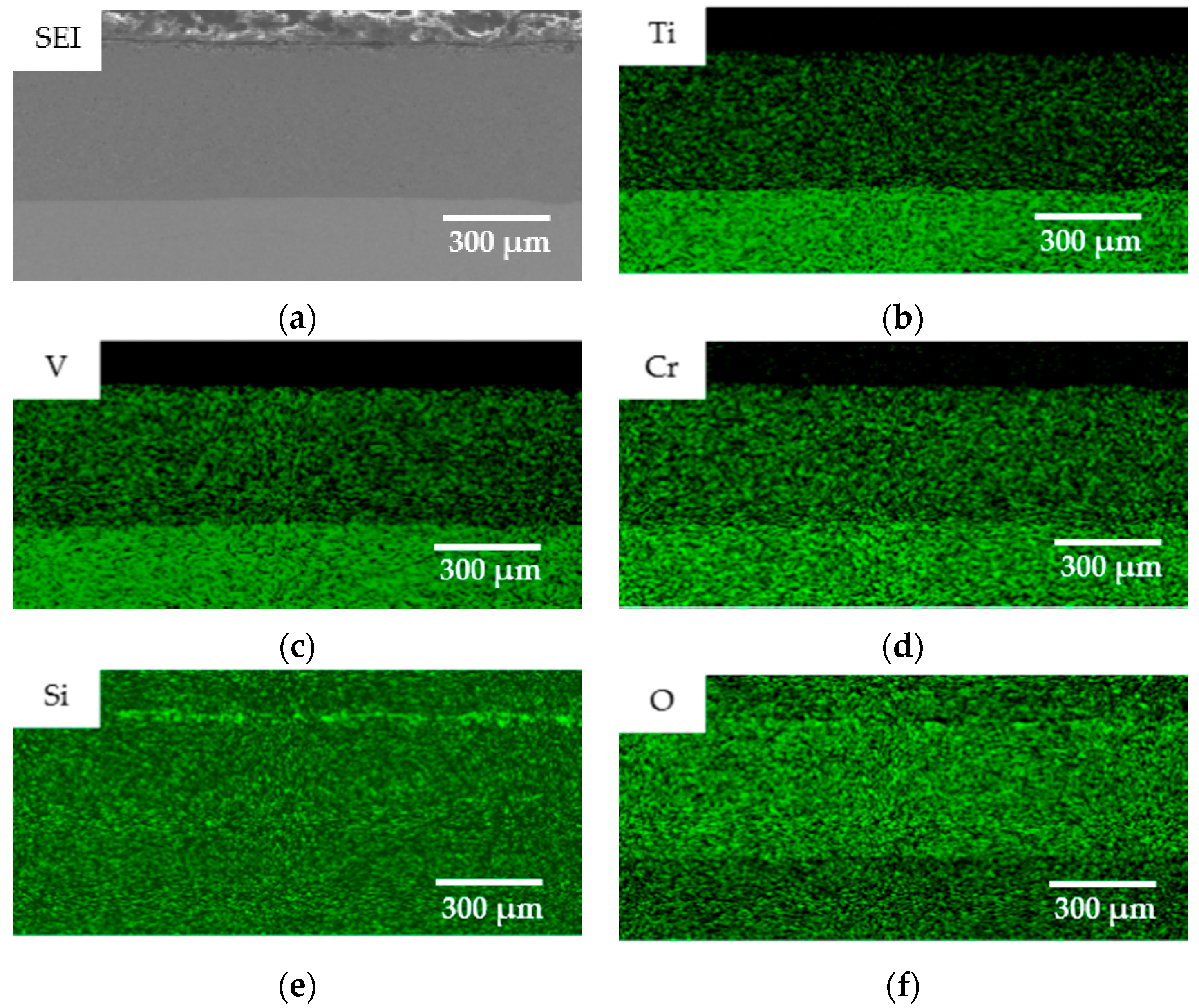
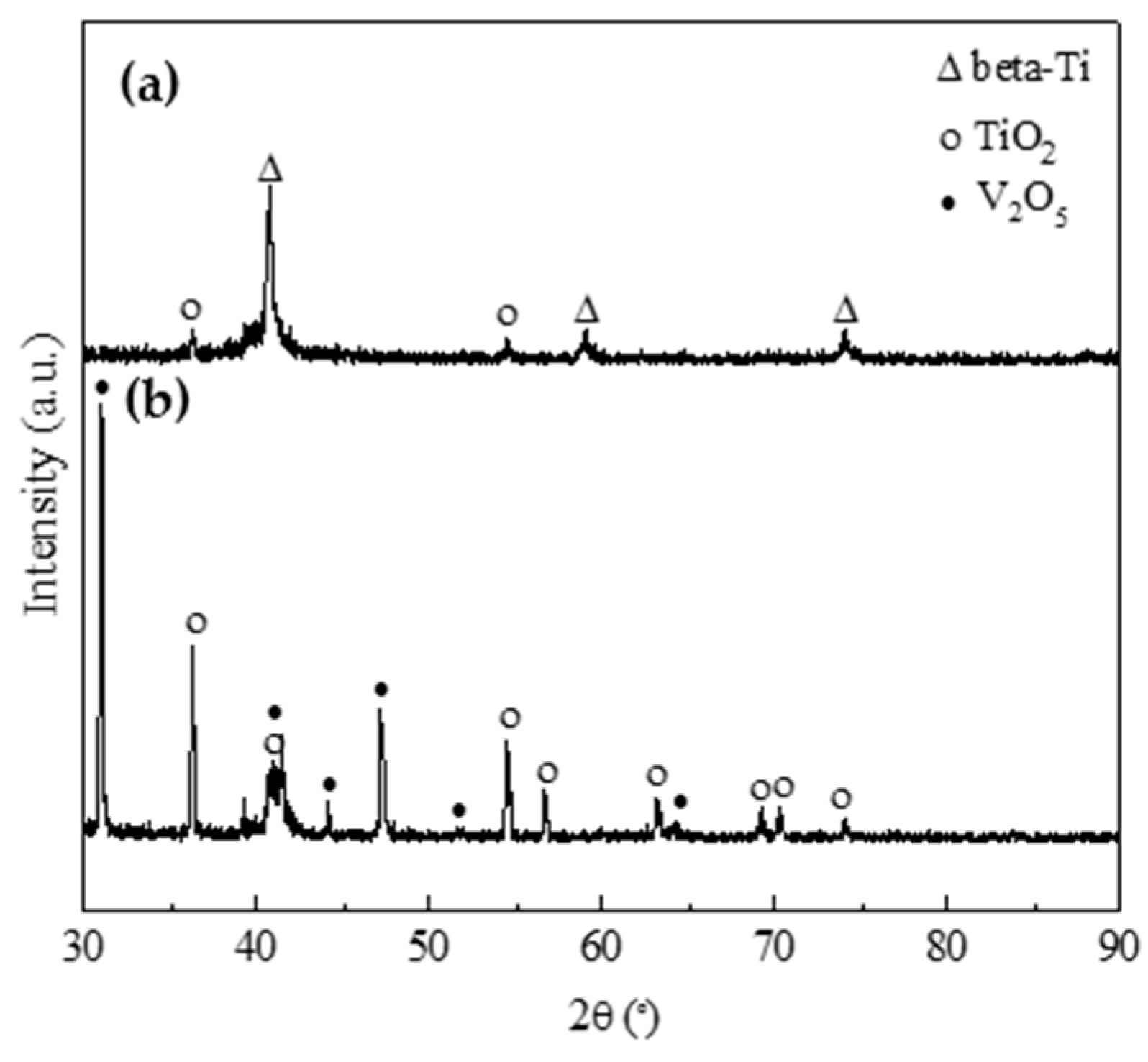
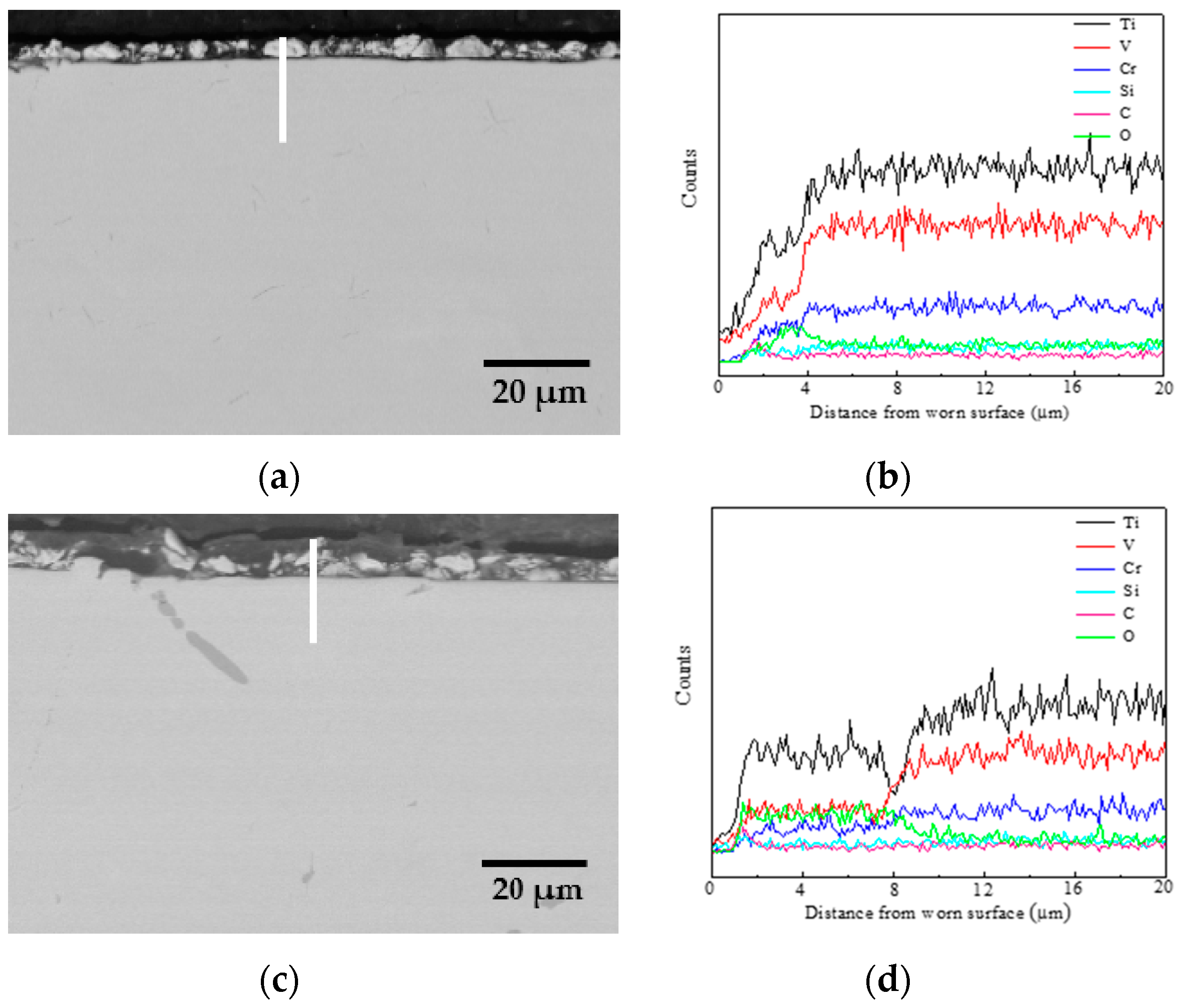
- The oxidation gain of TF550 alloy was much larger than that of Ti64 alloy, attributed the abundant addition of vanadium, which was regarded as a positive factor to improve the burn resistance. The oxidation weight gain of TF550 alloy after oxidation at 873 K for 100 h was much smaller than that at 1073 K for 100 h. The thin surface oxide film was identified as oxides of TiO2 and V2O5 at 873 K, while the thick one was detected to be TiO2 only at 1073 K. The oxidation reaction rate at 1073 K followed the linear law, indicating that the oxidation process of this alloy was mainly controlled by the oxidation reaction at the interface between the substrate and oxide layer.
- (2)
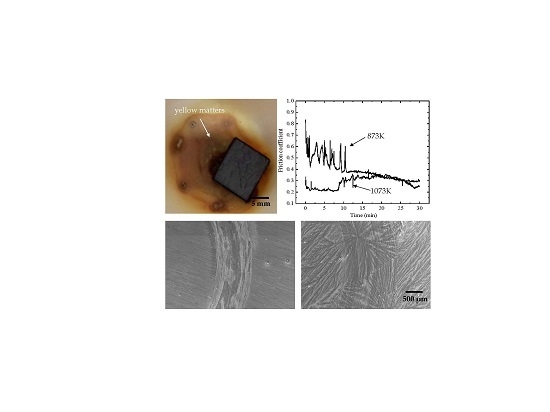
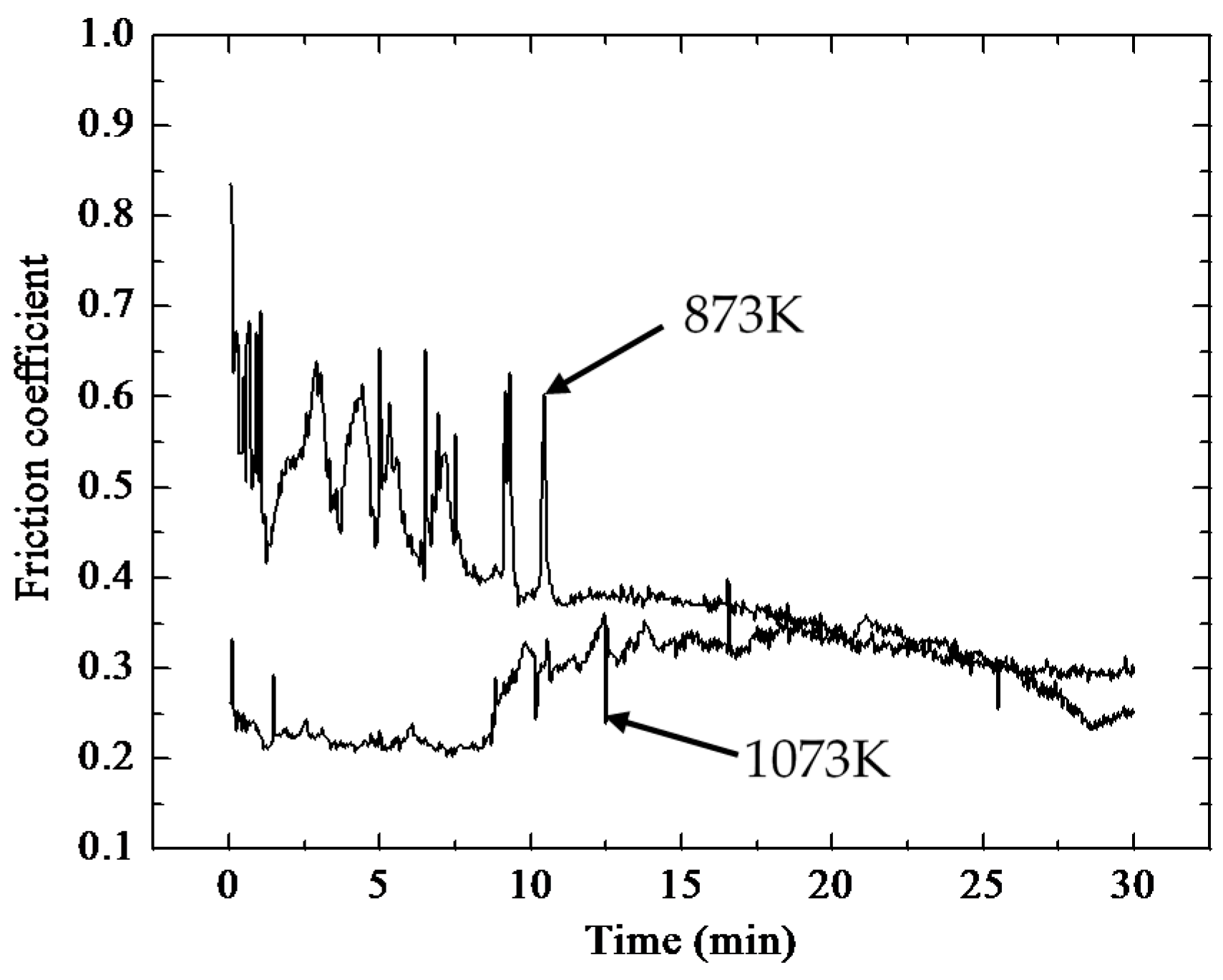
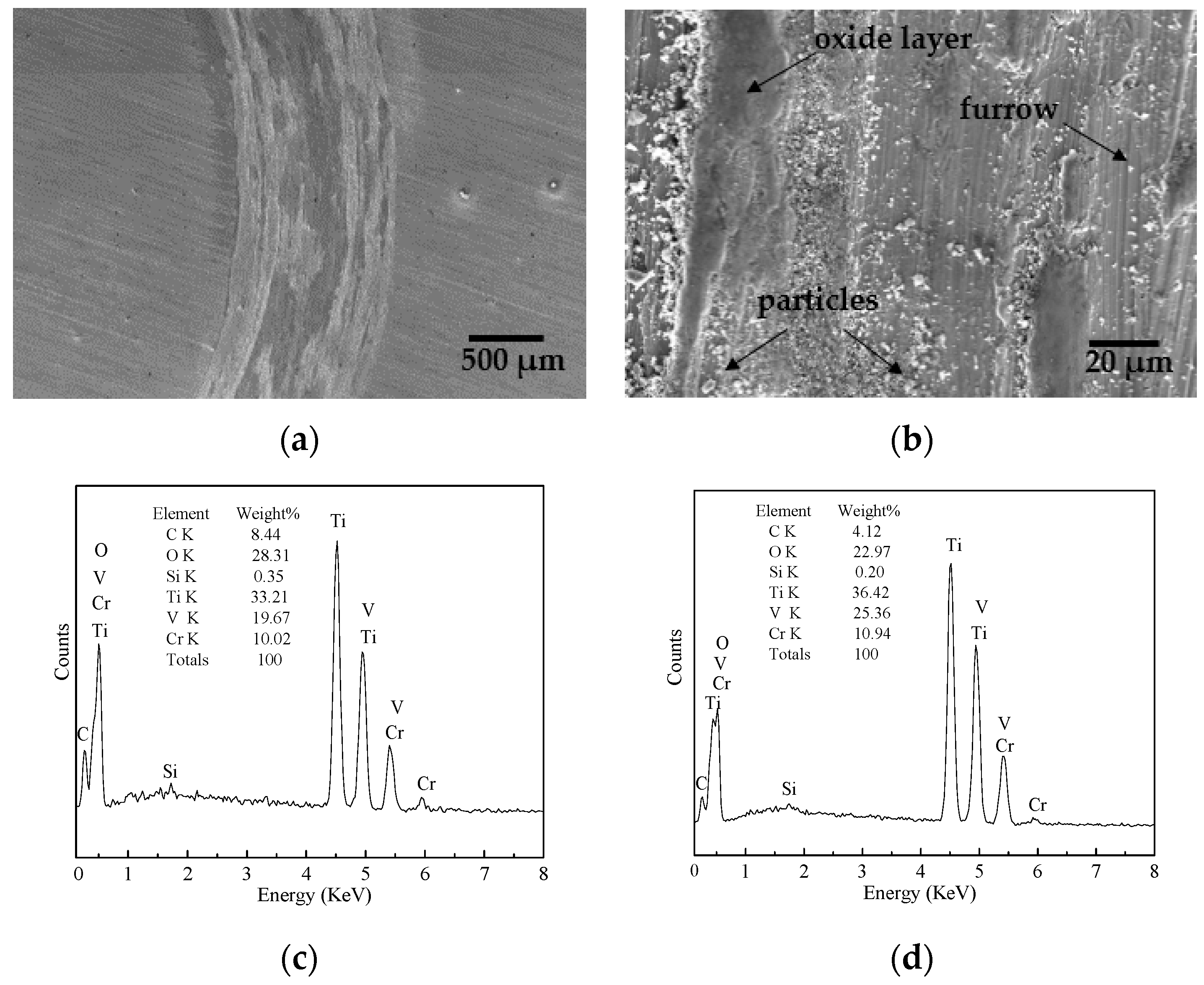
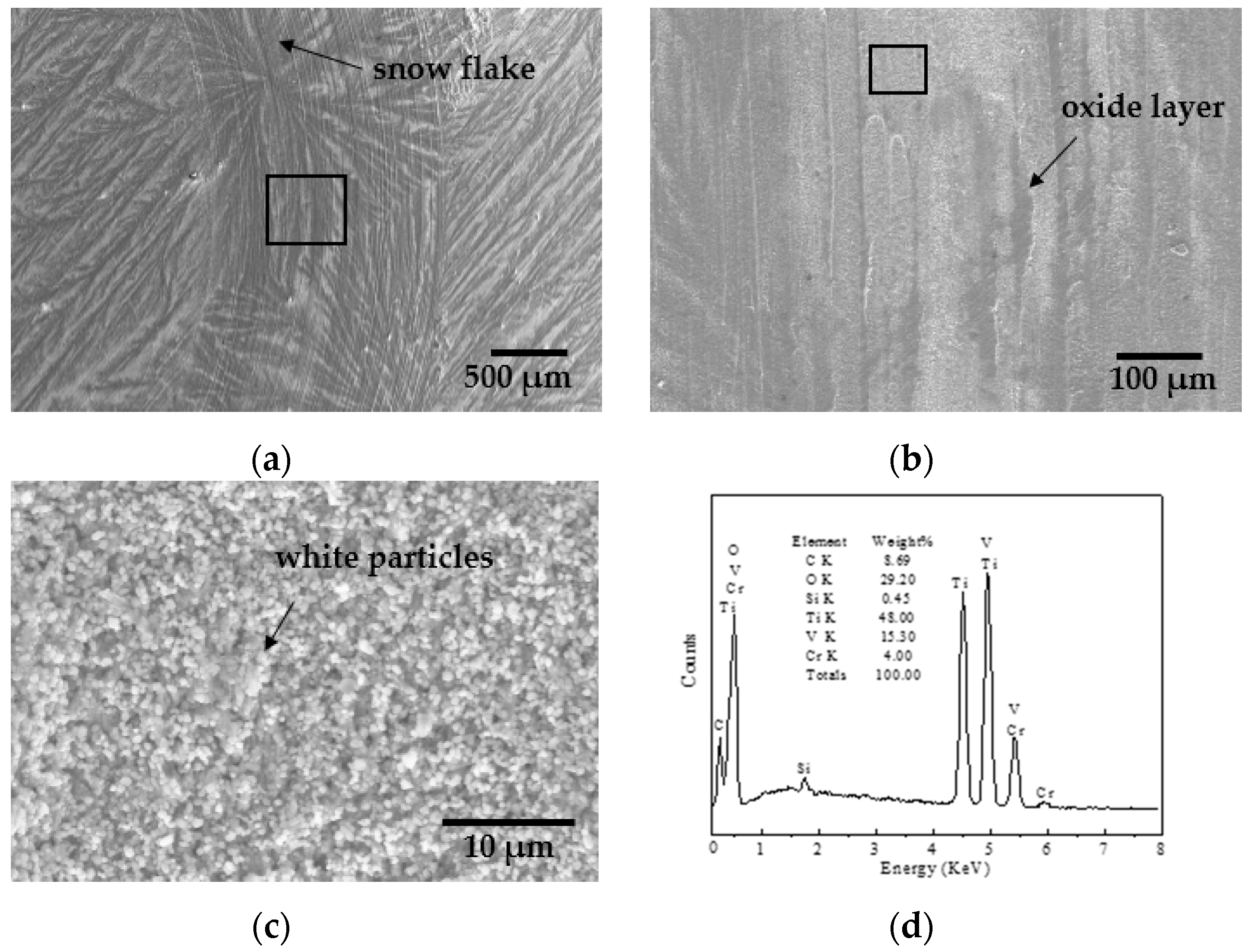
- The friction coefficient at 873 K was larger than that at 1073 K, while the volume wear rate at 1073 K was larger because a large amount of oxides formed on the worn surface. The TiO2 oxide was detected on the worn surface at 873 K, and both oxides of TiO2 and V2O5 were present at 1073 K.
- (3)
- The presence of furrows and a tribo-oxide layer at 873 K indicated that the wear mechanism was dominated by a combination of abrasive wear and oxidative wear. The continuous tribo-oxide layer was present at 1073 K, indicating that the wear mechanism was dominated by oxidative wear.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Min, X.H.; Tsuzaki, K.; Emura, S.; Tsuchiya, K. Heterogeneous twin formation and its effect on tensile properties in Ti–Mo based β titanium alloys. Mater. Sci. Eng. A 2012, 554, 53–60. [Google Scholar] [CrossRef]
- Min, X.H.; Emura, S.; Zhang, L.; Tsuzaki, K.; Tsuchiya, K. Improvement of strength-ductility tradeoff in β titanium alloy through pre-strain induced twins combined with brittle ω phase. Mater. Sci. Eng. A 2015, 646, 279–287. [Google Scholar] [CrossRef]
- Luo, Q.S.; Li, S.F.; Pei, H.P. Progress in titanium fire resistant technology for aero-engine. J. Aerosp. Power 2012, 27, 2763–2767. [Google Scholar]
- Zhang, P.Z.; Xu, Z.; Zhang, G.H.; He, Z.Y. Surface plasma chromized burn-resistant titanium alloy. Surf. Coat. Technol. 2007, 201, 4884–4887. [Google Scholar] [CrossRef]
- Lv, D.S.; Xu, J.H.; Ding, W.F.; Fu, Y.C.; Yang, C.Y.; Su, H.H. Tool wear in milling Ti40 burn-resistant titanium alloy using pneumatic mist jet impinging cooling. J. Mater. Process. Technol. 2016, 229, 641–650. [Google Scholar] [CrossRef]
- Zhang, X.J.; Cao, Y.H.; Ren, B.Y.; Tsubaki, N. Improvement of high-temperature oxidation resistance of titanium-based alloy by sol-gel method. J. Mater. Sci. 2010, 45, 1622–1628. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhao, Y.Q.; Zhou, L.; Zhang, D. Study on creep behavior of Ti–V–Cr burn resistant alloys. Mater. Lett. 2004, 58, 3248–3252. [Google Scholar] [CrossRef]
- Xin, S.W.; Zhao, Y.Q.; Zeng, W.D.; Wu, H. Research on thermal stability of Ti40 alloy at 550 °C. Mater. Sci. Eng. A 2008, 477, 372–378. [Google Scholar] [CrossRef]
- Li, Y.G.; Blenkinsop, P.A.; Loretto, M.H.; Walkern, N.A. Structure and stability of precipitates in 500 °C exposed in Ti–25V–15Cr–xAl. Acta Mater. 1998, 46, 5777–5794. [Google Scholar] [CrossRef]
- Seagle, S.R. The state of the USA titanium industry in 1995. Mater. Sci. Eng. A 1996, 213, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.N.; Huo, Y.Z.; Song, X.D.; Bi, Z.Z.; Gao, Y.; Zhao, Y.Q. Burn-resistant behavior and mechanism of Ti14 alloy. Int. J. Miner. Metall. Mater. 2016, 23, 215–221. [Google Scholar] [CrossRef]
- Campo, K.N.; Lima, D.D.; Lopes, E.S.N.; Caram, R. On the selection of Ti–Cu alloys for thixoforming processes: Phase diagram and microstructural evaluation. J. Mater. Sci. 2015, 50, 8007–8017. [Google Scholar] [CrossRef]
- Chen, Y.N.; Yang, W.Q.; Zhan, H.F.; Zhang, F.Y.; Huo, Y.Z.; Zhao, Y.Q.; Song, X.D.; Gu, Y.T. Tailorable burning behavior of Ti14 alloy by controlling semi-solid forging temperature. Materials 2016, 9, 697. [Google Scholar] [CrossRef]
- Mi, G.B.; Huang, X.; Cao, J.X.; Wang, B.; Cao, C.X. Microstructure characteristics of burning products of Ti–V–Cr fireproof titanium alloy by frictional ignition. Acta Phys. Sin. 2016, 65, 1–10. [Google Scholar]
- Cao, J.X.; Huang, X.; Mi, G.B.; Sha, A.X.; Wang, B. Research progress on application technique of Ti–V–Cr burn resistant titanium alloys. J. Aeronaut. Mater. 2014, 34, 92–97. [Google Scholar]
- Mi, G.B.; Cao, C.X.; Huang, X.; Cao, J.X.; Wang, B.; Sui, N. Non-isothermal oxidation characteristic and fireproof property prediction of Ti–V–Cr type fireproof titanium alloy. J. Mater. Eng. 2016, 44, 1–10. [Google Scholar]
- Molinari, A.; Straffelini, G.; Tesi, B.; Bacci, T. Dry sliding wear mechanisms of the Ti6Al4V alloy. Wear 1997, 208, 105–112. [Google Scholar] [CrossRef]
- Cui, X.H.; Mao, Y.S.; Wei, M.X.; Wang, S.Q. Wear Characteristics of Ti–6Al–4V Alloy at 20–400 °C. Tribol. Trans. 2012, 5, 185–190. [Google Scholar] [CrossRef]
- Sun, Q.C.; Hu, T.C.; Fan, H.Z.; Zhang, Y.S.; Hu, L.T. Dry sliding wear behavior of TC11 alloy at 500 °C: Influence oflaser surface texturing. Tribol. Int. 2015, 92, 136–145. [Google Scholar] [CrossRef]
- Mao, Y.S.; Wang, L.; Chen, K.M.; Wang, S.Q.; Cui, X.H. Tribo-layer and its role in dry sliding wear of Ti–6Al–4V alloy. Wear 2013, 297, 1032–1039. [Google Scholar] [CrossRef]
- Zeng, S.W.; Jiang, H.T.; Zhao, A.M. High Temperature Oxidation Behavior of TC4 Alloy. Rare Met. Mater. Eng. 2015, 44, 2812–2816. [Google Scholar]
- Zeng, S.W.; Zhao, A.M.; Jiang, H.T.; Fan, X.; Duan, X.G.; Yan, X.Q. Cyclic Oxidation Behavior of the Ti–6Al–4V Alloy. Oxid. Met. 2014, 81, 467–476. [Google Scholar] [CrossRef]
- Mendez, S.F.O.; Rodriguez, C.R.S.; Venegas, K.C.; Aquino, J.A.M.; Magana, F.E. Magnetism and decarburization-like diffusion process on V2O5-doped ZnO ceramics. Ceram. Int. 2015, 41, 6802–6806. [Google Scholar] [CrossRef]
- Li, Y.; Bai, C.Y.; Deng, X.Y.; Li, J.B.; Jing, Y.N.; Liu, Z.M. Effect of V2O5 addition on the properties of reaction-bonded porous SiC ceramics. Ceram. Int. 2014, 40, 16581–16587. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction of Metal Oxidation at High Temperature; Higher Education Press: Beijing, China, 2010; p. 43. [Google Scholar]
- Tomasi, A.; Gialanella, S. Oxidation phenomena in a Ti3Al base-alloy. Therm. Acta 1995, 269, 133–143. [Google Scholar] [CrossRef]
- Frangini, S.; Mignone, A.; De Riccardis, F. Various aspects of the air oxidation behavior of a Ti6Al4V alloy at temperatures in the range 600–700 °C. J. Mater. Sci. 1994, 29, 714–720. [Google Scholar] [CrossRef]
- Jia, W.J.; Zeng, W.D.; Zhang, X.M.; Zhou, Y.G.; Liu, J.R.; Wang, Q.J. Oxidation behavior and effect of oxidation on tensile properties of Ti60 alloy. J. Mater. Sci. 2011, 46, 1351–1358. [Google Scholar] [CrossRef]
- Huang, X.; Cao, C.X.; Ma, J.M.; Wang, B.; Gao, Y. High temperature oxidation behavior of a fire-resistant titanium alloy. Rare Met. Mater. Eng. 1997, 26, 27–30. [Google Scholar]
- Wang, L.; Zhang, Q.Y.; Li, X.X.; Cui, X.H.; Wang, S.Q. Dry Sliding Wear Behavior of Ti–6.5Al–3.5Mo–1.5Zr– 0.3Si Alloy. Metall. Mater. Trans. A 2014, 45A, 2284–2296. [Google Scholar] [CrossRef]
| Temperature (K) | fm | Wsd (10−13·m3/Nm) | Wsp (10−13·m3/Nm) |
|---|---|---|---|
| 873 | 0.394 ± 0.095 | 2.21 ± 0.35 | 0.197 ± 0.072 |
| 1073 | 0.286 ± 0.050 | - | 0.566 ± 0.039 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, G.; Yao, K.; Bai, P.; Cheng, C.; Min, X. High Temperature Oxidation and Wear Behaviors of Ti–V–Cr Fireproof Titanium Alloy. Metals 2017, 7, 226. https://doi.org/10.3390/met7060226
Mi G, Yao K, Bai P, Cheng C, Min X. High Temperature Oxidation and Wear Behaviors of Ti–V–Cr Fireproof Titanium Alloy. Metals. 2017; 7(6):226. https://doi.org/10.3390/met7060226
Chicago/Turabian StyleMi, Guangbao, Kai Yao, Pengfei Bai, Congqian Cheng, and Xiaohua Min. 2017. "High Temperature Oxidation and Wear Behaviors of Ti–V–Cr Fireproof Titanium Alloy" Metals 7, no. 6: 226. https://doi.org/10.3390/met7060226