Self-Propagating High Temperature Synthesis of TiB2–MgAl2O4 Composites
Abstract
:1. Introduction
2. Materials and Methods
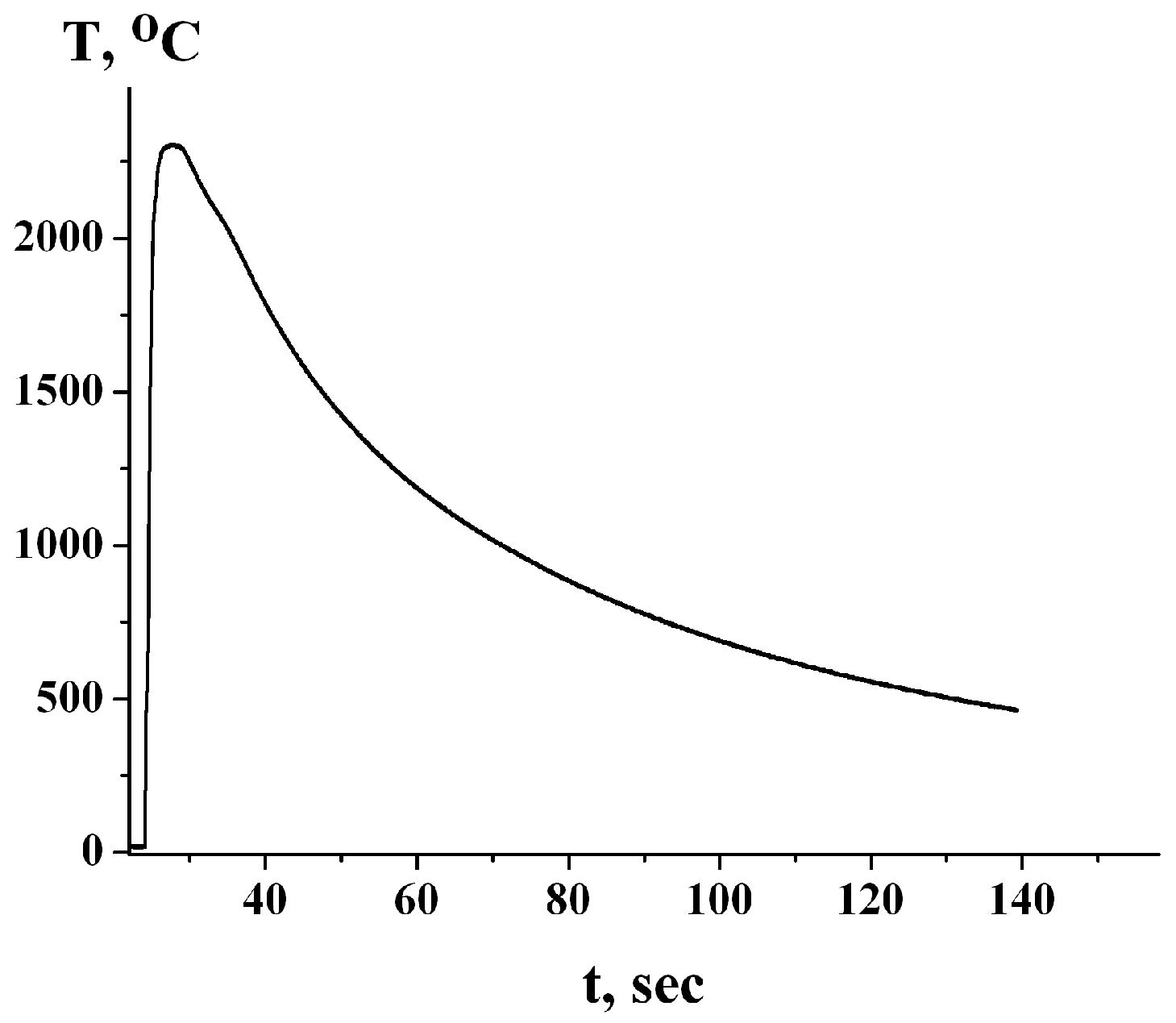
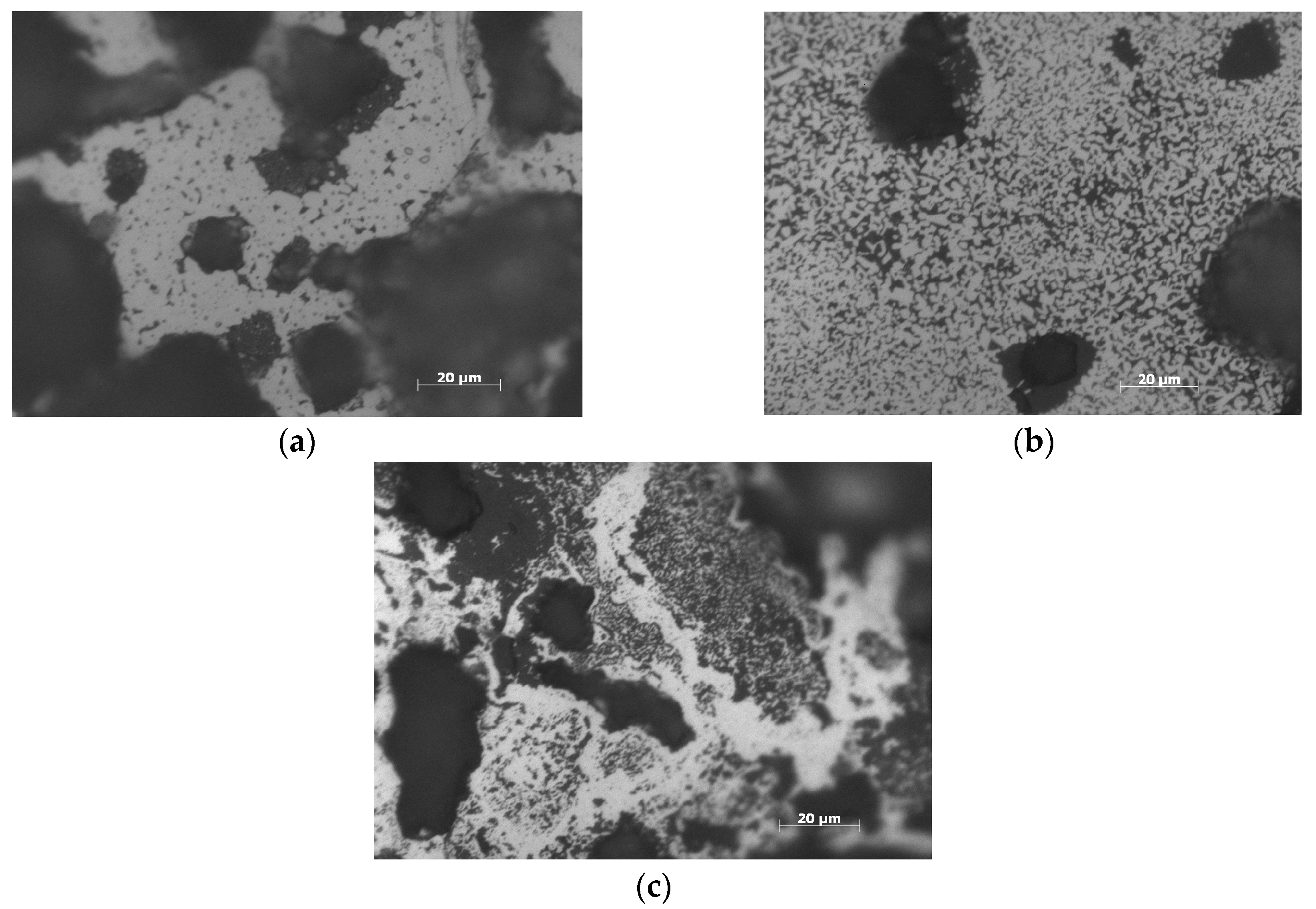
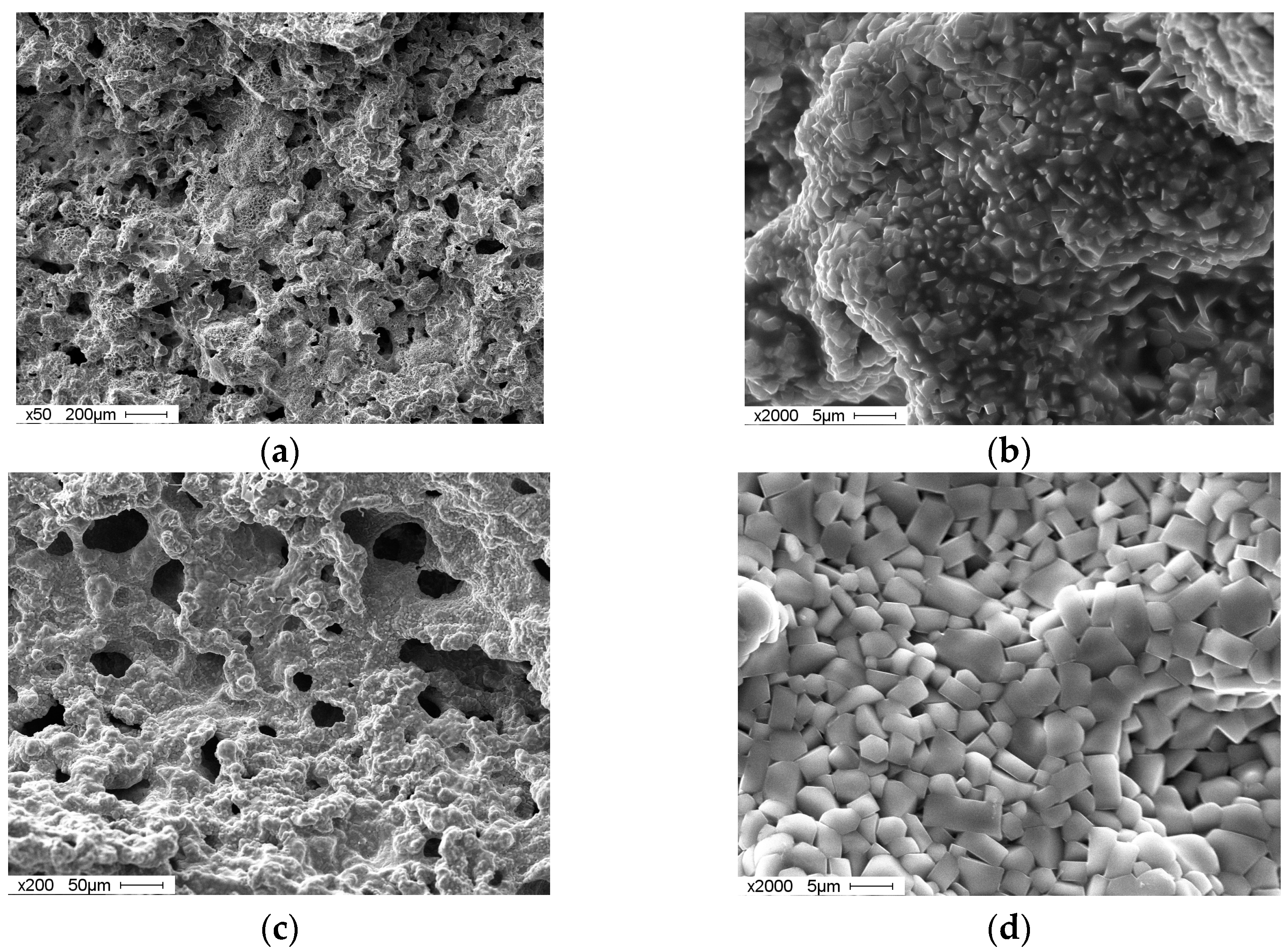
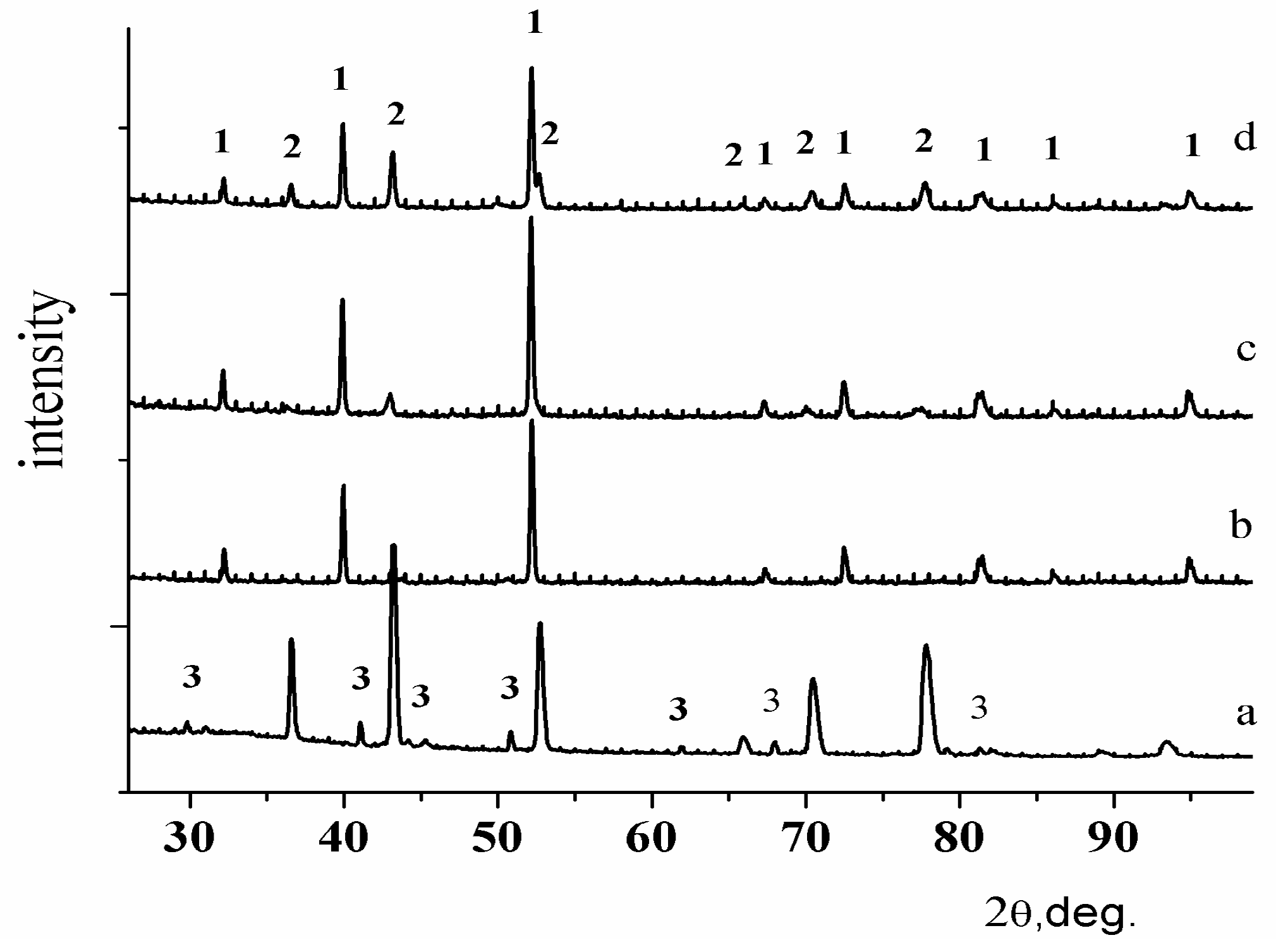
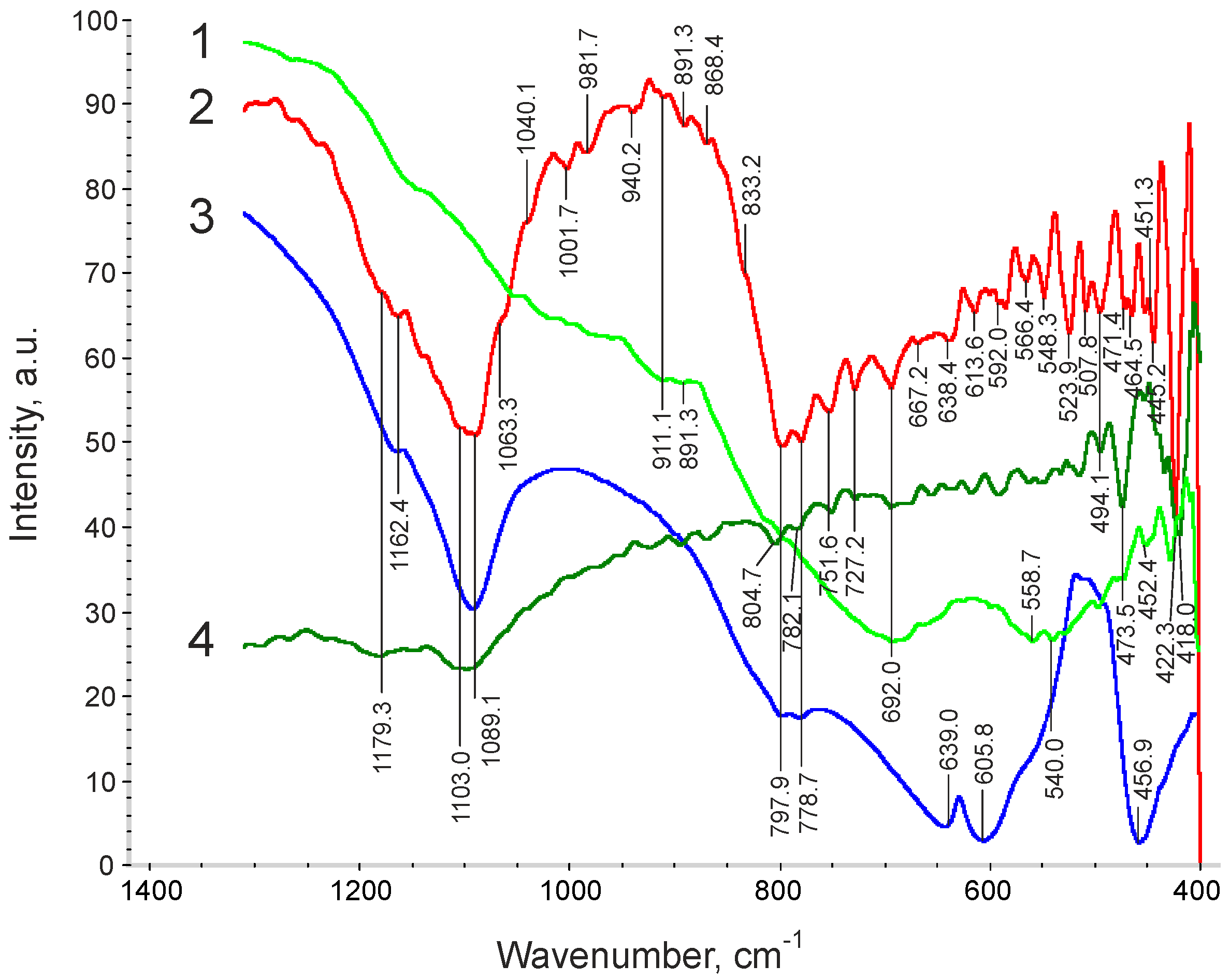
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.B.; Li, N.; Ruan, G.Z.; Li, X.H. Reaction in the aluminotheric reduction nitridation reaction to synthesize MgAl2O4/TiN. Ceram. Int. 2005, 31, 825–829. [Google Scholar] [CrossRef]
- Omid, E.K.; Naghizadeh, R.; Rezaie, H.R. Synthesis and comparison of MgAl2O4–Ti (C,N) composites using aluminothermic-carbothermal reduction and molten salts routes. J. Ceram. Process. Res. 2013, 14, 445–447. [Google Scholar]
- Bae, Y.; Jun, B. Preparation of ultrafine TiC, MgAl2O4 and AlON composite powder using chemical furnace. J. Ceram. Process. Res. 2008, 9, 661–665. [Google Scholar]
- Zaki, Z.I.; Ahmed, Y.M.Z.; Abdel Gawad, S.R. In situ synthesis of porous magnesia spinel/TiB2 composite by combustion technique. J. Ceram. Soc. Jpn. 2009, 117, 719–723. [Google Scholar] [CrossRef]
- Horvitz, D.; Gotman, I. Pressure-assisted DHD synthesis of MgAl2O4–TiAl in Situ composites with interpenetrating networks. Acta Mater. 2002, 50, 1961–1971. [Google Scholar] [CrossRef]
- Horoshavin, L.B. Spinel Nanorefractory Materials; UB RAS: Ekaterinburg, Russia, 2009; p. 600. [Google Scholar]
- Merzhanov, A.G. Processes of Burning and Materials Synthesis; ISMAN: Chernogolovka, Russia, 1998; p. 511. [Google Scholar]
- Lepakova, O.K.; Raskolenko, L.G.; Maksimov, Y.M. Self-propagating high-temperature synthesis of composite material TiB2–Fe. J. Mater. Sci. 2004, 39, 3723–3732. [Google Scholar] [CrossRef]
- Vadchenko, S.G.; Filimonov, I.A. Burning modes of diluted system Ti + 2B. Phys. Burn. Combust. 2003, 39, 48–55. [Google Scholar]
- Barabanov, V.F.; Goncharov, G.N.; Zorina, M.L. Modern Physical Methods on Geochemistry; Leningrad University: Pushkin, Russia, 1990; p. 390. [Google Scholar]
- Chernyakova, K.V.; Vrubelevskii, I.A.; Ivanovskaya, M.I.; Kotikov, D.A. Defective structure of anode alumina oxide, formed by method of bilateral anodic oxidation. J. Appl. Spectrosc. 2012, 79, 83–89. [Google Scholar]
- Nakamoto, K. IK-Spektry i Spektry KR Neorganicheskikh i Koordinatsionnykh Soedinenii (Infrared and Raman Spectra of Inorganic and Coordination Compounds); Mir: Moscow, Russia, 1991; p. 536. [Google Scholar]
- Solodkii, E.N.; Solodkii, N.F. Reasons for coloring corundum ceramics. Glass Ceram. 2000, 11, 24–26. [Google Scholar]
- Kulikov, I.S. Metals Deoxidation; Metallurgy: Moscow, Russia, 1975; p. 504. [Google Scholar]
| Compound | Melting Temperature, °C | Density, g/cm3 | −ΔH°form, kJ/mol |
|---|---|---|---|
| MgAl2O4 | 2135 | 3.8 | 2307.8 |
| TiB2 | 2850 | 4.45–4.50 | 293.3 |
| MgTiO3 | 1680 | 3.91 | 1573.6 |
| α–Al2O3 | 2045 | 3.99 | 1675.0 |
| Chemical Compounds in Eutectics | Al2O3 Composition, wt % | Melting Temperature, °C |
|---|---|---|
| MgO, MgAl2O4 | 55 | 1995 |
| MgAl2O4, Al2O3 | 98 | 1920 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radishevskaya, N.; Lepakova, O.; Karakchieva, N.; Nazarova, A.; Afanasiev, N.; Godymchuk, A.; Gusev, A. Self-Propagating High Temperature Synthesis of TiB2–MgAl2O4 Composites. Metals 2017, 7, 295. https://doi.org/10.3390/met7080295
Radishevskaya N, Lepakova O, Karakchieva N, Nazarova A, Afanasiev N, Godymchuk A, Gusev A. Self-Propagating High Temperature Synthesis of TiB2–MgAl2O4 Composites. Metals. 2017; 7(8):295. https://doi.org/10.3390/met7080295
Chicago/Turabian StyleRadishevskaya, Nina, Olga Lepakova, Natalia Karakchieva, Anastasiya Nazarova, Nikolai Afanasiev, Anna Godymchuk, and Alexander Gusev. 2017. "Self-Propagating High Temperature Synthesis of TiB2–MgAl2O4 Composites" Metals 7, no. 8: 295. https://doi.org/10.3390/met7080295







