Sintering Behavior and Microstructure of TiC-Me Composite Powder Prepared by SHS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Procedure
2.2. Characterisation
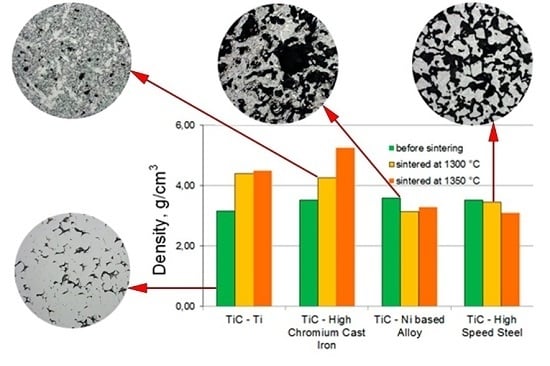
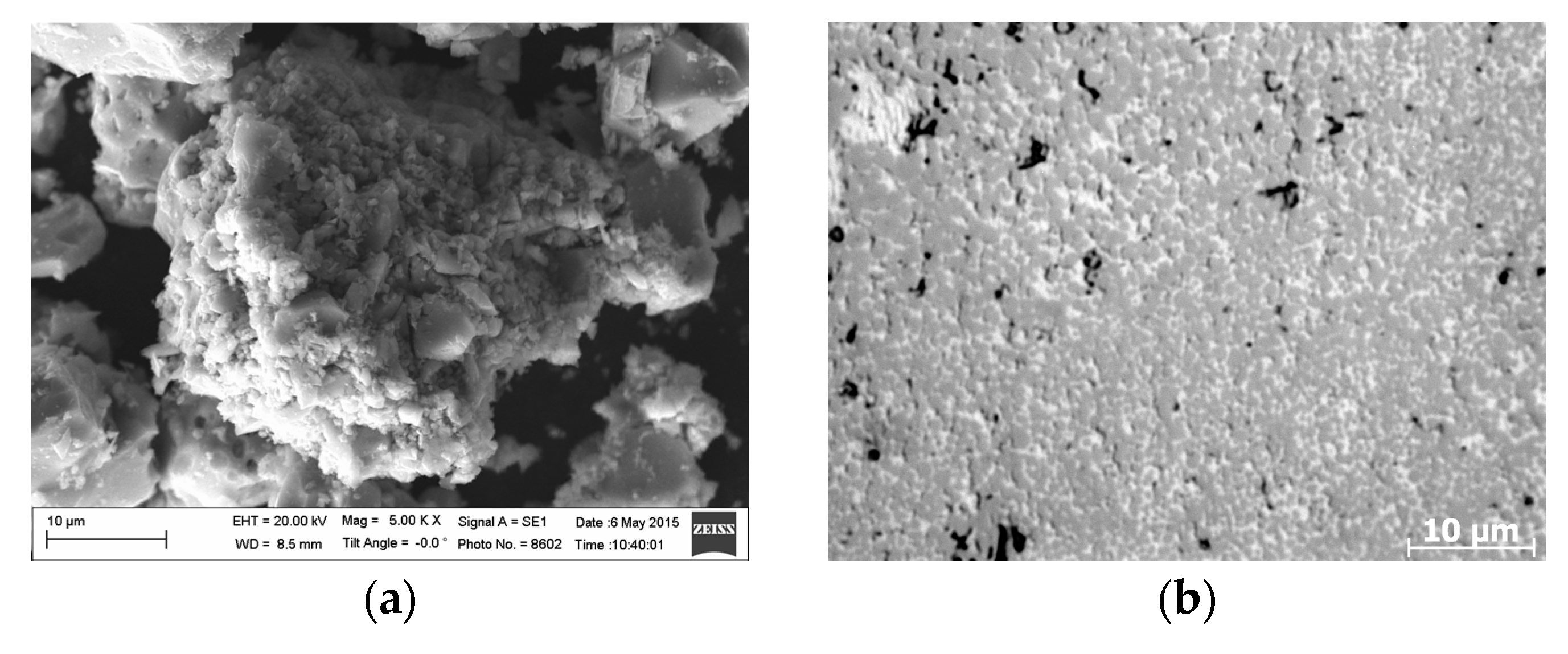
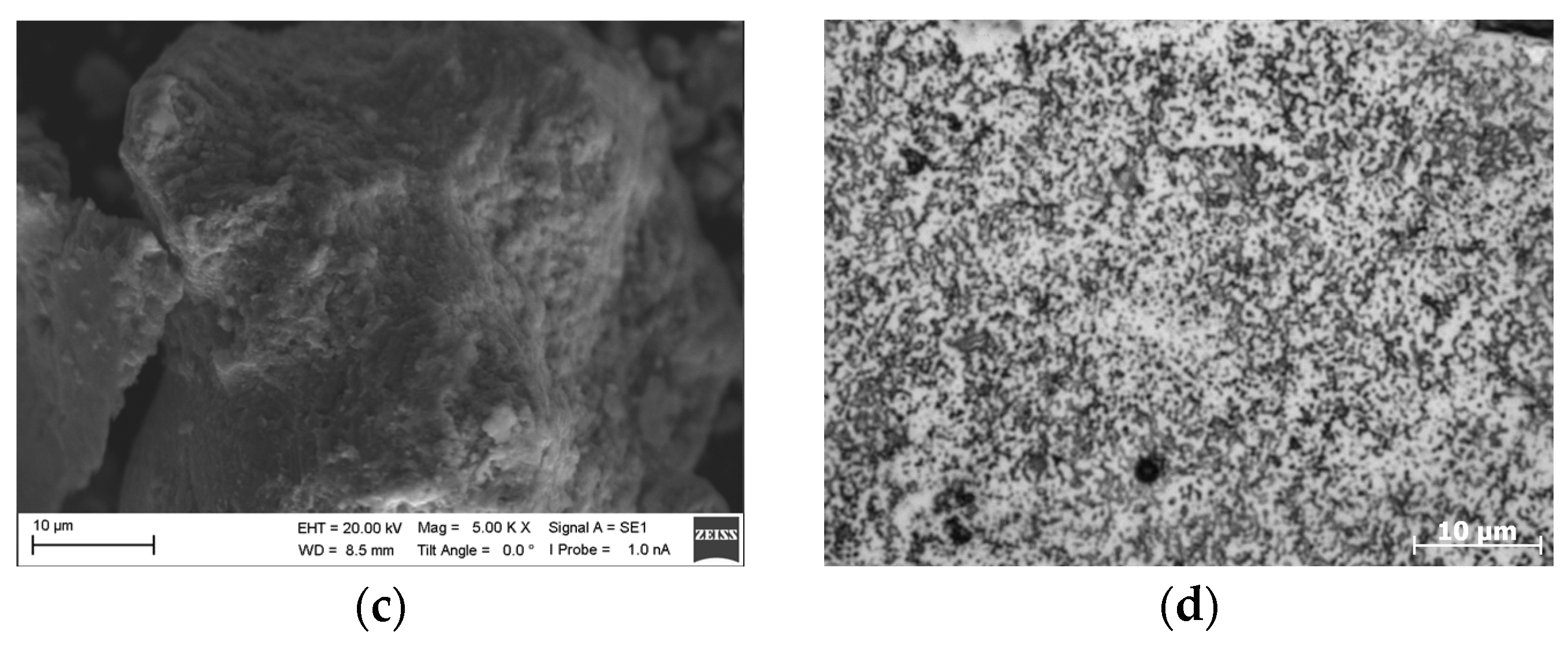
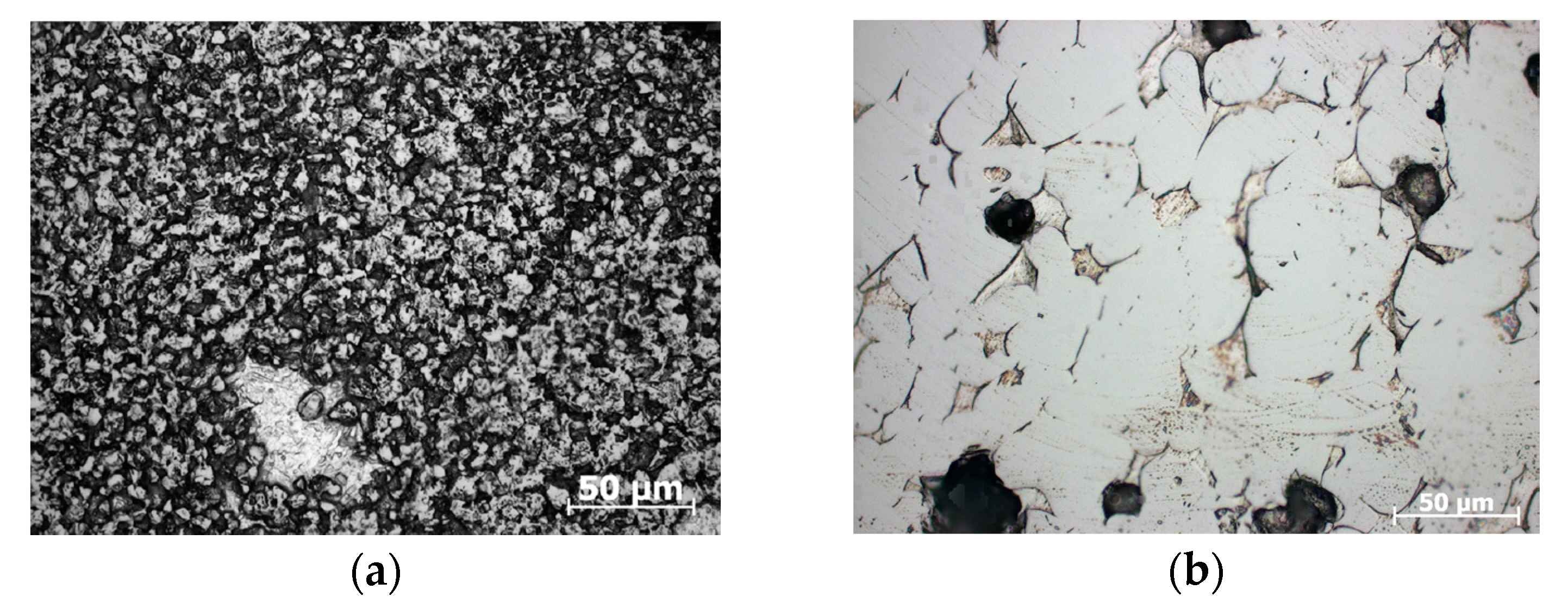
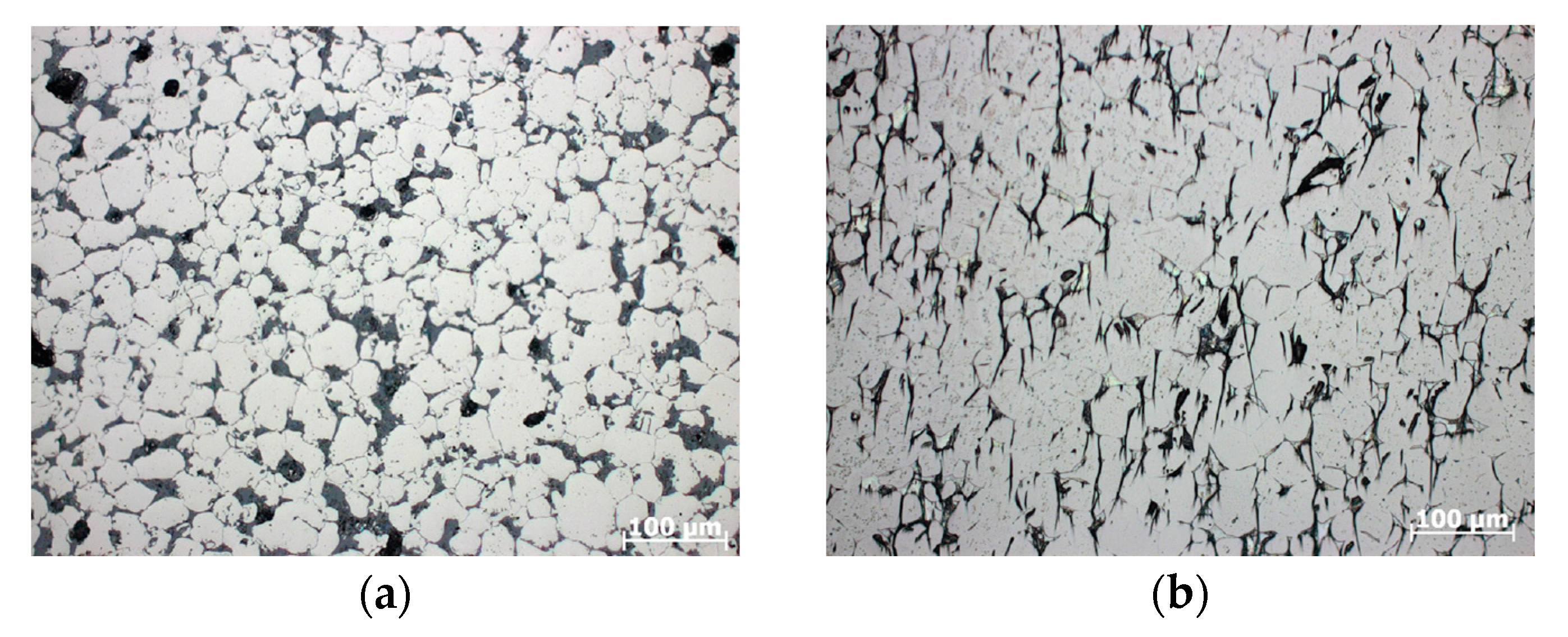
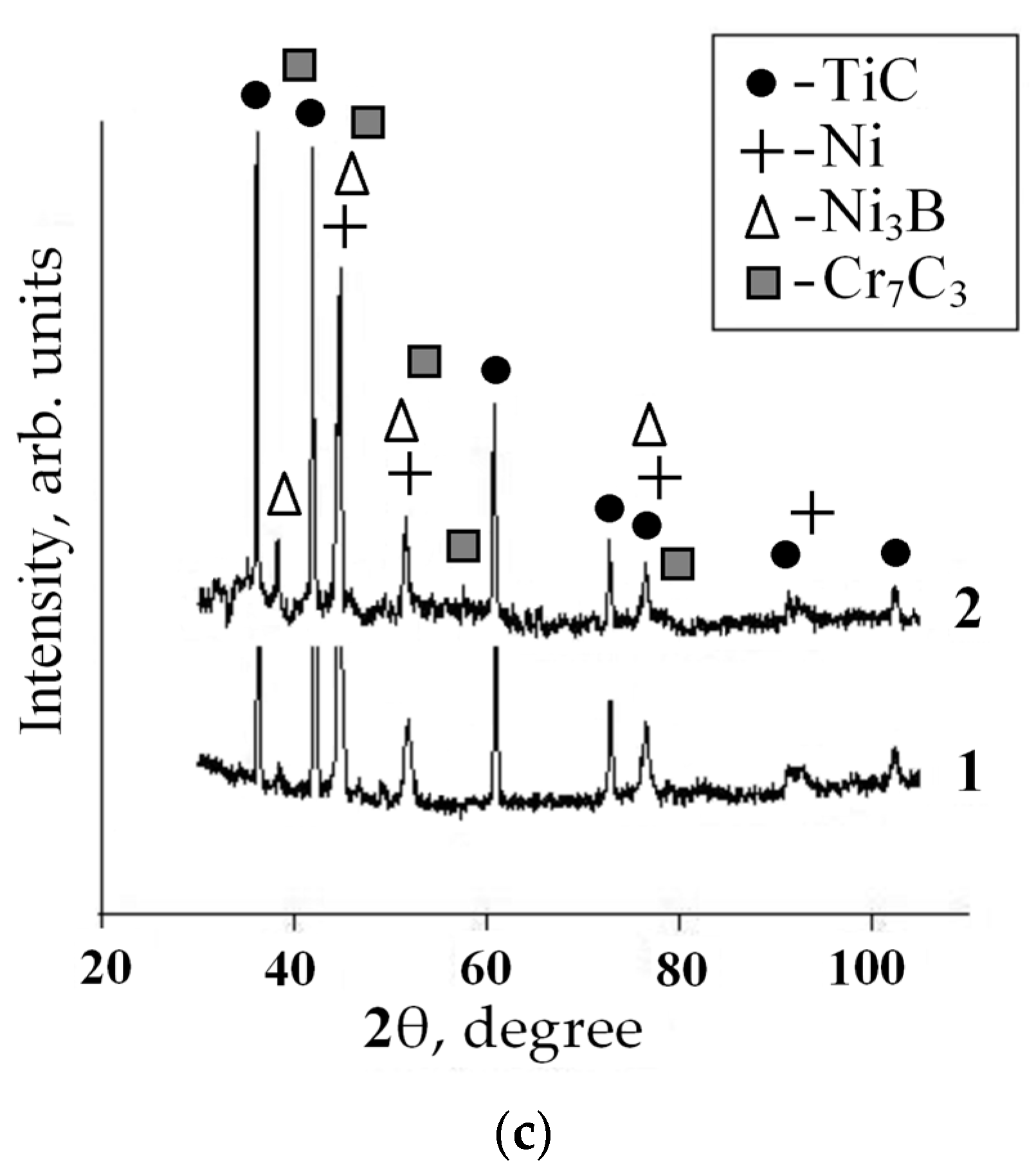
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cui, C.; Hu, B.; Zhao, L.; Liu, S. Titanium alloy production technology, market prospects and industry development. Mater. Des. 2011, 32, 1684–1691. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing and Applications, 1st ed.; Noyes publications: Westwood, NJ, USA, 1996. [Google Scholar]
- Weimer, A.W. Carbide, Nitride and Boride Materials Synthesis and Processing; Springer Science & Business Media: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Herderick, E. Additive manufacturing of metals: A review. Mater. Sci. Technol. 2011, 1413–1425. [Google Scholar]
- Gong, X.; Anderson, T.; Chou, K. Review on powder-based electron beam additive manufacturing technology. Manuf. Rev. 2014, 1. [Google Scholar] [CrossRef]
- Shabalin, I.L.; Luchka, M.V.; Shabalin, L.I. Vacuum SHS in systems with group IV transition metals for production of ceramic compositions. Phys. Chem. Solid State 2007, 8, 159–175. [Google Scholar]
- Okamoto, H. Phase Diagrams for Binary Alloys, 2nd ed.; ASM International: Materials Park, OH, USA, 2010; pp. 176–189. [Google Scholar]
- Wanjara, P.; Drew, R.A.L.; Root, J.; Yue, S. Evidence for stable stoichiometric Ti2C at the interface in TiC particulate reinforced Ti alloy composites. Acta Mater. 2000, 48, 1443–1450. [Google Scholar] [CrossRef]
- Quinn, C.J.; Kohlstedt, D.L. Solid-state reaction between titanium carbide and titanium metal. J. Am. Ceram. Soc. 1984, 67, 305–310. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Zhang, J.; Zhao, R.Y.; Liang, Y.H.; Jiang, Q.C. Lattice parameter and stoichiometry of TiCx produced in the Ti-C and Ni-Ti-C systems by self-propagating high-temperature synthesis. J. Am. Ceram. Soc. 2008, 91, 2736–2739. [Google Scholar] [CrossRef]
- Merzhanov, A.G. History and recent developments in SHS. Ceram. Int. 1995, 21, 371–379. [Google Scholar] [CrossRef]
- Merzhanov, A.G. Combustion processes that synthesize materials. J. Mater. Process. Technol. 1996, 56, 222–241. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U. Self-propagating exothermic reactions: the synthesis of high-temperature materials by combustion. Mater. Sci. Rep. 1989, 3, 277–365. [Google Scholar] [CrossRef]
- LaSalvia, J.C.; Meyer, L.W.; Meyers, M.A. Densification of reaction-synthesized titanium carbide by high-velocity forging. J. Am. Ceram. Soc. 1992, 75, 592–602. [Google Scholar] [CrossRef]
- Strutt, E.R.; Olevsky, E.A.; Meyers, M.A. Combustion synthesis/quasi-isostatic pressing of TiC–NiTi cermets: Processing and mechanical response. J. Mater. Sci. 2008, 43, 6513–6526. [Google Scholar] [CrossRef]
- Samokhin, A.V.; Alekseev, N.V.; Sinayskiy, M.A.; Tsvetkov, J.V. Thermodynamic model of high-temperature synthesis of oxygen-free titanium compounds from titanium tetrachloride. Contemp. Eng. Sci. 2015, 8, 1449–1460. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Lee, J.H.; Won, C.W. Self-propagating high-temperature synthesis of nano-sized titanium carbide powder. J. Mater. Res. 2002, 17, 2859–2864. [Google Scholar] [CrossRef]
- Kobashi, M.; Ichioka, D.; Kanetake, N. Combustion synthesis of porous TiC/Ti composite by a self-propagating mode. Materials 2010, 3, 3939–3947. [Google Scholar] [CrossRef]
- Gülsoy, H.O.; Gunay, V.; Baykara, T. Influence of TiC, TiN and TiC(N) additions on sintering and mechanical properties of injection moulded titanium based metal matrix composites. Powder Metall. 2015, 58, 30–35. [Google Scholar] [CrossRef]
- Kim, Y.J.; Chung, H.; Kang, S.J. In situ formation of titanium carbide in titanium powder compacts by gas-solid reaction. Compos. Part A Appl. Sci. Manuf. 2001, 32, 731–738. [Google Scholar] [CrossRef]
- Roger, J.; Gardiola, B.; Andrieux, J.; Viala, J.C.; Dezellus, O. Synthesis of Ti matrix composites reinforced with TiC particles: thermodynamic equilibrium and change in microstructure. J. Mater. Sci. 2017, 52, 4129–4141. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Structure and properties of nanocrystalline TiC full-density bulk alloy consolidated from mechanically reacted powders. J. Alloys Compd. 2000, 305, 225–238. [Google Scholar] [CrossRef]
- Winkler, B.; Juarez-Arellano, E.A.; Friedrich, A.; Bayarjargal, L.; Yan, J.; Clark, S.M. Reaction of titanium with carbon in a laser heated diamond anvil cell and reevaluation of a proposed pressure-induced structural phase transition of TiC. J. Alloys Compd. 2009, 478, 392–397. [Google Scholar] [CrossRef]
- Yan, Y.; Zheng, Y.; Yu, H.; Bu, H.; Cheng, X.; Zhao, N. Effect of sintering temperature on the microstructure and mechanical properties of Ti(C, N)-based cermets. Powder Metall. Met. Ceram. 2007, 46, 449–453. [Google Scholar] [CrossRef]
- Viljus, M.; Pirso, J.; Juhani, K.; Letunovitš, S. Structure Formation in Ti-C-Ni-Mo Composites during Reactive Sintering. Mater. Sci. 2012, 18, 62–65. [Google Scholar] [CrossRef]
- Luo, S.D.; Li, Q.; Tian, J.; Wang, C.; Yan, M.; Schaffer, G.B.; Qian, M. Self-assembled, aligned TiC nanoplatelet-reinforced titanium composites with outstanding compressive properties. Scr. Mater. 2013, 69, 29–32. [Google Scholar] [CrossRef]
- Li, S.; Sun, B.; Imai, H.; Kondoh, K. Powder metallurgy Ti-TiC metal matrix composites prepared by in situ reactive processing of Ti-VGCFs system. Carbon 2013, 61, 216–228. [Google Scholar] [CrossRef]
- Kalambaeva, S.S.; Korosteleva, E.N.; Pribytkov, G.A. Structure of Composite Powders “TiC-high Chromium Cast Iron Binder” Produced by SHS Method. In Proceedings of the International Conference on Mechanical Engineering, Automation and Control Systems, Tomsk, Russia, 16–18 October 2014. [Google Scholar] [CrossRef]
- Krinitcyn, M.G.; Pribytkov, G.A.; Durakov, V.G. Structure and properties of electron beam coatings, overlaid of SHS composite powders “TiC-Ti”, synthesized in air. Key Eng. Mater. 2016, 685, 719–723. [Google Scholar] [CrossRef]
- Korosteleva, E.N.; Pribytkov, G.A.; Krinitcyn, M.G.; Baranovskii, A.V.; Korzhova, V.V.; Strelnitskij, V.E. Fabrication of “TiC-HSS steel binder” composite powders by self-propagating high temperature synthesis. Key Eng. Mater. 2016, 712, 195–199. [Google Scholar] [CrossRef]
- Korosteleva, E.N.; Pribytkov, G.A.; Krinitcyn, M.G.; Baranovskii, A.V.; Korzhova, V.V. Problems of Development and Application of Metal Matrix Composite Powders for Additive Technologies. IOP Conf. Ser. Mater. Sci. Eng. 2016, 140. [Google Scholar] [CrossRef]
- Dudina, D.V.; Pribytkov, G.A.; Krinitcyn, M.G.; Korchagin, M.A.; Bulina, N.V.; Bokhonov, B.B.; Batraev, I.S.; Rybin, D.K.; Ulianitsky, V.Y. Detonation spraying behavior of TiCx-Ti powders and the role of reactive processes in the coating formation. Ceram. Int. 2016, 42, 690–696. [Google Scholar] [CrossRef]
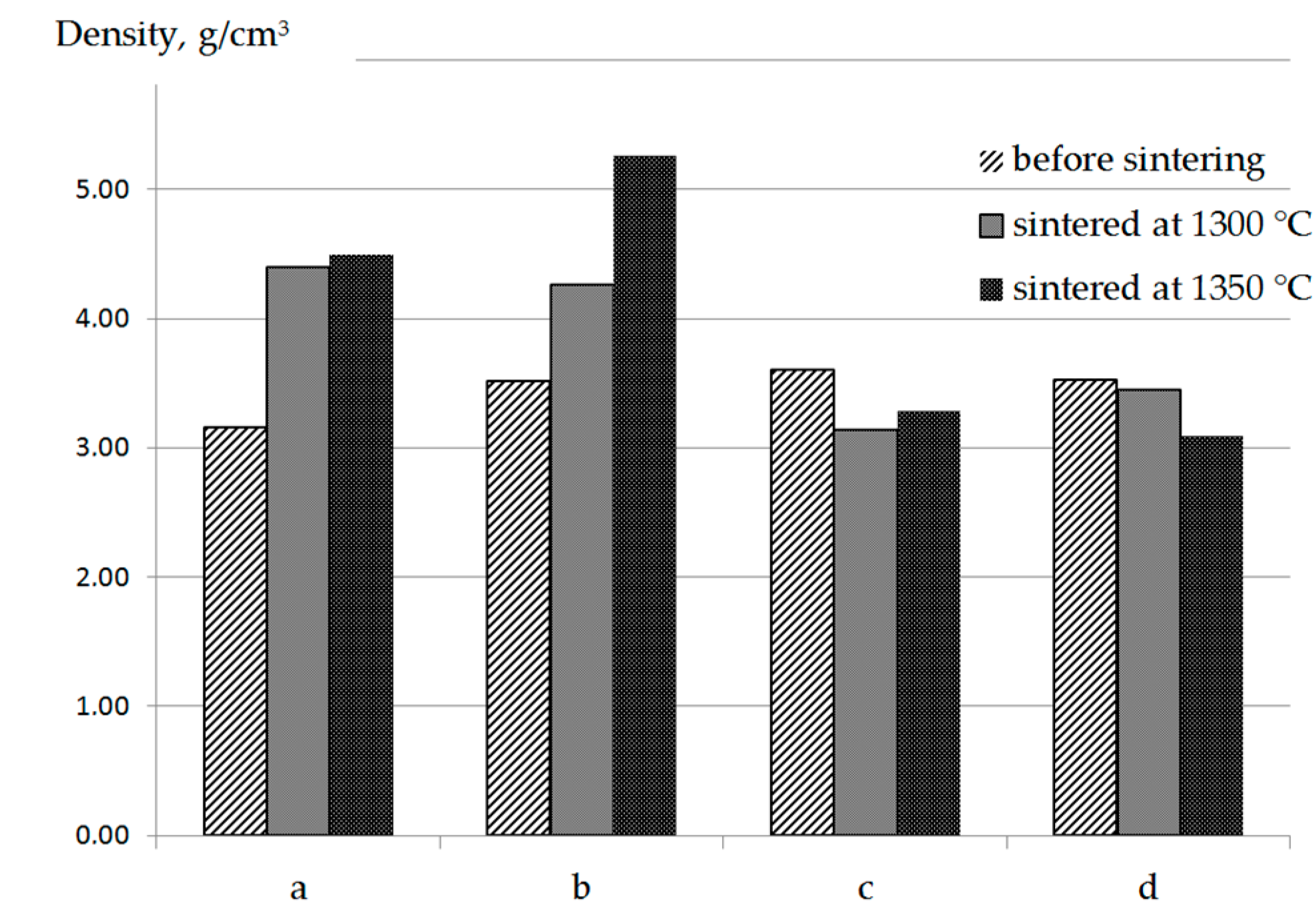




| Composition Number | The Calculated Content of Phases in the SHS Powders (with the Assumption of Equiatomic TiC Formation) |
|---|---|
| 1 | TiC + 50 vol % Ti |
| 2 | TiC + 60 vol % Ti |
| 3 | TiC + 50 vol % high-chromium cast iron |
| 4 | TiC + 50 vol % high-speed steel |
| 5 | TiC + 20 vol % nickel-based self-fluxing alloy |
| 6 | TiC + 30 vol % nickel-based self-fluxing alloy |
| 7 | TiC + 40 vol % nickel-based self-fluxing alloy |
| 8 | TiC + 50 vol % nickel-based self-fluxing alloy |
| Composition | Relative Densification, % | |
|---|---|---|
| Tsin = 1300 °C | Tsin = 1350 °C | |
| TiC + 50 vol % Ti | 40.1 | 42.0 |
| TiC + 60 vol % Ti | 43.2 | 50.6 |
| TiC + 20 vol % nickel-based self-fluxing alloy | −10.2 | −14.1 |
| TiC + 30 vol % nickel-based self-fluxing alloy | −11.3 | −12.5 |
| TiC + 40 vol % nickel-based self-fluxing alloy | −12.9 | −9.2 |
| TiC + 50 vol % nickel-based self-fluxing alloy | −13.5 | −8.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korosteleva, E.N.; Korzhova, V.V.; Krinitcyn, M.G. Sintering Behavior and Microstructure of TiC-Me Composite Powder Prepared by SHS. Metals 2017, 7, 290. https://doi.org/10.3390/met7080290
Korosteleva EN, Korzhova VV, Krinitcyn MG. Sintering Behavior and Microstructure of TiC-Me Composite Powder Prepared by SHS. Metals. 2017; 7(8):290. https://doi.org/10.3390/met7080290
Chicago/Turabian StyleKorosteleva, Elena N., Victoria V. Korzhova, and Maksim G. Krinitcyn. 2017. "Sintering Behavior and Microstructure of TiC-Me Composite Powder Prepared by SHS" Metals 7, no. 8: 290. https://doi.org/10.3390/met7080290