Preparing Ferro-Nickel Alloy from Low-Grade Laterite Nickel Ore Based on Metallized Reduction–Magnetic Separation
Abstract
:1. Introduction
2. Experiment
2.1. Raw Materials
2.2. Experimental Procedure
2.3. Thermodynamic Analysis
3. Results and Discussion
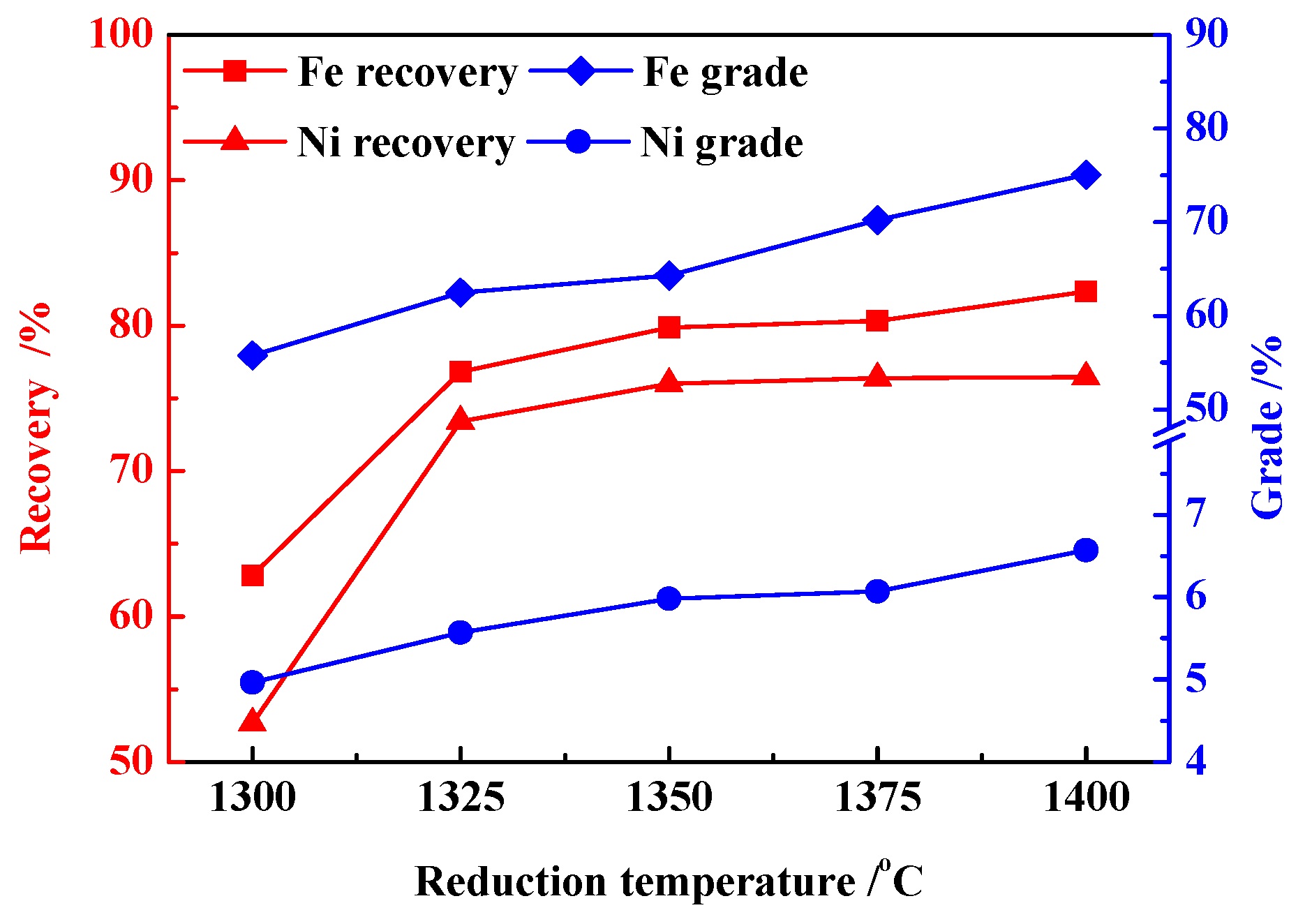
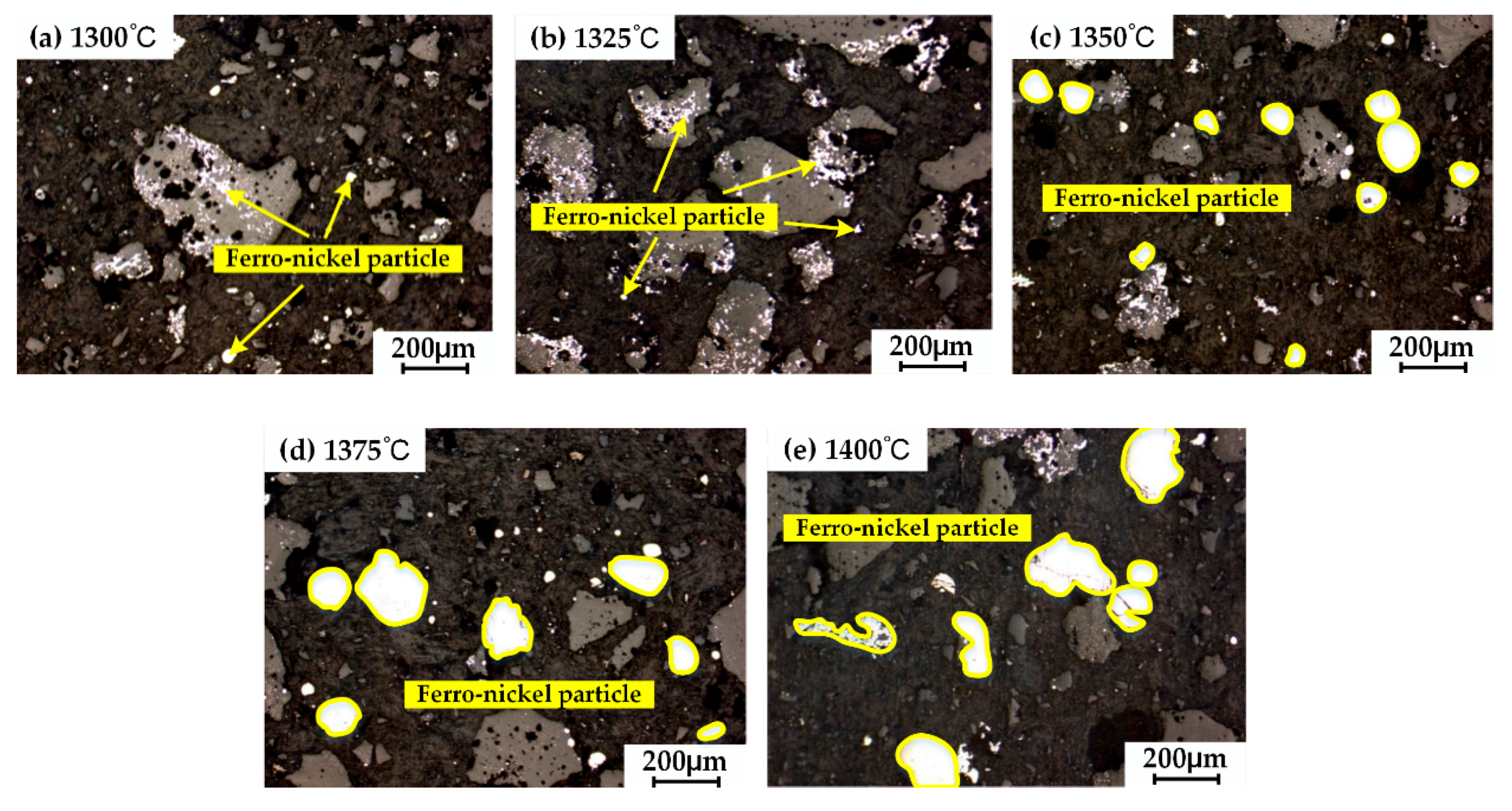
3.1. Effects of Reduction Temperature
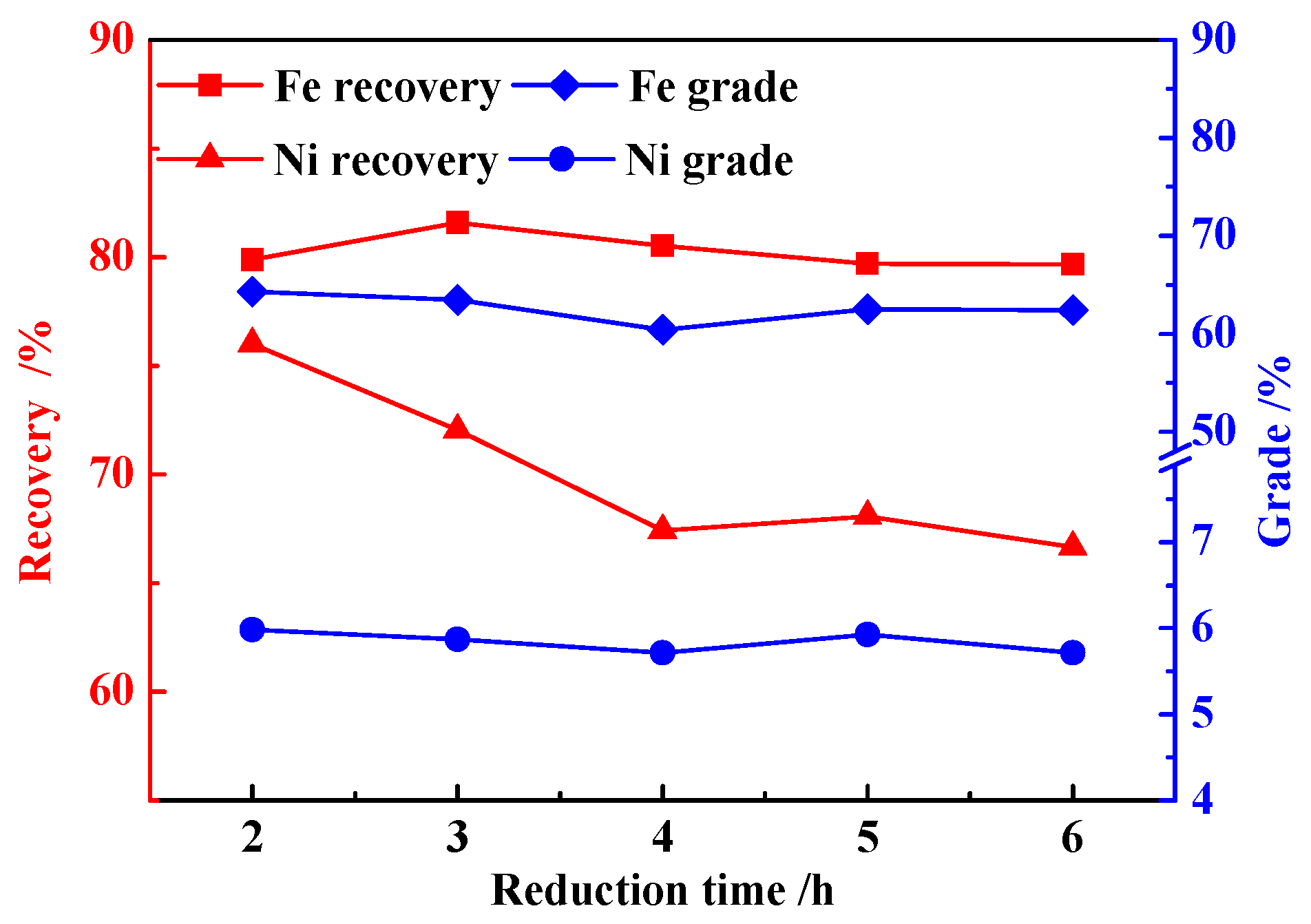
3.2. Effects of Reduction Time
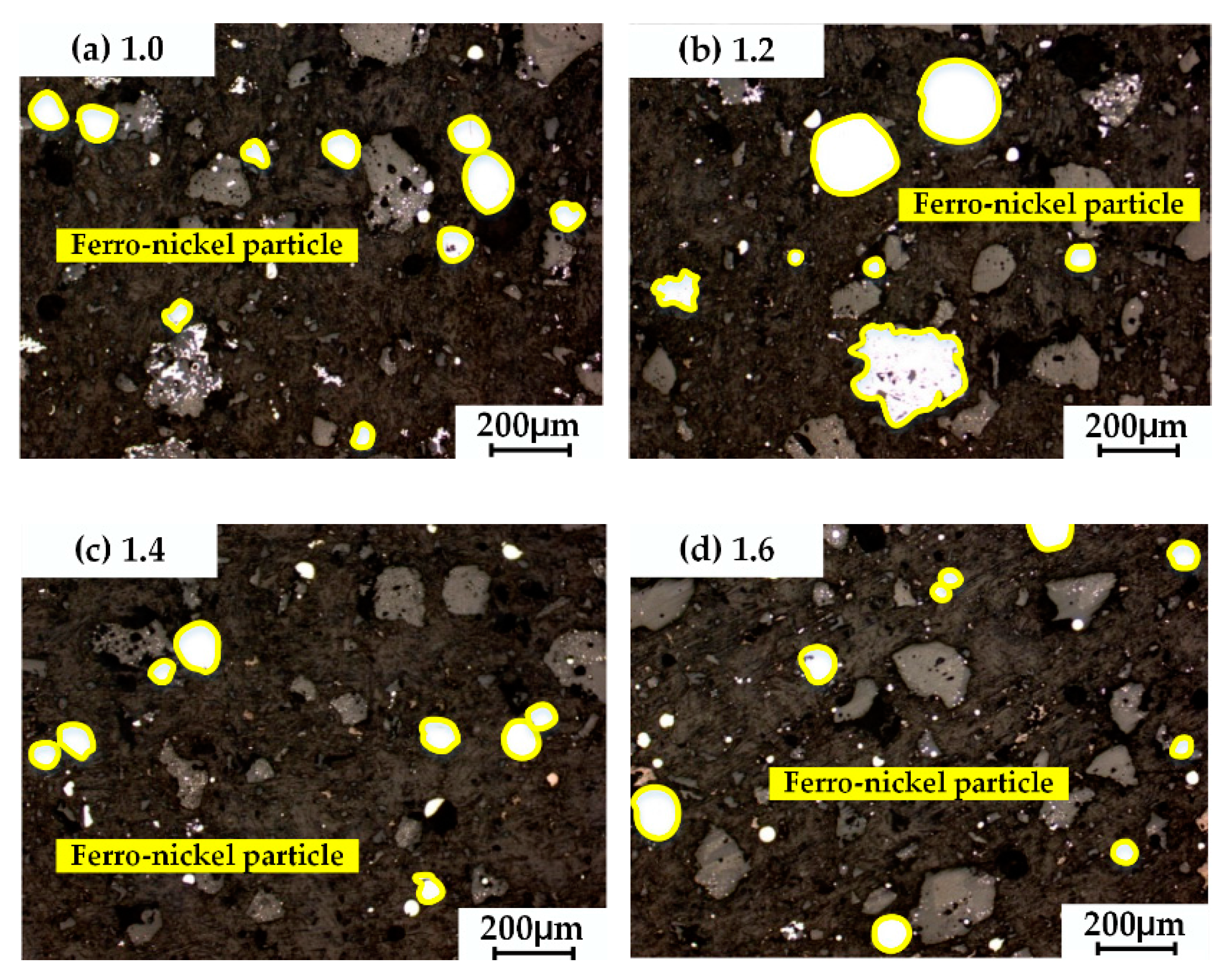
3.3. Effects of Carbon Ratio
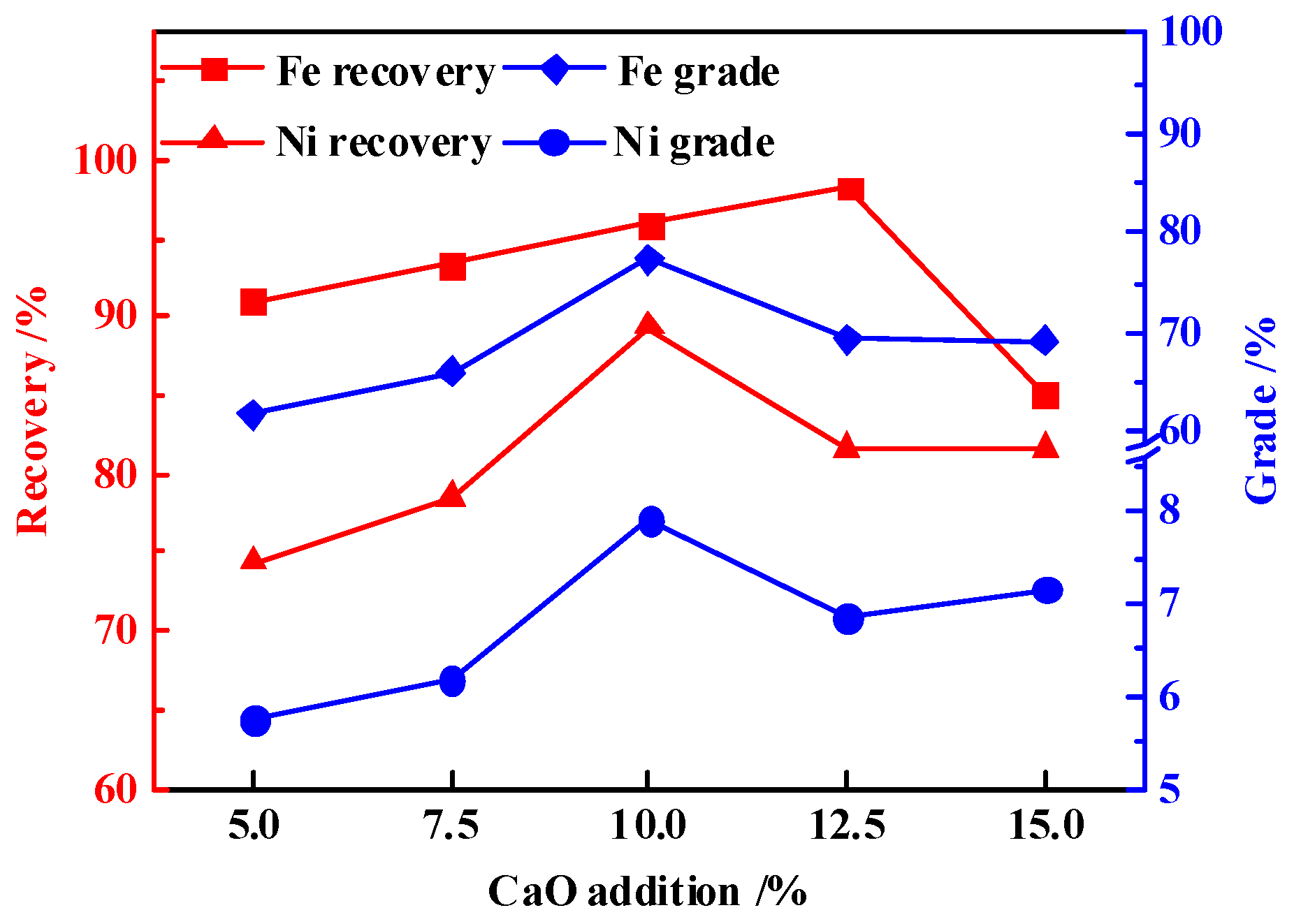
3.4. Effects of CaO Addition
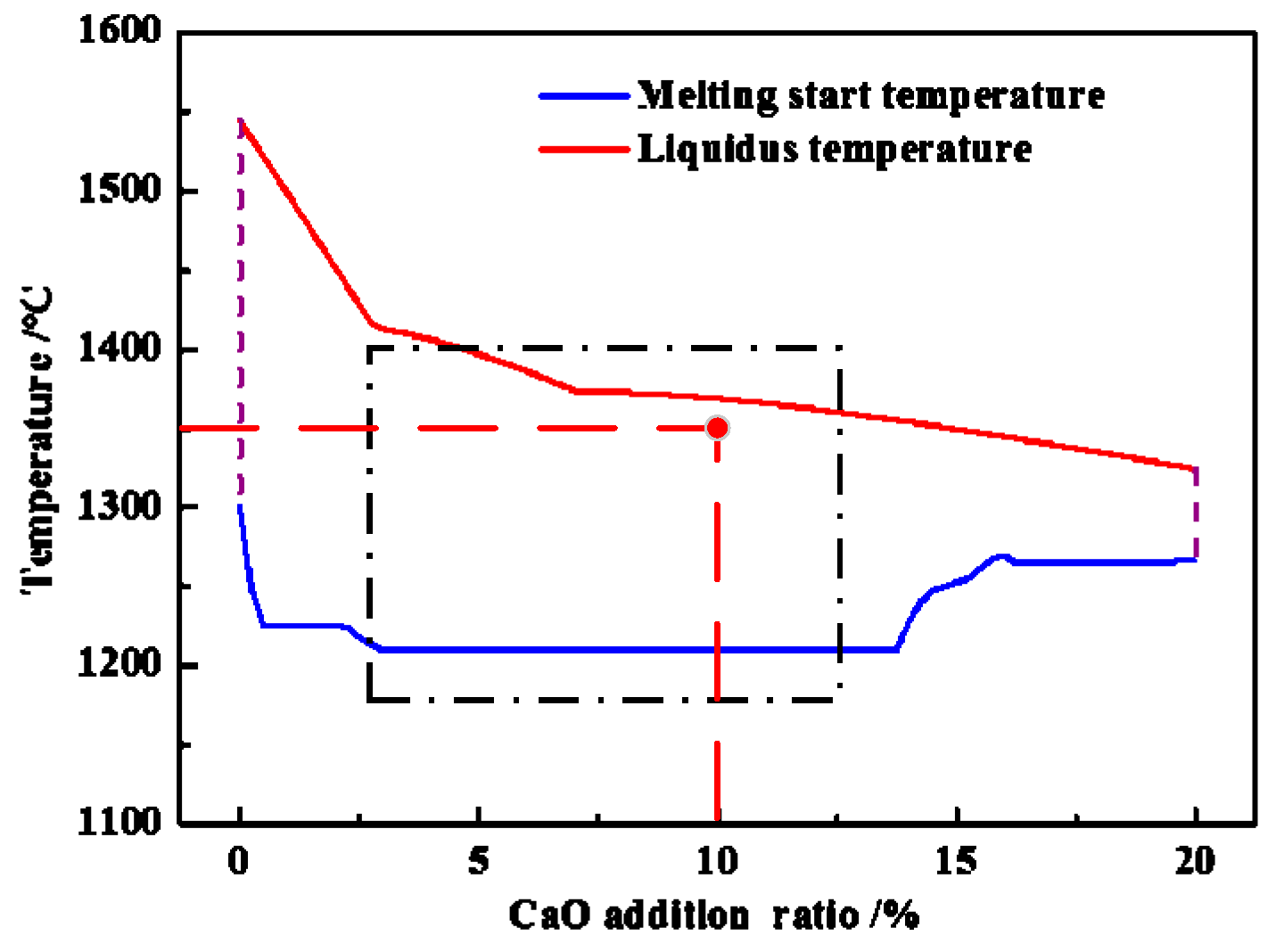
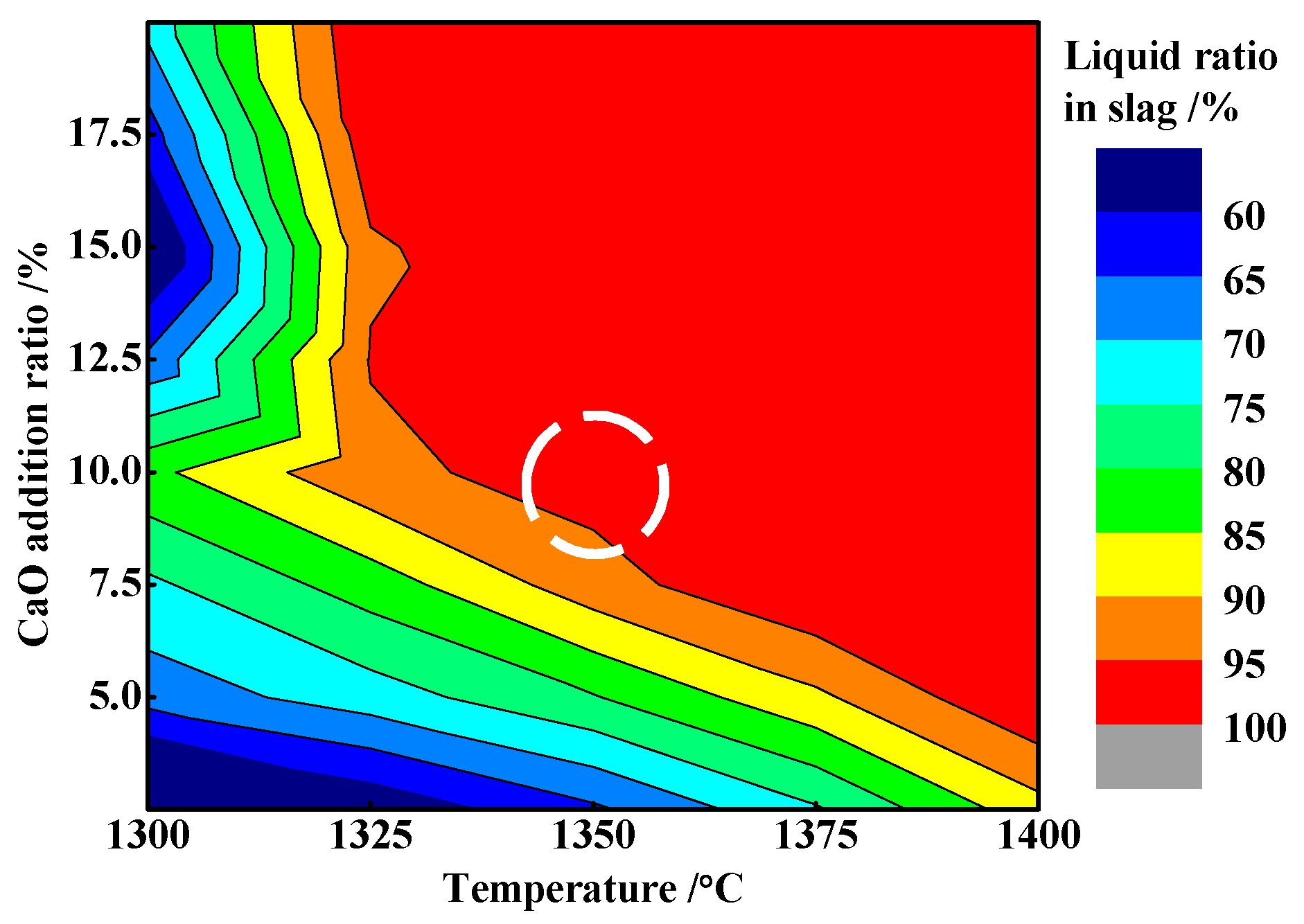
3.5. Function Mechanism of Adding CaO
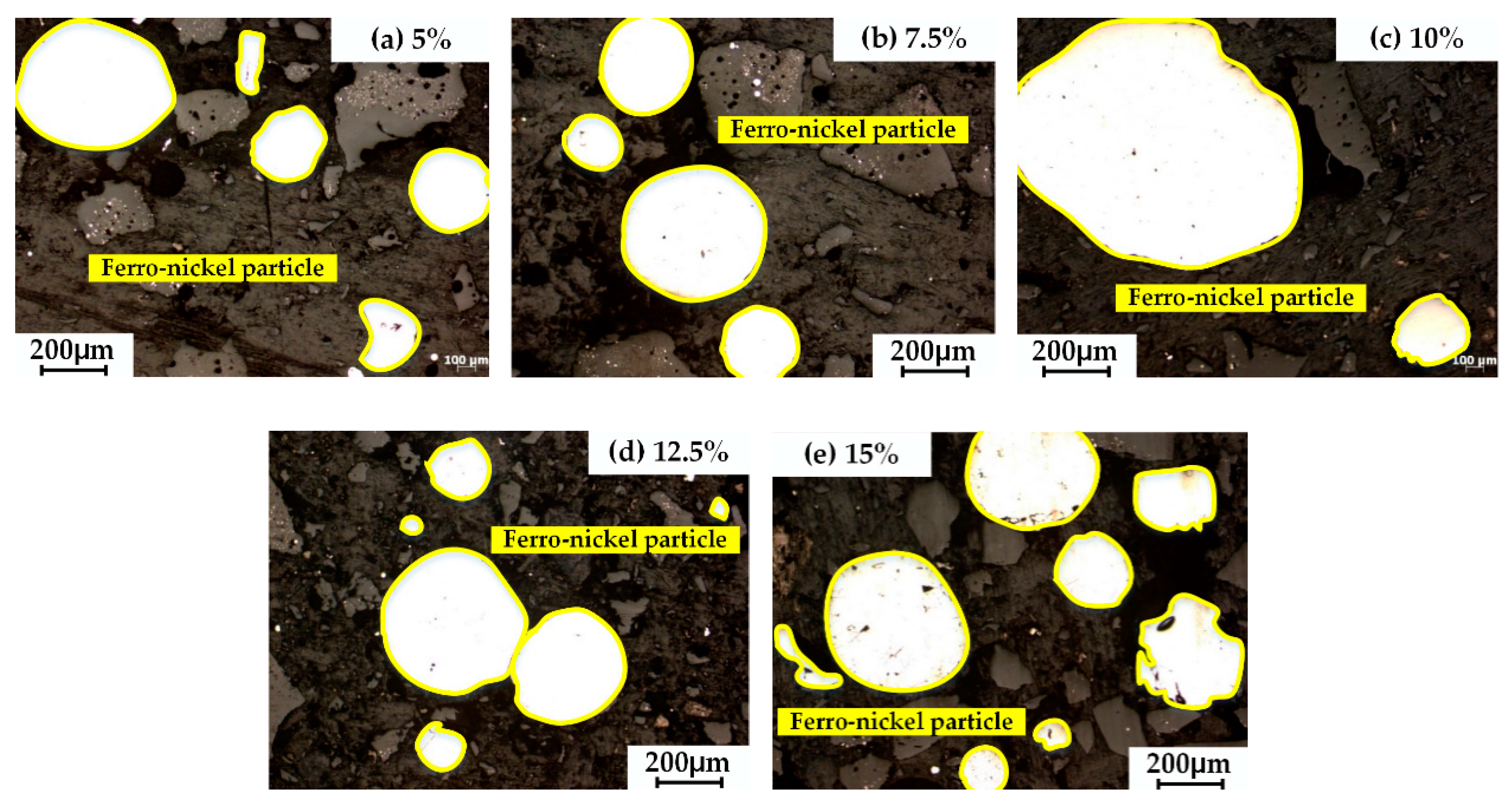
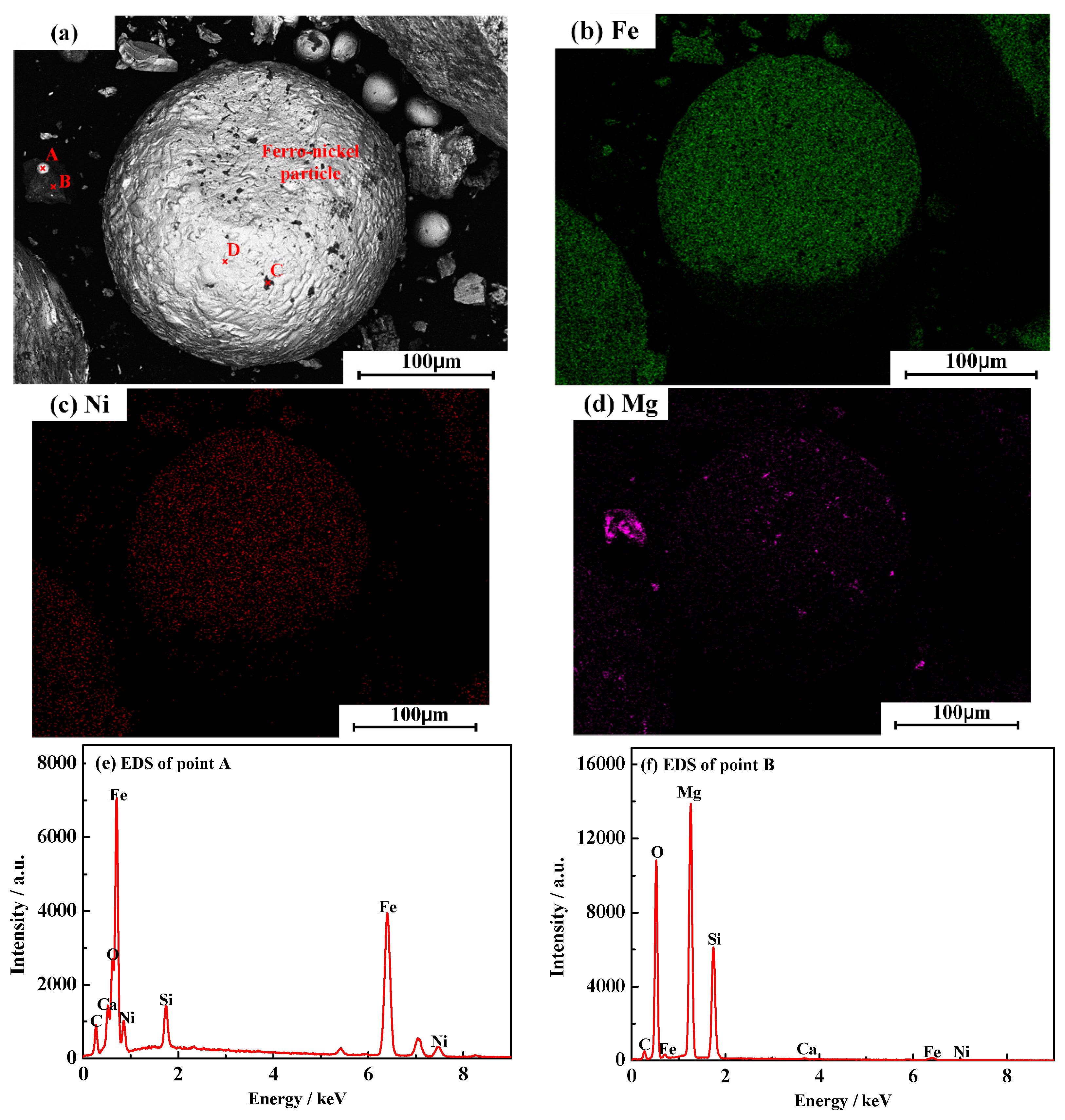
3.6. Characterization of Magnetic Product
4. Conclusions
- (1)
- The ferro-nickel alloy was prepared successfully from low-grade saprolitic laterite ore using a process of metallized reduction–magnetic separation.
- (2)
- The results indicated that, under the optimal process parameters of reduction temperature 1350 °C, reduction time 2 h, carbon ratio 1.2, and CaO addition ratio 10%, the nickel and iron grades in the magnetic product were 7.90% and 77.32%, respectively. Meanwhile, the nickel and iron recoveries were 89.36% and 95.87%, which reached high efficiency recovery of nickel and iron.
- (3)
- The appropriate amount of CaO contributed to liquid generation and slag viscosity. The conditions with a liquid generation ratio of 95% and a viscosity of slag 2–6 Pa·s at a reduction temperature of 1350 °C and CaO addition of 10% could favor the aggregation and growing up of ferro-nickel particles and improve recoveries of nickel and iron.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, G.L.; Zhu, D.Q.; Pan, J.; Li, Q.H. Pilot scale test of producing nickel concentrate from low-grade saprolitic laterite by direct reduction-magnetic separation. J. Cent. South Univ. 2014, 21, 1771–1777. [Google Scholar] [CrossRef]
- Yan, L.Y.; Wang, A.J. Analysis of global nickel supply and demand patterns. China Min. Mag. 2016, 25, 1–5. [Google Scholar]
- Li, J.H.; Li, Y.Y.; Zheng, S. Research review of laterite nickel ore metallurgy. Nonferrous Met. Sci. Eng. 2015, 6, 35–40. [Google Scholar]
- Yu, L. Development situation of Russian sulfide nickel ore resources. World Nonferrous Met. 2013, 3, 60–62. [Google Scholar]
- Yang, Z.Q.; Wang, Y.Q.; Gao, Q. Present situation and development strategy and key technologies of China’s nickel resources sustainable development. Conserv. Util. Miner. Res. 2016, 2, 58–69. [Google Scholar]
- Bai, X.Y. Exploitation and utilization situation and input strategy of nickel ore resources in China. China Trade 2014, 6, 123–125. [Google Scholar]
- Santos, R.M.; Audenaerde, A.V.; Chiang, Y.W.; Iacobescu, R.I.; Knops, P.; Gerven, T.V. Nickel extraction from olivine: Effect of carbonation pre-treatment. Metals 2015, 5, 1620–1644. [Google Scholar] [CrossRef] [Green Version]
- Dalvi, A.D.; Bacon, W.G.; Osborne, R.C. The past and the future of nickel laterites. In Proceedings of the PDAC 2005 International Convention, Toronto, ON, Canada, 6–9 March 2005. [Google Scholar]
- Charles, G. Mineral Commodity Summaries; U.S. Geological Survey: Washington, DC, USA, 2003.
- Rhamdhani, M.A.; Hayes, P.C.; Jak, E. Nickel laterite Part 1—Microstructure and phase characterizations during reduction roasting and leaching. Miner. Process. Extr. Metall. 2009, 3, 129–145. [Google Scholar] [CrossRef]
- Norgate, T.; Jahanshahi, S. Assessing the energy and greenhouse gas footprints of nickel laterite processing. Miner. Eng. 2011, 7, 698–707. [Google Scholar] [CrossRef]
- Cao, Z.C.; Sun, T.C.; Yang, H.F. Recovery of iron and nickel from nickel laterite ore by direct reduction roasting and magnetic separation. J. Univ. Sci. Technol. Beijing 2010, 6, 708–712. [Google Scholar]
- Shi, Q.X.; Qiu, G.X.; Wang, X.M. Study on direct reduction and enrichment of nickel from laterite-nickel ore. Gold 2009, 11, 46–49. [Google Scholar]
- Liang, W.; Wang, H.; Fu, J.G. High recovery of ferro-nickel from low grade nickel laterite ore. J. Cent. South Univ. Sci. Technol. 2011, 8, 2173–2177. [Google Scholar]
- Li, G.H.; Rao, M.J.; Jiang, T. Innovative process for preparing ferronickel materials from laterite ore by reduction roasting-magnetic separation. Chin. J. Nonferrous Met. 2011, 12, 3137–3142. [Google Scholar]
- Wang, C.Y. Chloridizing segregation of Yuanjiang lean nickel oxide ore. Min. Metall. 1997, 3, 55–59. [Google Scholar]
- Goodall, G. Nickel Recovery from Reject Laterite. Ph.D. Thesis, Mc Gill University, Montreal, QC, Canada, 2007. [Google Scholar]
- Warner, A.E.; Diaz, C.M.; Dalvi, A.D.; Mackey, P.J.; Tarasov, A.V. JOM world nonferrous smelter survey, part III: Nickel: Laterite. J. Min. Met. Mater. Soc. 2006, 58, 11–20. [Google Scholar] [CrossRef]
- Ishii, K. Development of ferro-nickel smelting from laterite in Japan. Int. J. Min. Process. 1987, 19, 15–24. [Google Scholar] [CrossRef]
- King, M.G.; Grund, G.; Schonewille, R. A mid-term report on Falconbridge’s 15 year technology plan for nickel. In Proceedings of the European Metallurgical Conference (EMC 2005), Dresden, Germany, 18–21 September 2005; Volume 3, pp. 935–954. [Google Scholar]
- Ahmed, H.M.; Viswanathan, N.; Bjorkman, B. Composite pellets—A potential raw material for iron-making. Steel Res. Int. 2014, 85, 293–306. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.C.; Liu, Z.Z.; Kou, J.; Xu, C.Y. Study on the strength of cold-bonded high-phosphorus oolitic hematite-coal composite briquettes. Int. J. Miner. Metall. Mater. 2014, 5, 423–430. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Zhu, D.Q. New technology of coal-based direct reduction for unfired pellet and its application. Res. Iron Steel 2003, 1, 58–62. [Google Scholar]
- Kasai, A.; Matsui, Y.; Noma, F. Cold strength enhancement mechanism of carbon composite iron ore hot briquet. Iron Steel 2009, 87, 313–319. [Google Scholar] [CrossRef]
- Chu, M.; Nogami, H.; Jun, I.Y. Numerical analysis on blast furnace performance under operation with top gas recycling and carbon composite agglomerates charging. ISIJ Int. 2004, 12, 2159–2167. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chu, M.S. Metalizing reduction and magnetic separation of vanadium titano-magnetite based on hot briquetting. Int. J. Miner. Metall. Mater. 2014, 3, 225–233. [Google Scholar] [CrossRef]
- Chu, M.S.; Liu, Z.G.; Wang, Z.C. Fundamental study on carbon composite iron ore hot briquette used as blast furnace burden. Steel Res. Int. 2011, 5, 521–528. [Google Scholar]
- Matsui, Y.; Sawayama, M.; Kasai, A. Reduction behavior of carbon composite iron ore hot briquette in shaft furnace and scope on blast furnace performance reinforcement. ISIJ Int. 2007, 12, 1904–1912. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ueno, T.; Okumura, K. Reaction behavior of coal rich composite iron ore hot briquettes under load at high temperatures until 1400.DEG.C. ISIJ Int. 2011, 8, 1240–1246. [Google Scholar] [CrossRef]
- Suzuki, H.; Mizoguchi, H.; Hayashi, S. Influence of ore reducibility on reaction behavior of ore bed mixed with coal composite iron ore hot briquettes. ISIJ Int. 2011, 8, 1255–1261. [Google Scholar] [CrossRef]
- Long, H.M.; Chun, T.J.; Di, Z.X.; Wang, P.; Meng, Q.M.; Li, J.X. Preparation of metallic iron powder from pyrite cinder by carbothermic reduction and magnetic separation. Metals 2016, 6, 88. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.J.; Shi, T. Technology and Theory of Non-Blast Furnace Ironmaking; Metallurgical Industry Press: Beijing, China, 2010; p. 1. [Google Scholar]
- Li, M.; Peng, B.; Chai, L.Y.; Peng, N.; Yan, H.; Hou, D.K. Recovery of iron from zinc leaching residue by selective reduction roasting with carbon. J. Hazard. Mater. 2012, 237, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Noguchi, T.; Hino, M. Reduction and carburization of iron oxide by carbonaceous materials. Steel Res. Int. 2010, 81, 834–840. [Google Scholar] [CrossRef]
- Einstein, A. Eine neue bestimmung der moleküldimensionen. Ann. Phys. 1906, 324, 289–306. [Google Scholar] [CrossRef]
- Roscoe, R. The viscosity of suspensions of rigid spheres. Br. J. Appl. Phys. 1952, 8, 267–269. [Google Scholar] [CrossRef]
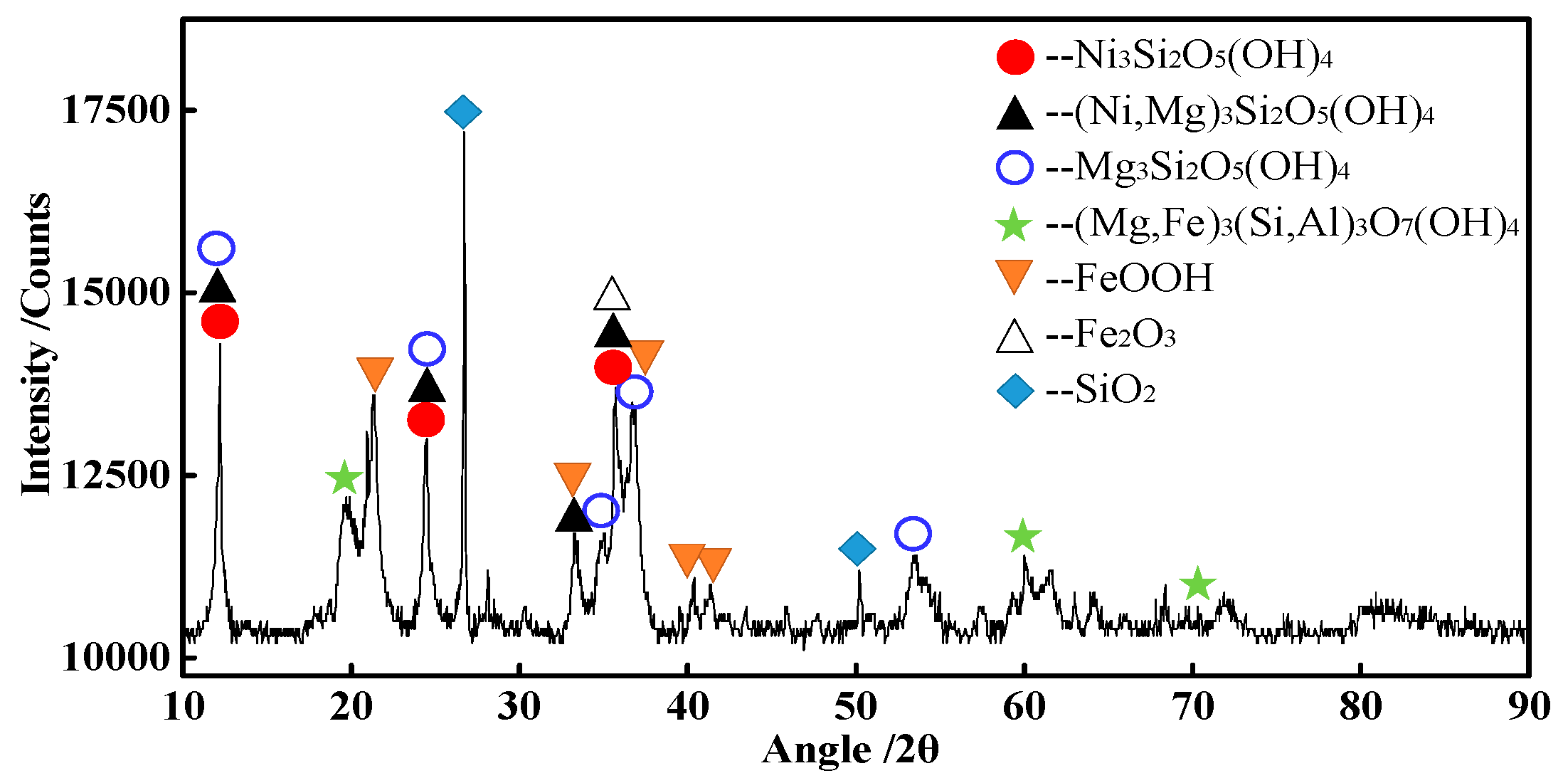
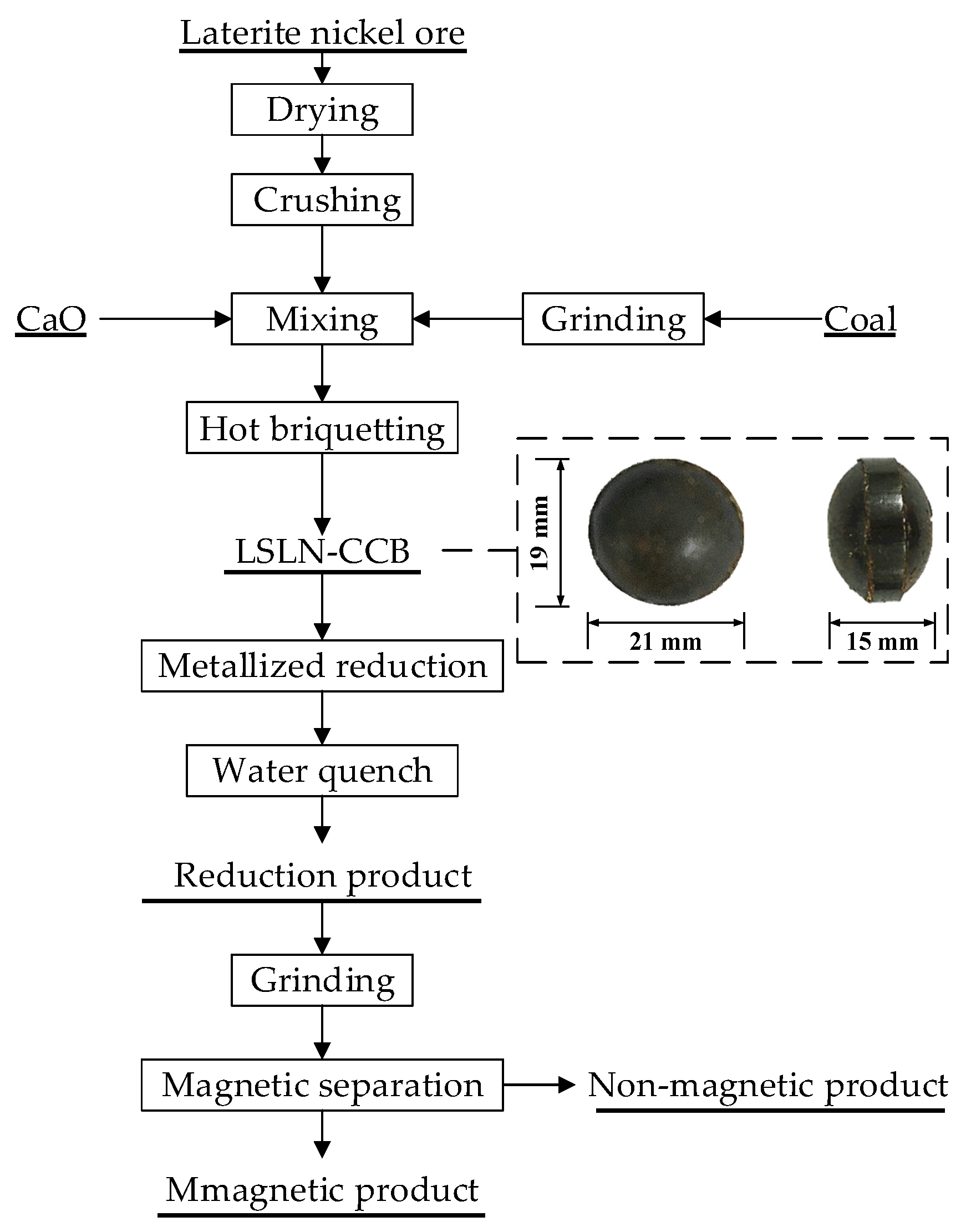
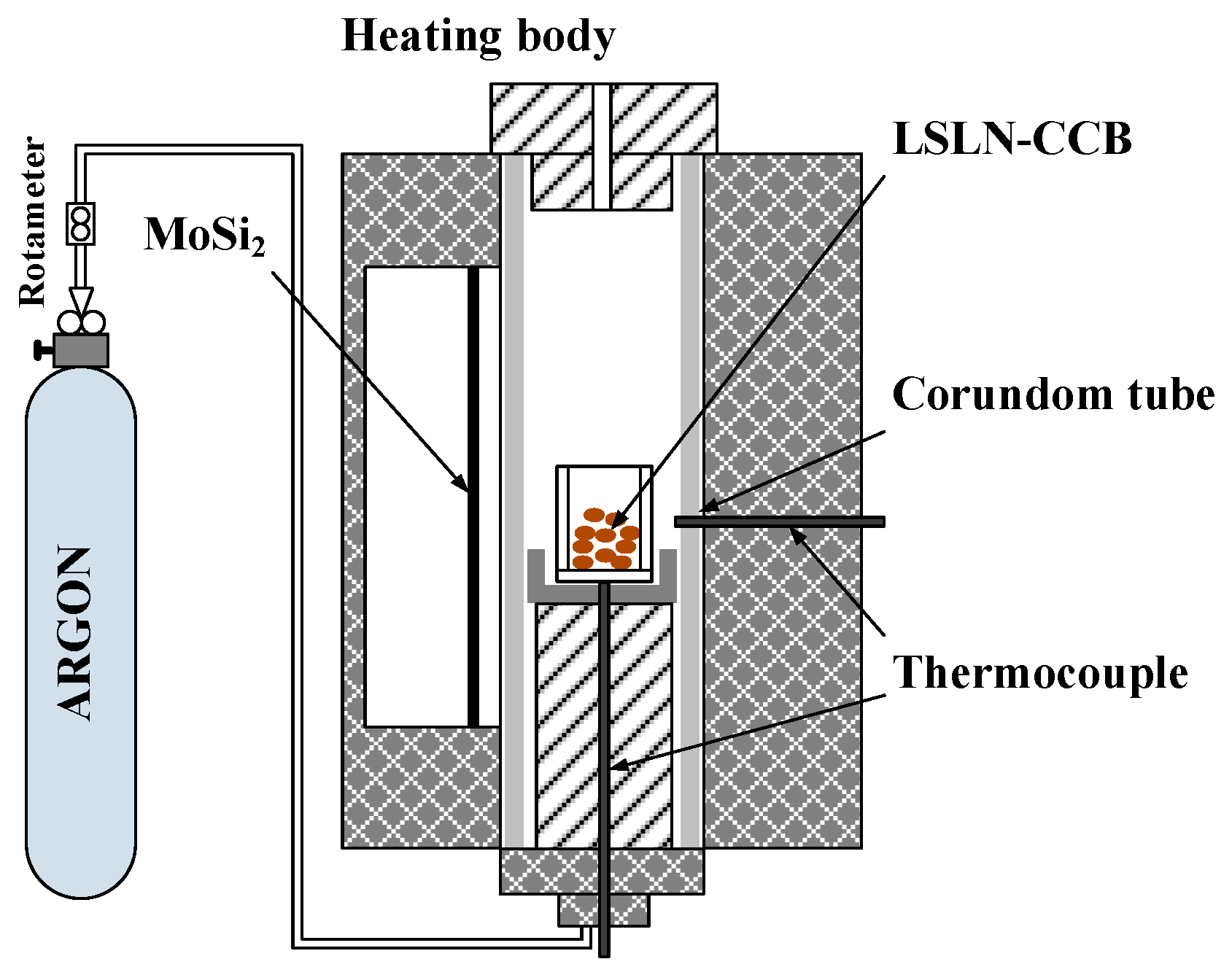
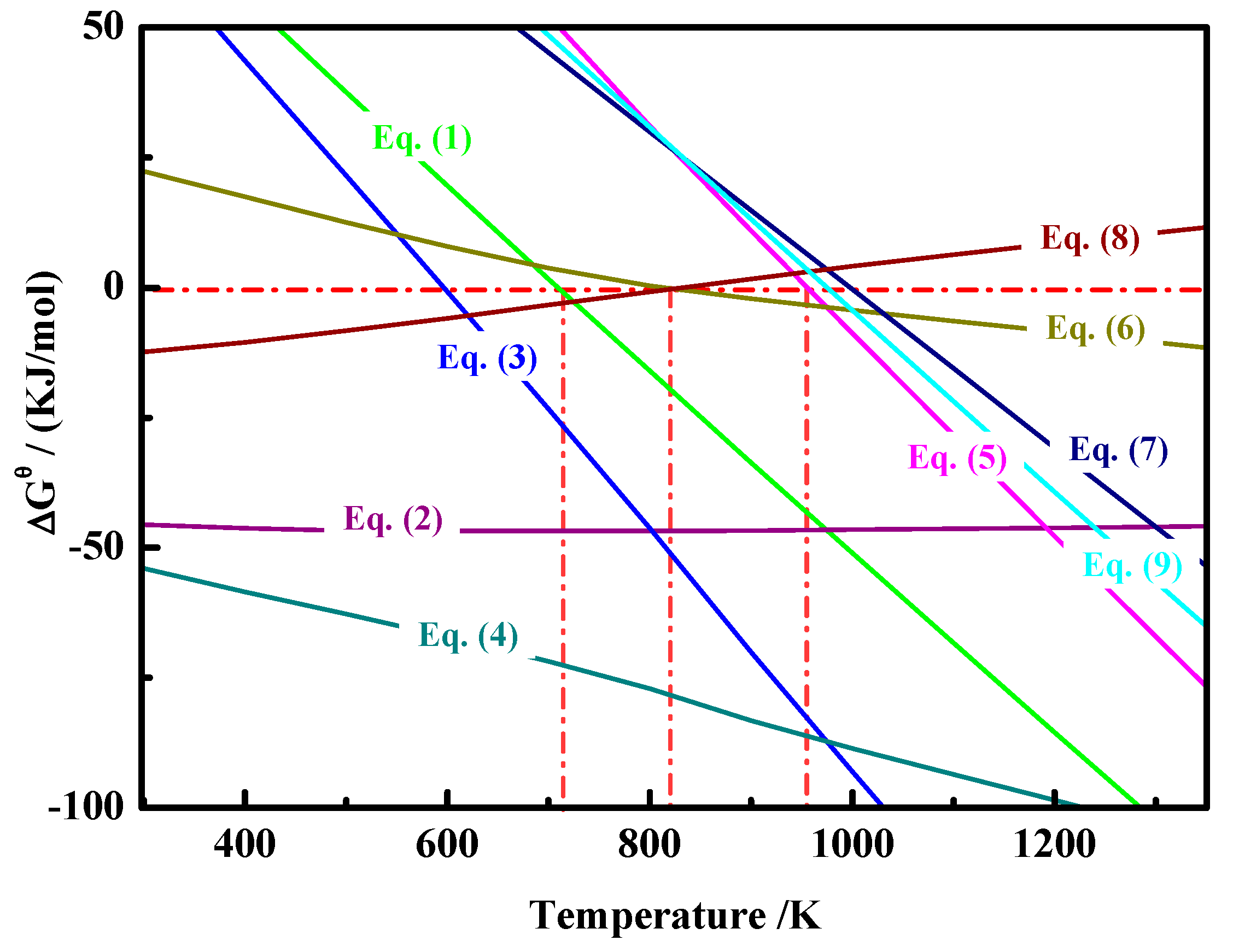




| Element | TFe | FeO | Ni | CaO | SiO2 | MgO | Al2O3 | Cr2O3 | P | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 19.57 | 0.21 | 1.82 | 0.09 | 34.78 | 12.98 | 4.00 | 1.40 | 0.003 | 0.028 |
| Item | Fixed Carbon | Ash | Volatile | Total Sulfur | Moisture | Ash | |||
|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | MgO | Al2O3 | ||||||
| Content | 60.86 | 8.19 | 30.45 | 0.14 | 0.5 | 2.95 | 51.91 | 1.08 | 29.97 |
| Item | Parameter | ||||
|---|---|---|---|---|---|
| Reduction temperature (°C) | 1300 | 1325 | 1350 | 1375 | 1400 |
| Redution time (h) | 2 | 3 | 4 | 5 | 6 |
| Carbon ratio | 1.0 | 1.2 | 1.4 | 1.6 | - |
| CaO addition (%) | 5 | 7.5 | 10 | 12.5 | 15 |
| Element | TFe | Ni | C | P | S |
|---|---|---|---|---|---|
| Content | 77.32 | 7.90 | 2.50 | 0.002 | 0.056 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chu, M.; Liu, Z.; Wang, H.; Zhao, W.; Gao, L. Preparing Ferro-Nickel Alloy from Low-Grade Laterite Nickel Ore Based on Metallized Reduction–Magnetic Separation. Metals 2017, 7, 313. https://doi.org/10.3390/met7080313
Wang Z, Chu M, Liu Z, Wang H, Zhao W, Gao L. Preparing Ferro-Nickel Alloy from Low-Grade Laterite Nickel Ore Based on Metallized Reduction–Magnetic Separation. Metals. 2017; 7(8):313. https://doi.org/10.3390/met7080313
Chicago/Turabian StyleWang, Zhihao, Mansheng Chu, Zhenggen Liu, Hongtao Wang, Wei Zhao, and Lihua Gao. 2017. "Preparing Ferro-Nickel Alloy from Low-Grade Laterite Nickel Ore Based on Metallized Reduction–Magnetic Separation" Metals 7, no. 8: 313. https://doi.org/10.3390/met7080313





