Electrolytic Plasma Polishing of Pipe Inner Surfaces
Abstract
:1. Introduction
2. Theoretical Fundamentals: Surface Finishing Processes
2.1. Electrolytic Polishing Process
2.2. Electro Polishing
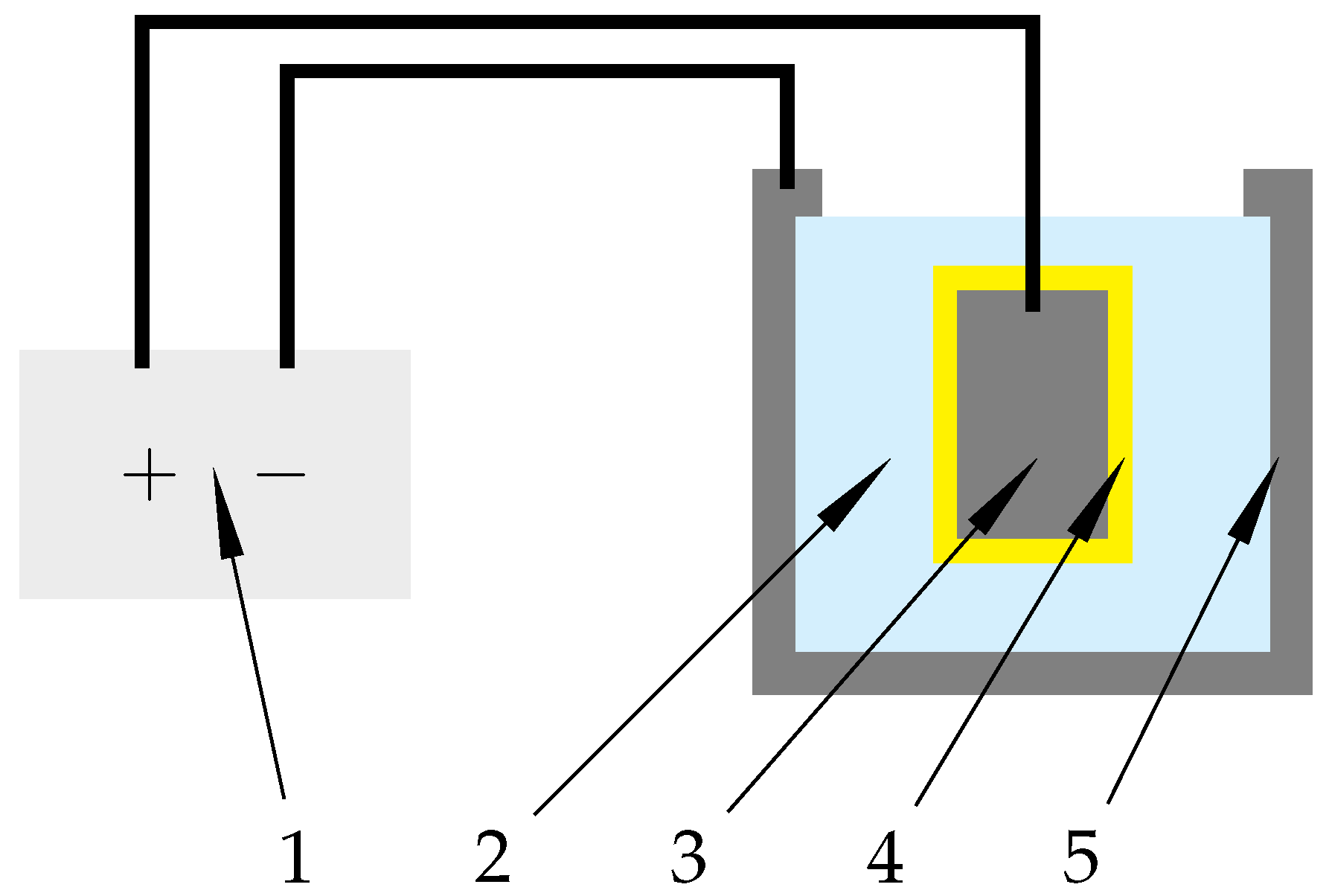
2.3. Electrolytic Plasma Polishing (EPP)
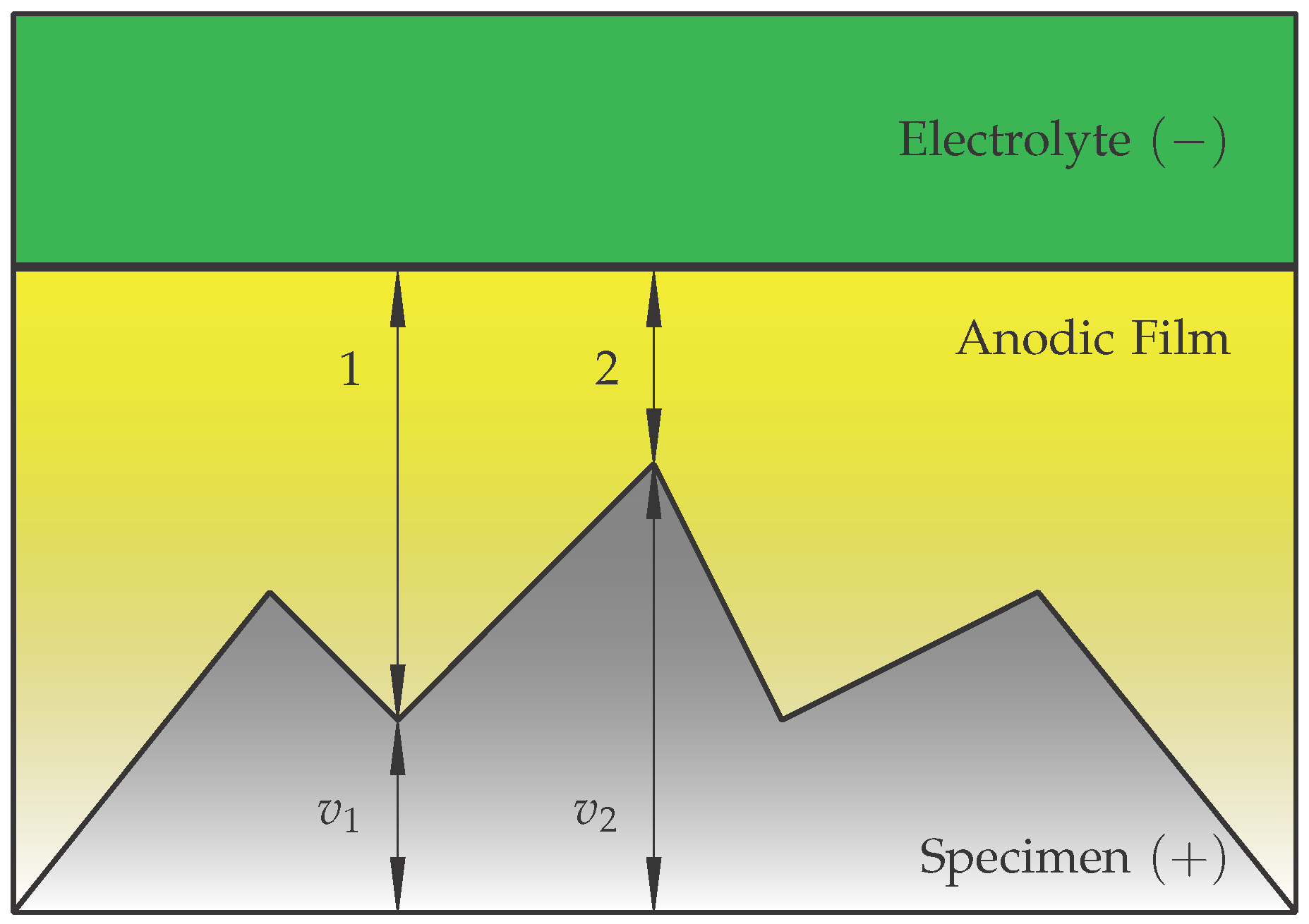
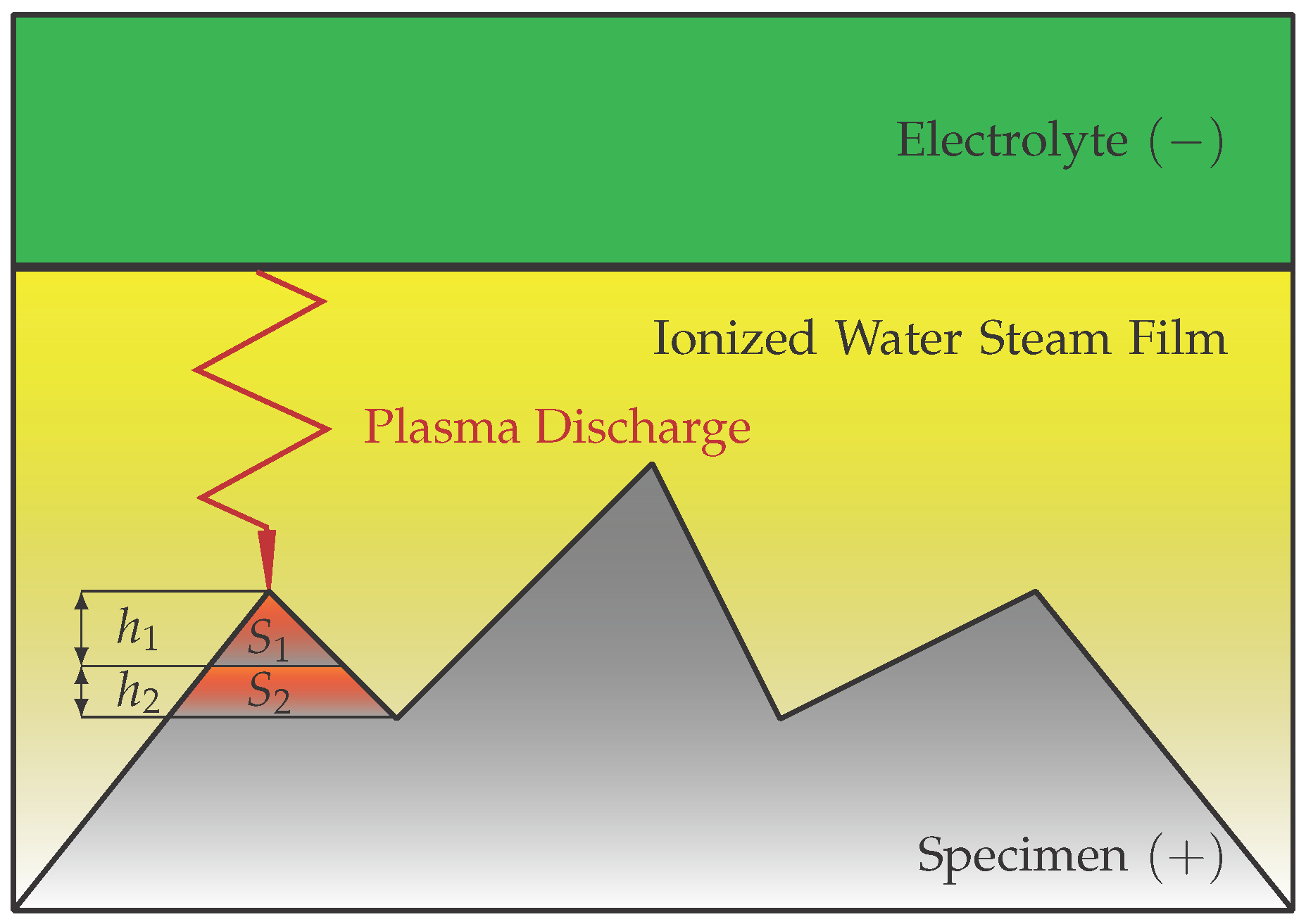
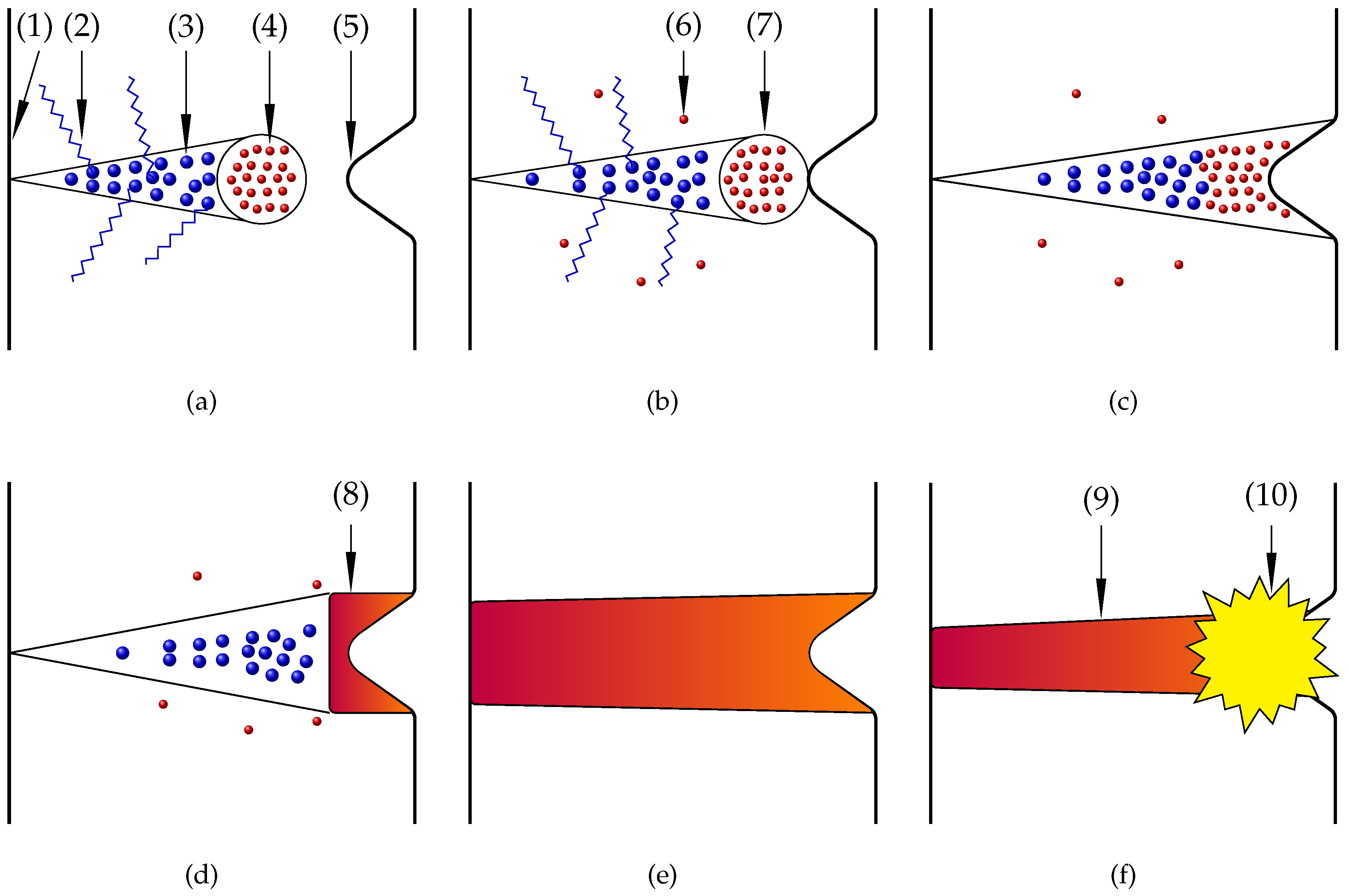
2.4. Material Removal During the EPP According to the Streamer Theory
3. Materials and Methods
3.1. Electrolytic Plasma Polishing of Inner Surfaces
3.2. Sample Material
3.3. Parameters of Electrolytic Plasma Polishing
3.4. Measurement Methods to Characterize the Surface Roughness
4. Results and Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Gils, S.; Le Pen, C.; Hubin, A.; Terryn, H.; Stijns, E. Electropolishing of copper in H3PO4: Ex situ and in situ optical characterization. J. Electrochem. Soc. 2007, 154, C175–C180. [Google Scholar] [CrossRef]
- Duradji, V.; Kaputkin, D. Metal Surface Treatment in Electrolyte Plasma during Anodic Process. J. Electrochem. Soc. 2016, 163, E43–E48. [Google Scholar] [CrossRef]
- Buhlert, M. Elektropolieren und Elektrostrukturieren von Edelstahl, Messing und Aluminium: Untersuchung des transpassiven Abtragprozesses Einschließlich unerwünschter Nebeneffekte; VDI-Verlag: Düsseldorf, Germany, 2000. [Google Scholar]
- Buhlert, M. Elektropolieren: Elektrolytisches Glänzen, Glätten und Entgraten von Edelstahl, Stahl, Messing, Kupfer, Aluminium und Titan; mit 4 Tabellen; Leuze: Bad Saulgau, Germany, 2009. [Google Scholar]
- Podhorský, S.; Malík, A. The Possibilities of Plasma Polishing of the Steel DIN 1.0570 in Electrolyte. In Proceedings of the 19th Conference Metal 2010, Zlín Region, Czech Republic, 18–20 May 2010. [Google Scholar]
- Kuhn, A. The electropolishing of titanium and its alloys. Met. Finish. 2004, 102, 80–86. [Google Scholar] [CrossRef]
- Böttger-Hiller, F.; Nestler, K.; Zeidler, H.; Glowa, G.; Lampke, T. Plasma electrolytic polishing of metalized carbon fibers. Aims Mater. Sci. 2016, 3, 260–269. [Google Scholar] [CrossRef]
- Zeidler, H.; Böttger-Hiller, F.; Edelmann, J.; Schubert, A. Surface finish machining of medical parts using Plasma electrolytic Polishing. Procedia CIRP 2016, 49, 83–87. [Google Scholar] [CrossRef]
- Zeidler, H.; Nestler, K.; Böttger-Hiller, F.; Schubert, A.; Previtali, B.; Demir, A. Finishing of laser-machined coronary stents by plasma electrolytic polishing. In Proceedings of the 16th International Conference of the European Society for Precision Engineering and Nanotechnology, EUSPEN, Nottingham, UK, 30 May–3 June 2016. [Google Scholar]
- Weingarten, C.; Schmickler, A.; Willenborg, E.; Wissenbach, K.; Poprawe, R. Laser polishing and laser shape correction of optical glass. J. Laser Appl. 2017, 29, 011702. [Google Scholar] [CrossRef]
- Ostholt, R.; Willenborg, E.; Wissenbach, K. Laser polishing of freeform surfaces. In Proceedings of the 5th International WLT-Conference on Lasers in Manufacturing, Munich, Germany, 15–18 June 2009; pp. 397–402. [Google Scholar]
- Gora, W.; Tian, Y.; Cabo, A.; Ardron, M.; Maier, R.; Prangnell, P.; Weston, N.; Hand, D. Enhancing surface finish of additively manufactured titanium and cobalt chrome elements using laser based finishing. Phys. Procedia 2016, 83, 258–263. [Google Scholar] [CrossRef]
- Bordatchev, E.; Hafiz, A.; Tutunea-Fatan, O. Performance of laser polishing in finishing of metallic surfaces. Int. J. Adv. Manuf. Technol. 2014, 73, 35–52. [Google Scholar] [CrossRef]
- Nestler, K.; Böttger-Hiller, F.; Adamitzki, W.; Glowa, G.; Zeidler, H.; Schubert, A. Plasma Electrolytic Polishing—An Overview of Applied Technologies and Current Challenges to Extend the Polishable Material Range. Procedia CIRP 2016, 42, 503–507. [Google Scholar] [CrossRef]
- Landolt, D. Fundamental Aspects of Electropolishing. Electrochim. Acta 1987, 32, 1–11. [Google Scholar] [CrossRef]
- Kißling, S. Chemische und Elektrochemische Methoden zur Oberflächenbearbeitung von Galvanogeformten Nickel-Mikrostrukturen; KIT Scientific Publishing: Karlsruhe, Germany, 2010; Volume 6. [Google Scholar]
- Parfenov, E.; Farrakhov, R.; Mukaeva, V.; Gusarov, A.; Nevyantseva, R.; Yerokhin, A. Electric field effect on surface layer removal during electrolytic plasma polishing. Surf. Coat. Technol. 2016, 307, 1329–1340. [Google Scholar] [CrossRef]
- Parfenov, E.; Yerokhin, A.; Nevyantseva, R.; Gorbatkov, M.; Liang, C.J.; Matthews, A. Towards smart electrolytic plasma technologies: An overview of methodological approaches to process modelling. Surf. Coat. Technol. 2015, 269, 2–22. [Google Scholar] [CrossRef]
- Yerokhin, A.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Nestler, K.; Adamitzki, W.; Glowa, G.; Zeidler, H. Plasma Electrolytic Surface Treatment of Copper and Copper Alloys [Plasma-elektrolytische Oberflächenbehandlung von Kupfer und Kupferlegierungen]. Metall 2014, 68, 73–93. [Google Scholar]
- DIN 10528:2009-06. Lebensmittelhygiene–Anleitung für die Auswahl von Werkstoffen für den Kontakt mit Lebensmitteln–Allgemeine Grundsätze; Deutsches Institut für Normung e.V.: Berlin, Germany, 2009. [Google Scholar]
- ISO 4287:1997-04. Geometrical Product Specifications (GPS)–Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters; International Organization for Standardization (ISO): Geneva, Switzerland, 1997. [Google Scholar]
- ISO 12085:1996-08. Geometrical Product Specifications (GPS)–Surface Texture: Profile Method—Motif Parameters; International Organization for Standardization (ISO): Geneva, Switzerland, 1996. [Google Scholar]
- Wang, J.; Suo, L.; Guan, L.; Fu, Y. Optimization of Processing Parameters for Electrolysis and Plasma Polishing. Appl. Mech. Mater. 2012, 217–219, 1368–1371. [Google Scholar] [CrossRef]
- Vaňa, D.; Podhorský, S.; Hurajt, M.; Hanzen, V. Surface Properties of the Stainless Steel X10 CrNi 18/10 after Application of Plasma Polishing in Electrolyte. Int. J. Mod. Eng. Res. 2013, 3, 788–792. [Google Scholar]
- Vaňa, D.; Podhorský, S.; Suba, R.; Hurajt, M. The Change of Surface Properties on tested Smooth Stainless Steel Surfaces after Plasma Polishing. Int. J. Eng. Sci. Invent. 2013, 2, 2319–6734. [Google Scholar]
- Wang, J.; Suo, L.; Guan, L.; Fu, Y. Analytical Study on Mechanism of Electrolysis and Plasma Polishing. Adv. Mater. Res. 2012, 472–475, 350–353. [Google Scholar] [CrossRef]
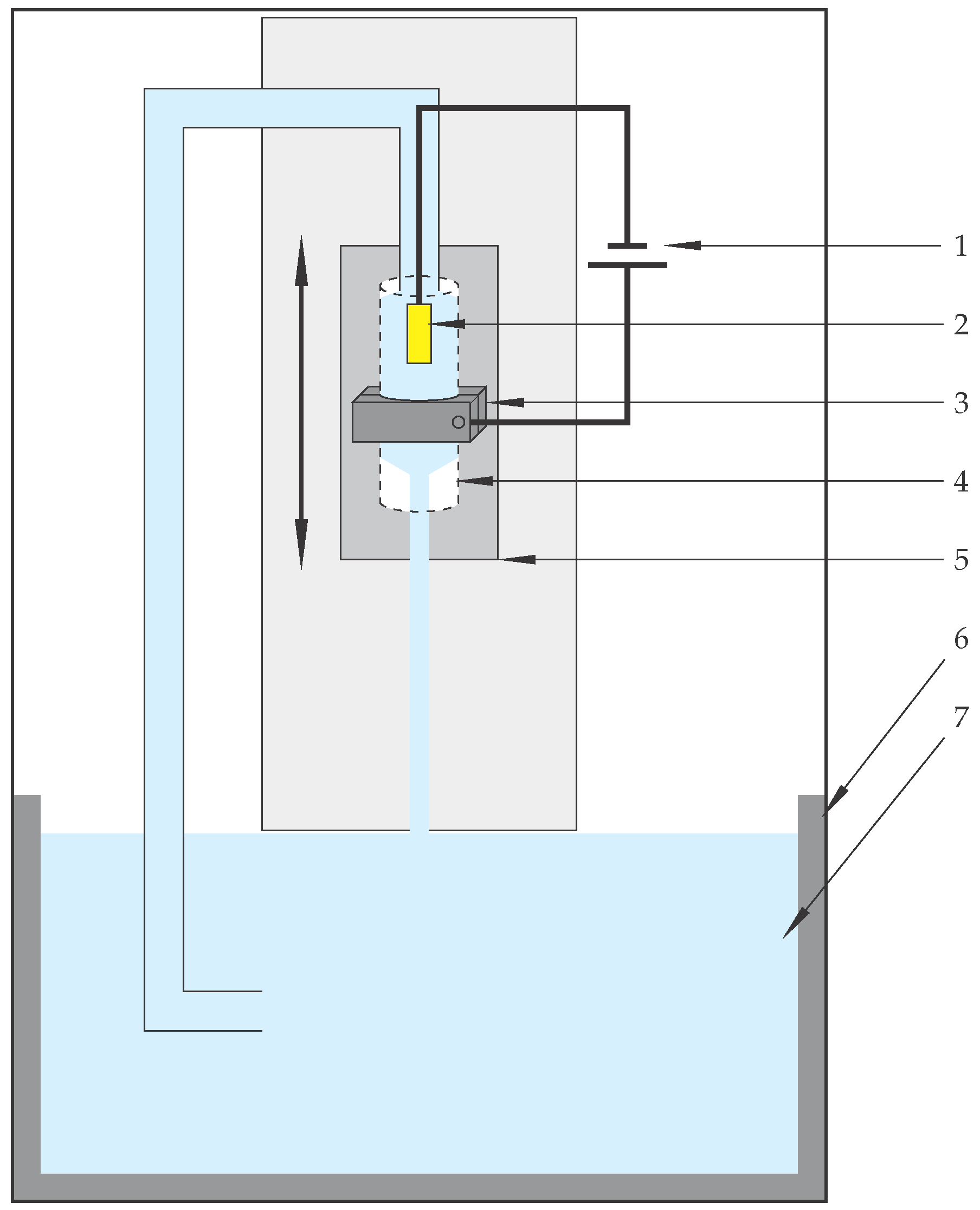

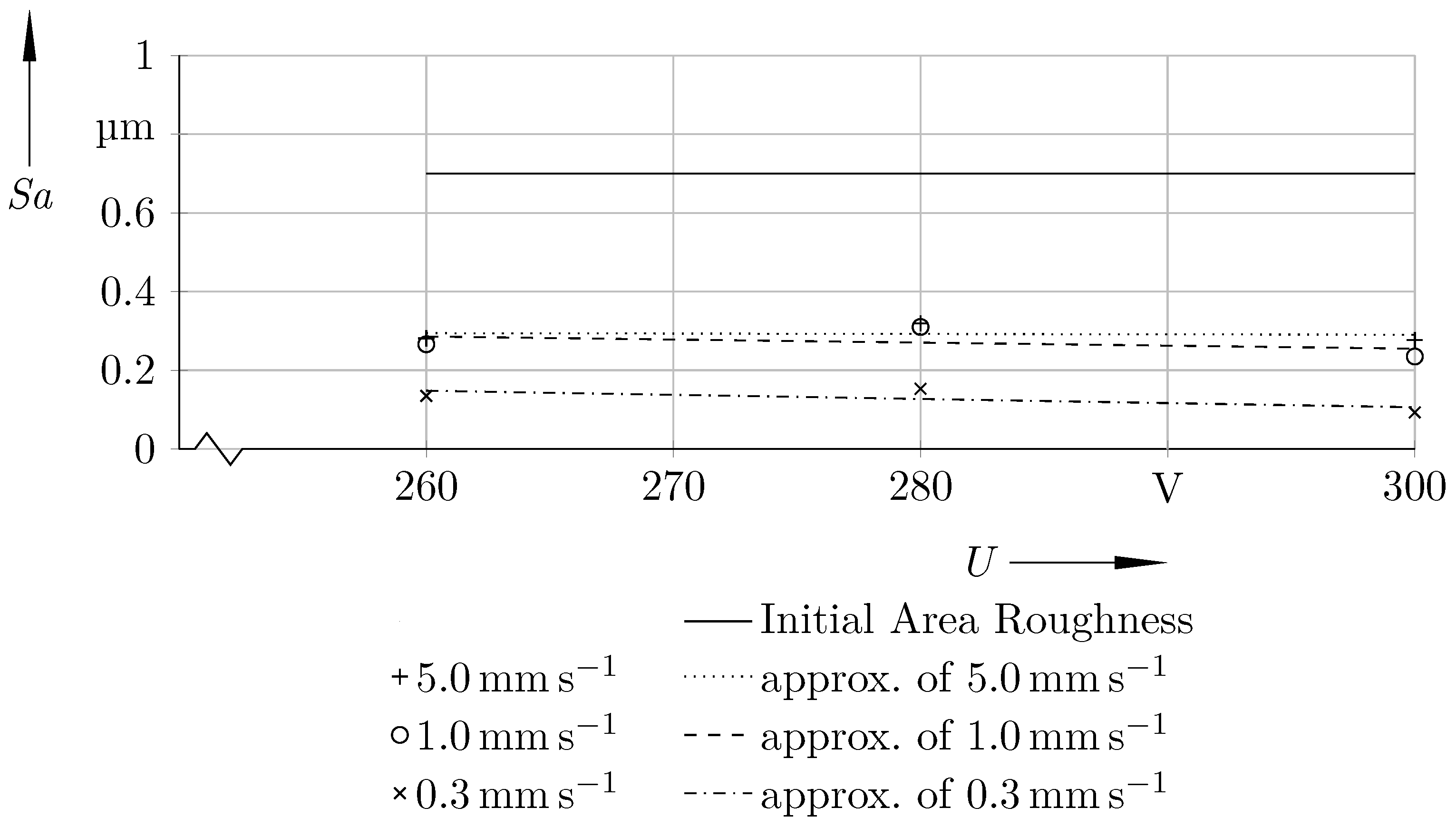



| Velocity of the Adjustable Head Gap in mm s−1 | U = 260 V | U = 280 V | U = 300 V |
|---|---|---|---|
| 5.0 | 0.281 ± 0.067 | 0.319 ± 0.075 | 0.277 ± 0.047 |
| 1.0 | 0.266 ± 0.031 | 0.310 ± 0.010 | 0.235 ± 0.041 |
| 0.3 | 0.135 ± 0.028 | 0.153 ± 0.035 | 0.093 ± 0.029 |
| Gap Width in mm | U = 260 V | U = 280 V | U = 300 V | U = 320 V |
|---|---|---|---|---|
| 2 | Sa = 0.129 ± 0.063 | Sa = 0.057 ± 0.006 | Sa = 0.076 ± 0.015 | Sa = 0.120 ± 0.041 |
| 4 | Sa = 0.159 ± 0.087 | Sa = 0.129 ± 0.047 | Sa = 0.060 ± 0.013 | Sa = 0.065 ± 0.002 |
| 6 | Sa = 0.049 ± 0.006 | Sa = 0.068 ± 0.002 | Sa = 0.070 ± 0.001 | Sa = 0.072 ± 0.008 |
| 8 | Sa = 0.180 ± 0.080 | Sa = 0.102 ± 0.003 | Sa = 0.095 ± 0.013 | Sa = 0.090 ± 0.002 |
| 10 | Sa = 0.057 ± 0.006 | Sa = 0.060 ± 0.006 | Sa = 0.063 ± 0.001 | Sa = 0.066 ± 0.005 |
| 2 | I = 14.1 ± 14.1 | I = 8.2 ± 6.2 | I = 6.9 ± 2.9 | I = 5.7 ± 1.4 |
| 4 | I = 14.1 ± 15.7 | I = 10.8 ± 11.0 | I = 7.5 ± 3.0 | I = 6.9 ± 1.9 |
| 6 | I = 4.7 ± 1.2 | I = 6.3 ± 1.9 | I = 5.3 ± 1.3 | I = 5.3 ± 1.1 |
| 8 | I = 6.4 ± 7.2 | I = 4.7 ± 3.7 | I = 6.1 ± 4.0 | I = 5.7 ± 1.6 |
| 10 | I = 5.5 ± 1.4 | I = 6.5 ± 1.4 | I = 5.5 ± 1.0 | I = 5.1 ± 1.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornelsen, M.; Deutsch, C.; Seitz, H. Electrolytic Plasma Polishing of Pipe Inner Surfaces. Metals 2018, 8, 12. https://doi.org/10.3390/met8010012
Cornelsen M, Deutsch C, Seitz H. Electrolytic Plasma Polishing of Pipe Inner Surfaces. Metals. 2018; 8(1):12. https://doi.org/10.3390/met8010012
Chicago/Turabian StyleCornelsen, Matthias, Carolin Deutsch, and Hermann Seitz. 2018. "Electrolytic Plasma Polishing of Pipe Inner Surfaces" Metals 8, no. 1: 12. https://doi.org/10.3390/met8010012






