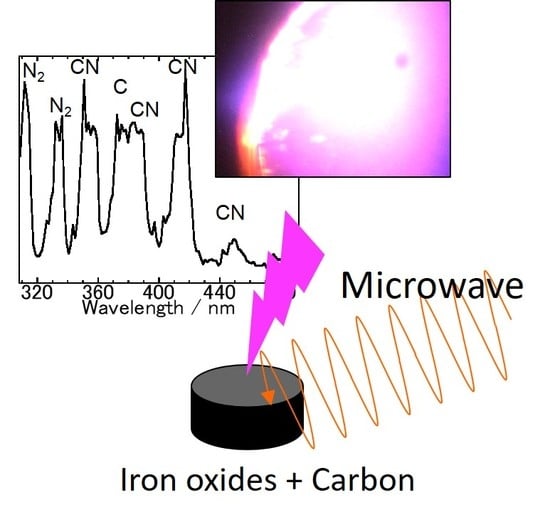
In Situ Spectroscopic Analysis of the Carbothermal Reduction Process of Iron Oxides during Microwave Irradiation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
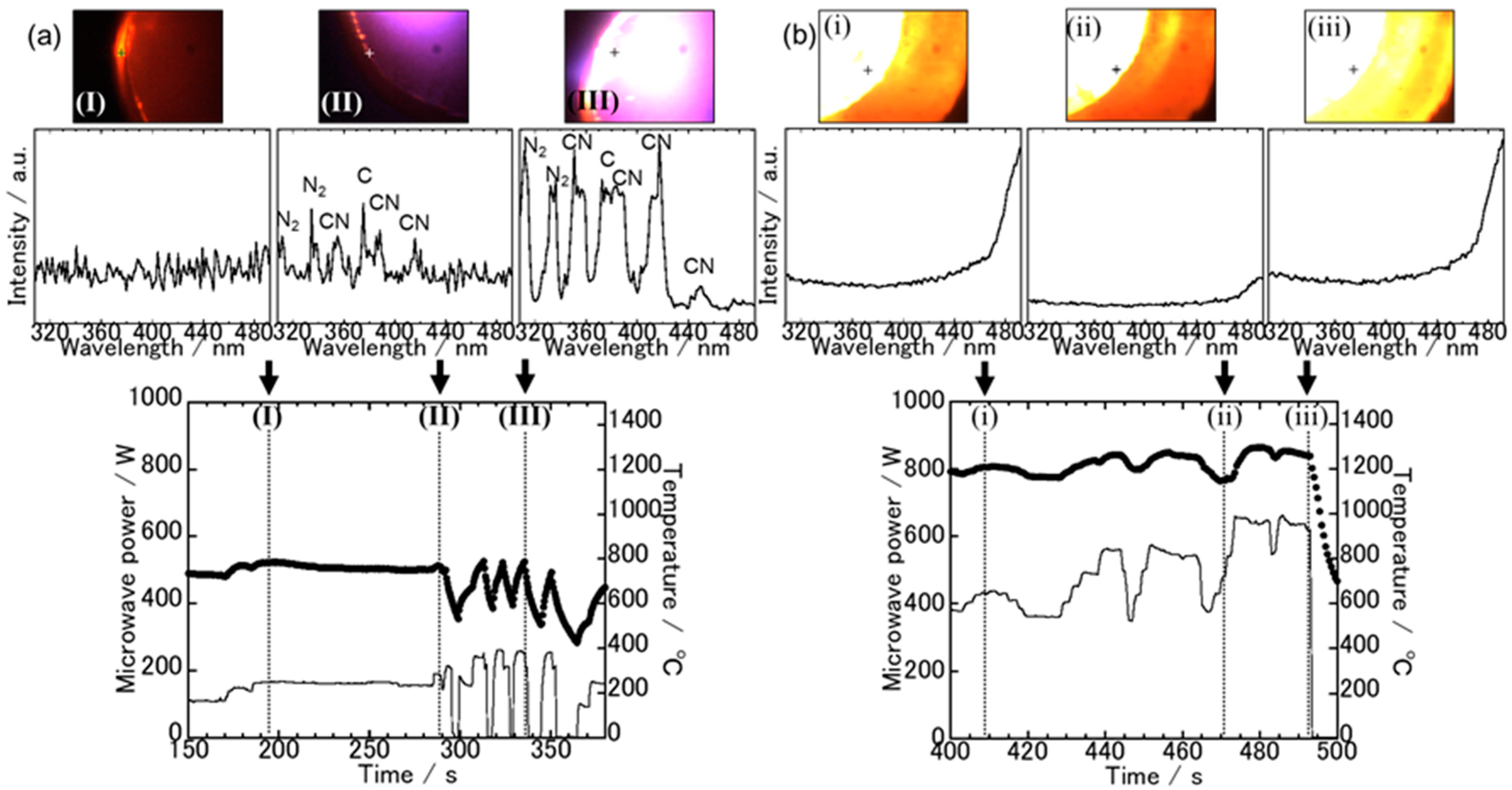
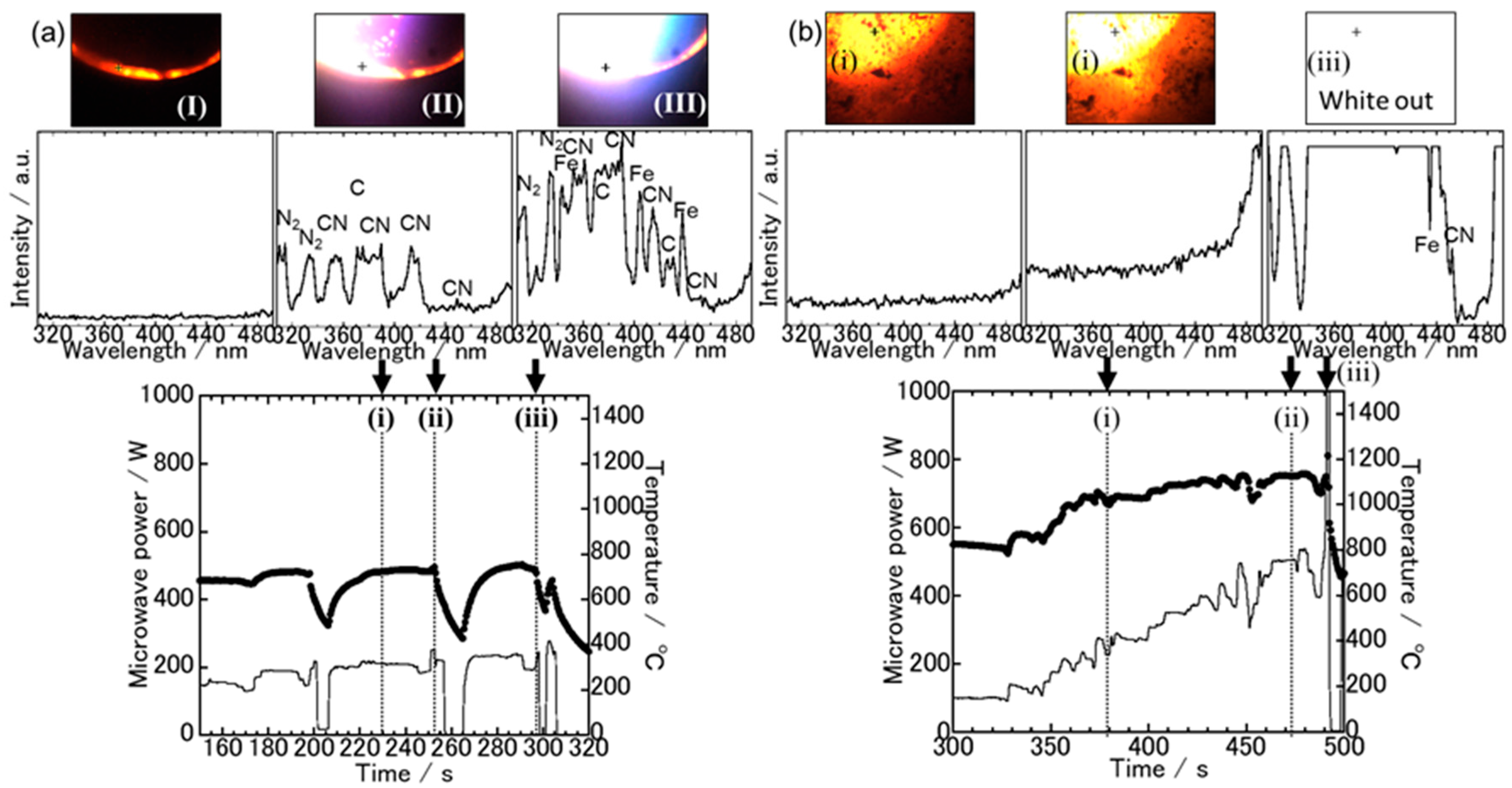
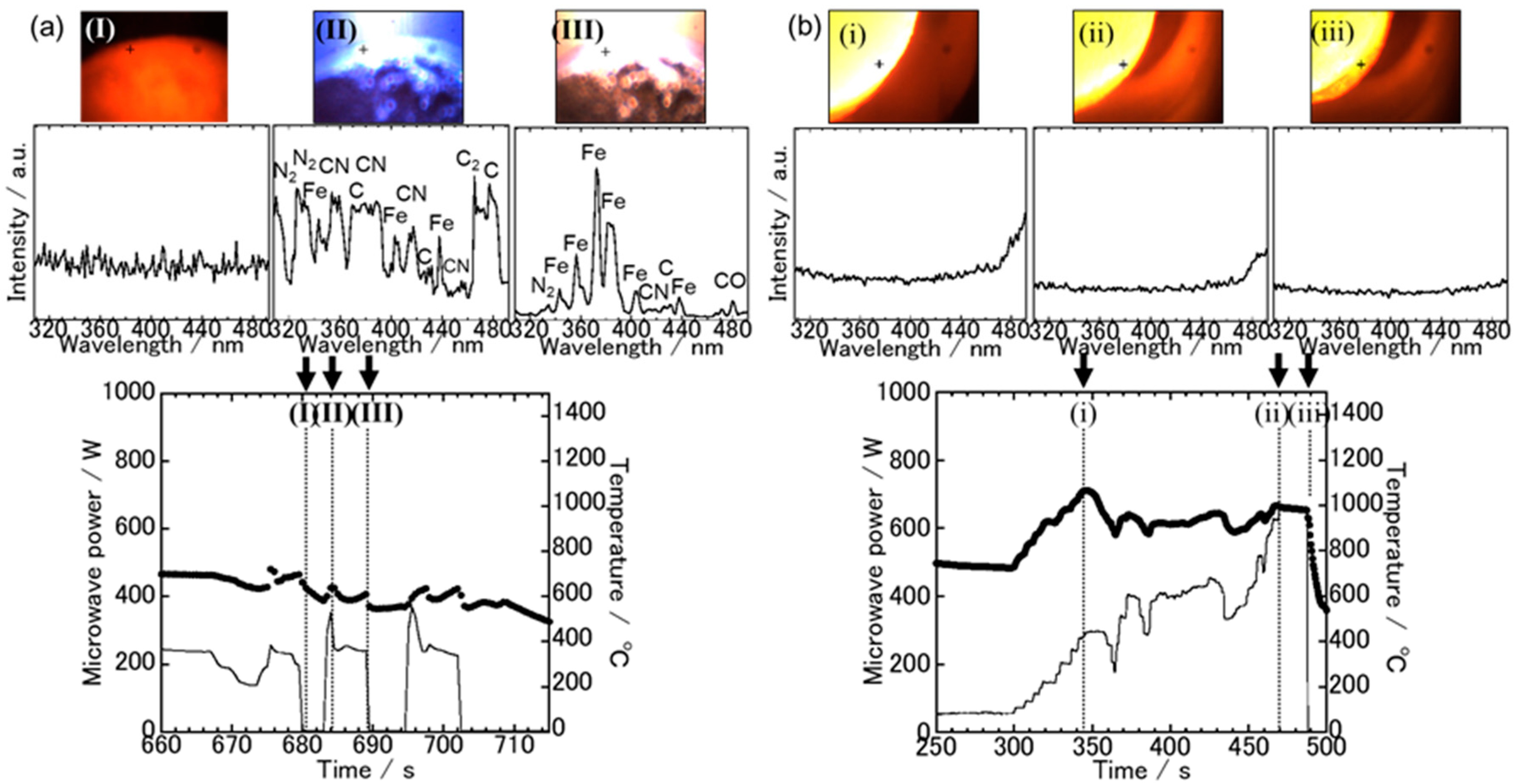
3.1. Spectroscopic Measurements during Microwave Irradiation
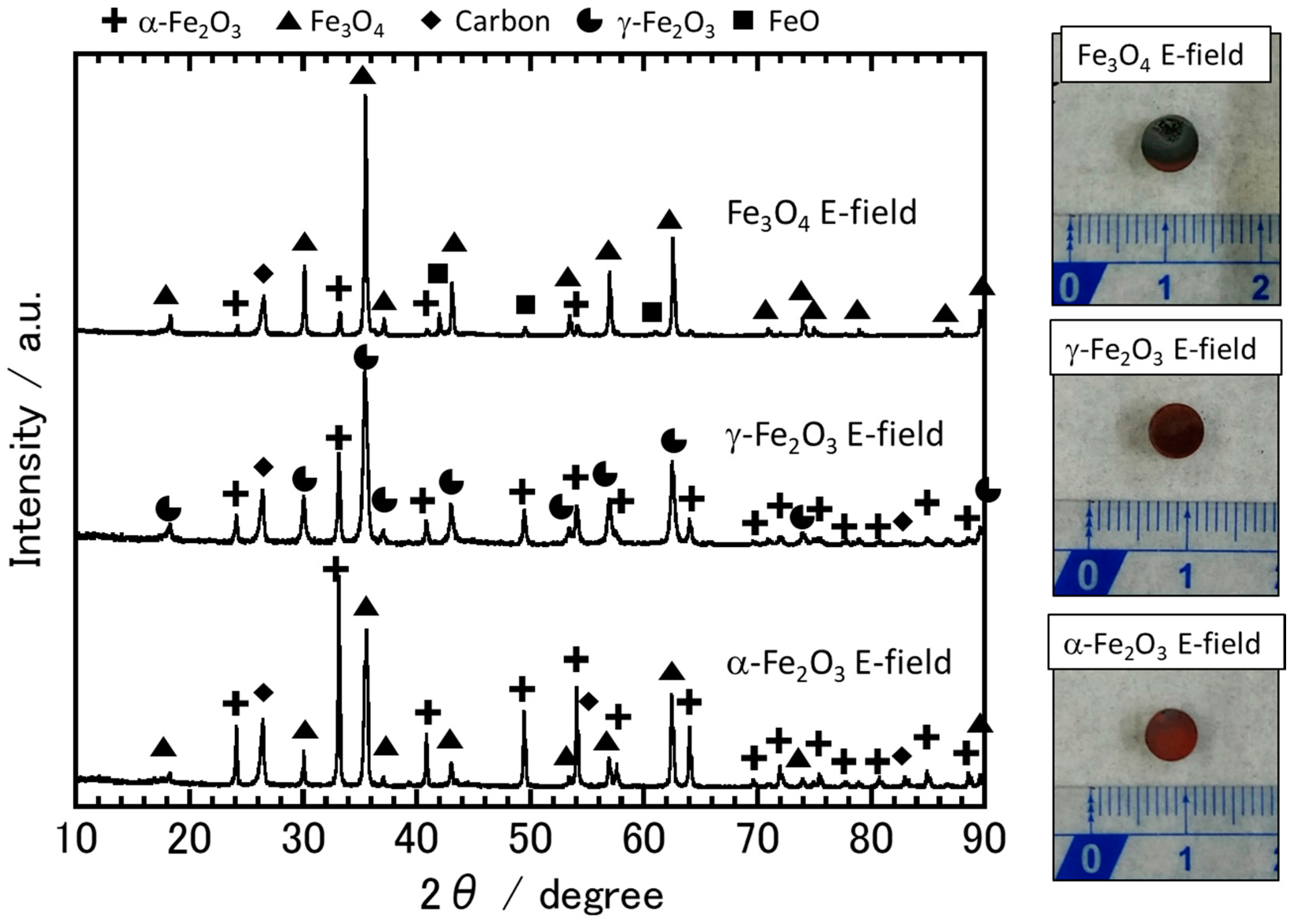
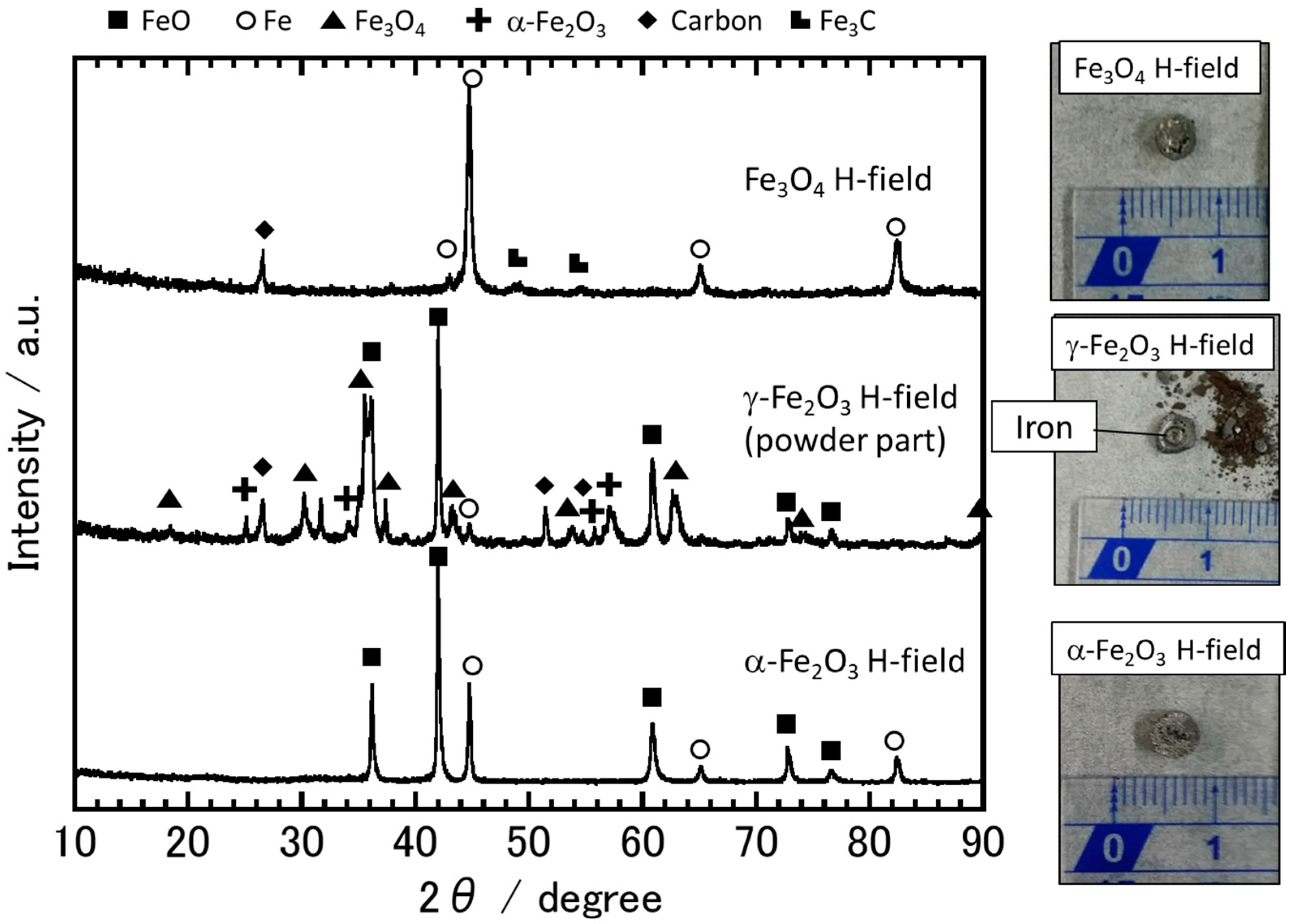
3.2. The Sample Reduction State after Microwave Irradiation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ishizaki, K.; Nagata, K.; Hayashi, T. Localized Heating and Reduction of Magnetite Ore with Coal in Composite Pellets Using Microwave Irradiation. ISIJ Int. 2007, 47, 817–822. [Google Scholar] [CrossRef]
- Kashimura, K.; Sato, M.; Hotta, M.; Agrawal, D.K.; Nagata, K.; Hayashi, M.; Mitani, T.; Shinohara, N. Iron production from Fe3O4 and graphite by applying 915 MHz microwaves. Mater. Sci. Eng. A 2012, 556, 977–979. [Google Scholar] [CrossRef]
- Castro, E.R.D.; Moura, M.B.; Jermolovicius, Ã.L.A.; Takano, C. Carbothermal reduction of iron ore applying microwave energy. Steel Res. Int. 2012, 83, 131–138. [Google Scholar] [CrossRef]
- Hara, K.; Hayashi, M.; Sato, M.; Nagata, K. Continuous Pig Iron Making by Microwave. J. Microw. Power Electromagn. Energy 2011, 45, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Hayashi, M.; Sato, M.; Nagata, K. Pig Iron Making by Focused Microwave Beams with 20 kW at 2.45 GHz. ISIJ Int. 2012, 52, 2149–2157. [Google Scholar] [CrossRef]
- Sabelström, N.; Hayashi, M.; Yokoyama, Y.; Watanabe, T.; Nagata, K. XRD In Situ Observation of Carbothermic Reduction of Magnetite Powder in Microwave Electric and Magnetic Fields. Steel Res. Int. 2013, 84, 975–981. [Google Scholar] [CrossRef]
- Nagata, K.; Sato, M.; Hara, K.; Hotta, T.; Kitamura, Y.; Hayashi, M.; Kashimura, K.; Mitani, T.; Fukushima, J. Microwave Blast Furnace and Its Refractories. J. Tech. Assoc. Refract. Jpn. 2014, 34, 66–73. [Google Scholar]
- Kashimura, K.; Nagata, K.; Sato, M. Concept of Furnace for Metal Refining by Microwave Heating—A Design of Microwave Smelting Furnace with Low CO2 Emission. Mater. Trans. 2010, 51, 1847–1853. [Google Scholar] [CrossRef]
- Ishizaki, K.; Stir, M.; Gozzo, F.; Catalá-Civera, J.M.; Vaucher, S.; Nicula, R. Magnetic microwave heating of magnetite-carbon black mixtures. Mater. Chem. Phys. 2012, 134, 1007–1012. [Google Scholar] [CrossRef]
- Stir, M.; Ishizaki, K.; Vaucher, S.; Nicula, R. Mechanism and kinetics of the reduction of magnetite to iron during heating in a microwave E-field maximum. J. Appl. Phys. 2009, 105, 124901. [Google Scholar] [CrossRef]
- Matsubara, A.; Takayama, S.; Okajima, S.; Sato, M. Evolution of the Near-UV Emission Spectrum Associated with the Reduction Process in Microwave Iron Making. J. Microw. Power Electromagn. Energy 2008, 42, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, A.; Nakayama, K.; Okajima, S.; Sato, M. Microscopic and Spectroscopic Observations of Plasma Generation in the Microwave Heating of Powder Material. Plasma Fusion Res. 2010, 5, 041. [Google Scholar] [CrossRef]
- Sonobe, T.; Mitani, T.; Shinohara, N.; Hachiya, K.; Yoshikawa, S. Plasma emission and surface reduction of titanium dioxides by microwave irradiation. Jpn. J. Appl. Phys. 2009, 48, 116003. [Google Scholar] [CrossRef]
- Sabat, K.C.; Paramguru, R.K.; Mishra, B.K. Reduction of Oxide Mixtures of (Fe2O3 + CuO) and (Fe2O3 + Co3O4) by Low-Temperature Hydrogen Plasma. Plasma Chem. Plasma Process. 2017, 37, 979–995. [Google Scholar] [CrossRef]
- Sabat, K.C.; Rajput, P.; Paramguru, R.K.; Bhoi, B.; Mishra, B.K. Reduction of Oxide Minerals by Hydrogen Plasma: An Overview. Plasma Chem. Plasma Process. 2014, 34, 1–23. [Google Scholar] [CrossRef]
- Kitamura, T.; Shibata, K.; Takeda, K. In-fright Reduction of Fe2O3, Cr2O3, TiO2 and Al2O3 by Ar-H2 and Ar-CH4 Plasma. ISIJ Int. 1993, 33, 1150–1158. [Google Scholar] [CrossRef]
- Fukushima, J.; Kashimura, K.; Takayama, S.; Sato, M.; Sano, S.; Hayashi, Y.; Takizawa, H. In-situ kinetic study on non-thermal reduction reaction of CuO during microwave heating. Mater. Lett. 2013, 91, 252–254. [Google Scholar] [CrossRef]
- Dong, M.; Lu, J.; Yao, S.; Zhong, Z.; Li, J.; Li, J.; Lu, W. Experimental study on the characteristics of molecular emission spectroscopy for the analysis of solid materials containing C and N. Opt. Express 2011, 19, 17021–17029. [Google Scholar] [CrossRef] [PubMed]
- Wasowicz, T.J.; Kivimäki, A.; Coreno, M.; Zubek, M. Superexcited states in the vacuum-ultraviolet photofragmentation of isoxazole molecules. J. Phys. B Atom. Mol. Opt. Phys. 2012, 45, 205103. [Google Scholar] [CrossRef]
- Pearse, R.W.B.; Gaydon, A.G. The Identification of Molecular Spectra, 3rd ed.; Chapman and Hall: London, UK, 1965. [Google Scholar]
- Voevodin, A.A.; Jones, J.G.; Zabinski, J.S.; Hultman, L. Plasma characterization during laser ablation of graphite in nitrogen for the growth of fullerene-like CNx films. J. Appl. Phys. 2002, 92, 724–735. [Google Scholar] [CrossRef]
- Bourquard, F.; Maddi, C.; Donnet, C.; Loir, A.-S.; Barnier, V.; Wolski, K.; Garrelie, F. Effect of nitrogen surrounding gas and plasma assistance on nitrogen incorporation in a-C:N films by femtosecond pulsed laser deposition. Appl. Surf. Sci. 2016, 374, 104–111. [Google Scholar] [CrossRef]
- Kraus, M.; Egli, W.; Haffner, K.; Eliasson, B.; Kogelschatz, U.; Wokaun, A. Investigation of mechanistic aspects of the catalytic CO2 reforming of methane in a dielectric-barrier discharge using optical emission. Phys. Chem. Chem. Phys. 2002, 4, 668–675. [Google Scholar] [CrossRef]
- Chase, M.W.J. (Ed.) NIST-JANAF Thermochemical Tables, 4th ed.; American Institute of Physics: College Park, MD, USA, 1998. [Google Scholar]
- Ishizaki, K.; Nagata, K. Microwave Induced Solid—Solid Reactions between Fe3O4 and Carbon Black Powders. ISIJ Int. 2008, 48, 1159–1164. [Google Scholar] [CrossRef]
- Fukushima, J.; Kashimura, K.; Sato, M. Chemical bond cleavage induced by electron heating Gas emission behavior of titanium-metalloid compounds (titanium nitride and oxide) in a microwave field. Mater. Chem. Phys. 2011, 131, 178–183. [Google Scholar] [CrossRef]
- Fukushima, J.; Kashimura, K.; Takayama, S.; Sato, M. Microwave-energy Distribution for Reduction and Decrystallization of Titanium Oxides. Chem. Lett. 2012, 41, 39–41. [Google Scholar] [CrossRef]
- Tanaka, M.; Kono, H.; Maruyama, K. Selective heating mechanism of magnetic metal oxides by a microwave magnetic field. Phys. Rev. B 2009, 79, 104420. [Google Scholar] [CrossRef]
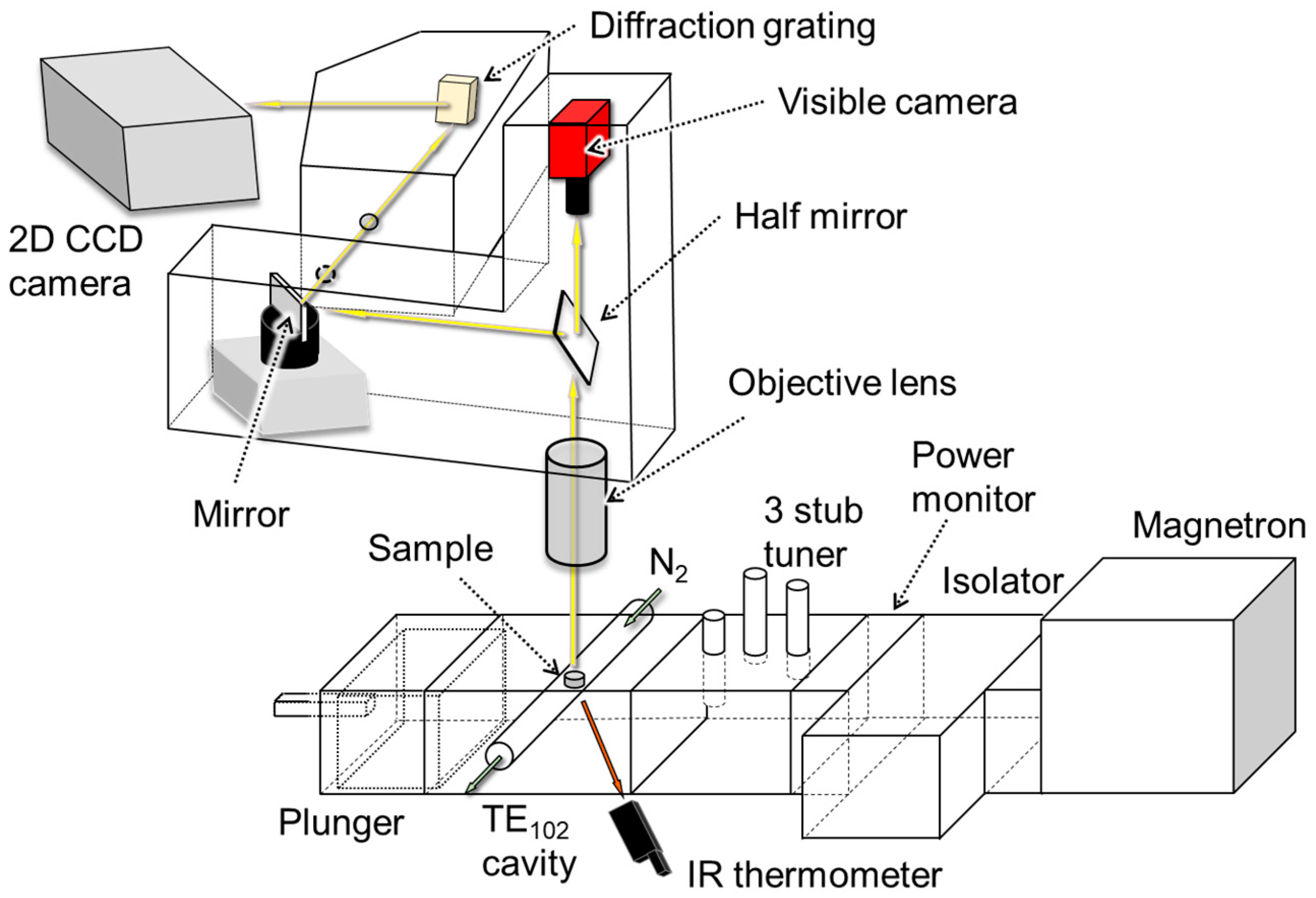
| Spectrometer | Visible Camera | |
|---|---|---|
| Exposure time | 0.5 s | 0.5 s |
| Spatial resolution | 4.9 μm2 | 25 μm2 |
| Wavelength resolution | 0.5 nm | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukushima, J.; Takizawa, H. In Situ Spectroscopic Analysis of the Carbothermal Reduction Process of Iron Oxides during Microwave Irradiation. Metals 2018, 8, 49. https://doi.org/10.3390/met8010049
Fukushima J, Takizawa H. In Situ Spectroscopic Analysis of the Carbothermal Reduction Process of Iron Oxides during Microwave Irradiation. Metals. 2018; 8(1):49. https://doi.org/10.3390/met8010049
Chicago/Turabian StyleFukushima, Jun, and Hirotsugu Takizawa. 2018. "In Situ Spectroscopic Analysis of the Carbothermal Reduction Process of Iron Oxides during Microwave Irradiation" Metals 8, no. 1: 49. https://doi.org/10.3390/met8010049