Surface Modification of Q195 Structure Carbon Steel by Electrolytic Plasma Processing
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Q195 Structural Carbon Steel Sample Preparation
2.2. Instruments
2.3. Q195 Structural Carbon Steel Treatment by EPP
2.3.1. Configuration of Sodium Bicarbonate Saturated Solution
2.3.2. EPP Treatment Process
2.3.3. Sample Post-Processing
2.3.4. Preparation of Zinc-Coated Q195 Structural Carbon Steel
3. Results and Discussion
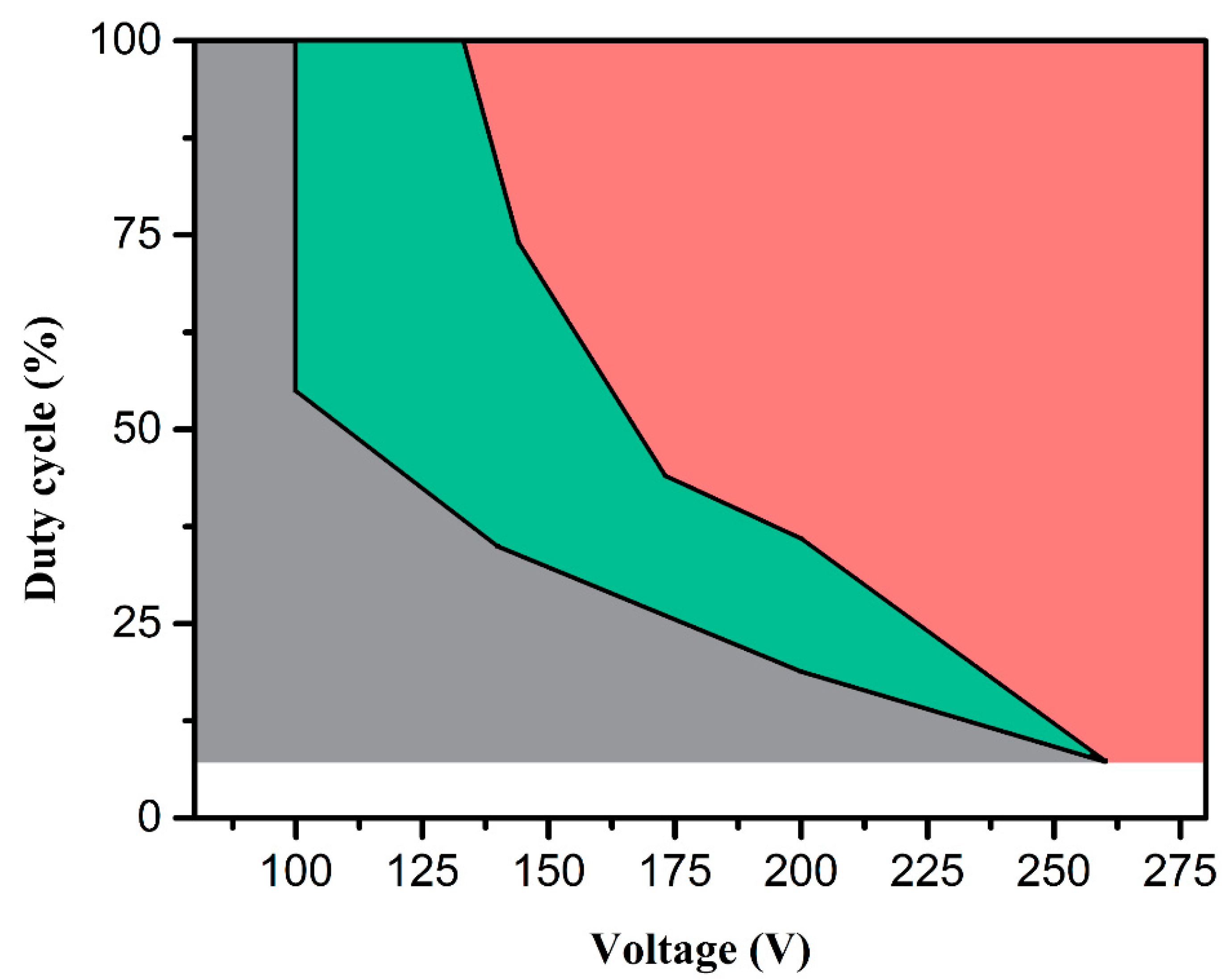
3.1. Characterization of Electrolytic Plasma Processing
3.2. Surface Microstructural Morphology Analysis
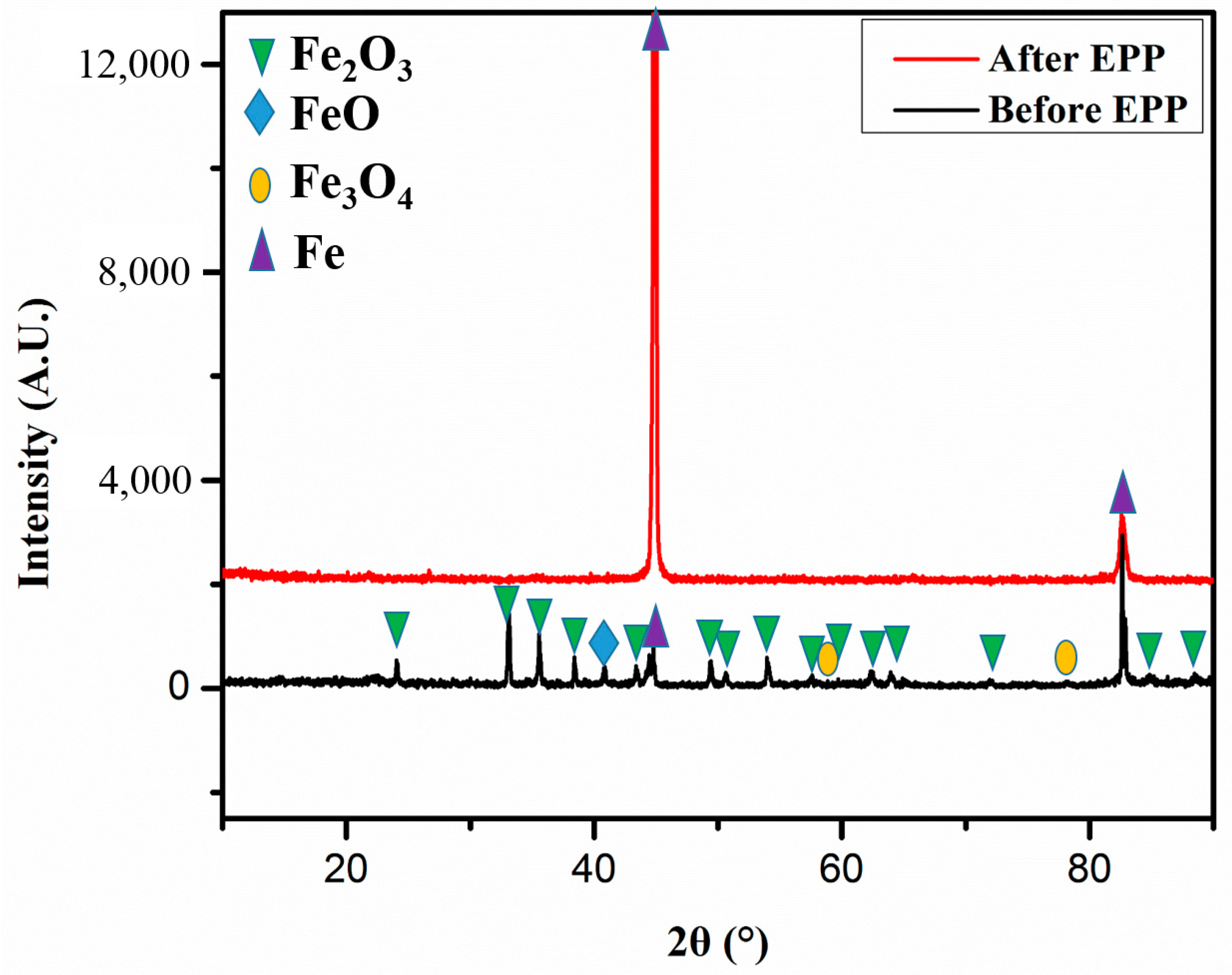
3.3. X-ray Diffraction (XRD) Pattern Characterization
3.4. Mechanical Properties Testing
3.4.1. Fracture Morphology Characterization
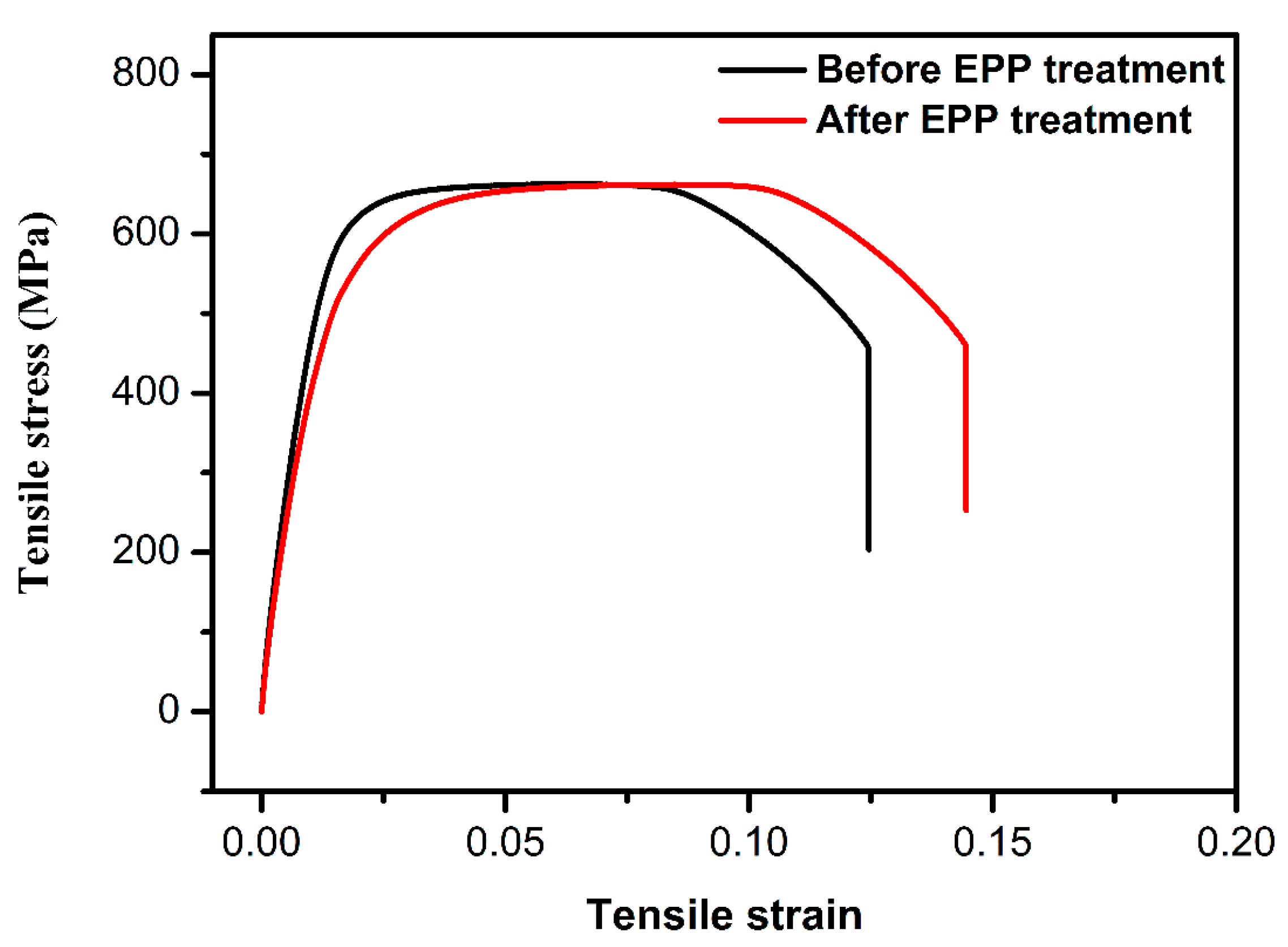
3.4.2. Tensile Mechanical Properties
3.4.3. Elastic Modulus and Hardness
3.5. Zinc-Coating Preparation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karmakar, A.; Mandal, M.; Mandal, A.; Sk, B.; Mukherjee, S.; Chakrabarti, D. Effect of starting microstructure on the grain refinement in cold-rolled low-carbon steel during annealing at two different heating rates. Metall. Mater. Trans. A 2015, 47, 268–281. [Google Scholar] [CrossRef]
- Lakshminarayanan, A.K.; Annamalai, V.E.; Elangovan, K. Identification of optimum friction stir spot welding process parameters controlling the properties of low carbon automotive steel joints. J. Mater. Res. Technol. 2015, 4, 262–272. [Google Scholar] [CrossRef]
- Rotellaa, G.; Alfanoa, M.; Candamano, S. Surface modification of Ti6Al4V alloy by pulsed Yb-laser irradiation for enhanced adhesive bonding. CIRP Ann. Manuf. Tech. 2015, 64, 527–530. [Google Scholar] [CrossRef]
- Etminanfar, M.R.; Khalil-Allafi, J. On the electrodeposition of Ca-P coatings on nitinol alloy: A comparison between different surface modification methods. J. Mater. Eng. Perform. 2016, 25, 466–473. [Google Scholar] [CrossRef]
- El-Hossary, F.M.; Negm, N.Z.; Abd El-Rahman, A.M.; Raaif, M.; Elmula, A.A.A. Properties of titanium oxynitride prepared by RF plasma. Adv. Chem. Eng. Sci. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Mo, M.H.; Wu, L.K.; Cao, H.Z.; Lin, J.-P.; Zheng, G.-Q. Halogen effect for improving high temperature oxidation resistance of Ti-50Al by anodization. Appl. Surf. Sci. 2017, 407, 246–254. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Z.P.; Hao, G.J.; Liang, Y.F.; Ding, X.F.; Lin, J.P. Ni-doped Al2O3, coatings prepared by cathode plasma electrolysis deposition on Ti-45Al-8.5 Nb alloys. Appl. Surf. Sci. 2018, 455, 144–152. [Google Scholar] [CrossRef]
- Cheng, F.; Li, S.S.; Gui, W.Y.; Lin, J. Surface modification of Ti-45Al-8.5Nb alloys by microarc oxidation to improve high-temperature oxidation resistance. Prog. Nat. Sci. 2018, 28, 386–390. [Google Scholar] [CrossRef]
- Ueda, M.; Oliveira, R.M.; Rossi, J.O. Improvements of plasma immersion ion implantation (PIII) and deposition (PIII&D) processing for materials surface modification. Surf. Coat. Technol. 2013, 229, 97–104. [Google Scholar]
- Eddy, N.O. Ethanol extract of phyllanthus amarus as a green inhibitor for the corrosion of mild steel in H2SO4. Portugaliae Electrochim. Acta 2009, 27, 579–589. [Google Scholar] [CrossRef]
- Kumar, S.; Mathur, S.P. Corrosion inhibition and adsorption properties of ethanolic extract of calotropis for corrosion of aluminium in acidic media. ISRN Corros. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Pushilina, N.S.; Kudiiarov, V.N.; Laptev, R.S.; Lider, A.M.; Teresov, A.D. Microstructure changes in Zr–1Nb alloy after pulsed electron beam surface modification and hydrogenation. Surf. Coat. Technol. 2015, 284, 63–68. [Google Scholar] [CrossRef]
- Yan, L.; Yin, X.; Zhang, J.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar]
- Qing, Y.; Yang, C.; Hu, C.; Zheng, Y.; Liu, C. A facile method to prepare superhydrophobic fluorinated polysiloxane/ZnO nanocomposite coatings with corrosion resistance. Appl. Surf. Sci. 2015, 326, 48–54. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.; Gao, Y.; Yeung, W.K.; Strumban, E.; Leshchinsky, V.; Chu, P.-J.; Matthews, A.; Yerokhin, A.; Maev, R.G. Characterization and corrosion evaluation of TiO2:n-HA coatings on titanium alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2015, 269, 258–265. [Google Scholar] [CrossRef]
- Rudnev, V.S. Micro and nano-formations on the surface of plasma electrolytic oxide coatings on aluminum and titanium. Surf. Coat. Technol. 2013, 235, 134–143. [Google Scholar] [CrossRef]
- Simka, W.; Krząkała, A.; Korotin, D.M. Modification of a Ti–Mo alloy surface via plasma electrolytic oxidation in a solution containing calcium and phosphorus. Electrochim. Acta 2013, 96, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.; Kelton, R.; Meletis, E.I. Deposition of Ni Coatings by Electrolytic Plasma Processing. Plasma Chem. Plasma Process. 2015, 35, 963–978. [Google Scholar] [CrossRef]
- Dewan, M.W.; Liang, J.D.; Wahab, M.A. Effect of post-weld heat treatment and electrolytic plasma processing on tungsten inert gas welded AISI 4140 alloy steel. Mater. Des. 2014, 54, 6–13. [Google Scholar] [CrossRef]
- Masiha, H.R.; Bagheri, H.R.; Gheytani, M. Effect of surface nanostructuring of aluminum alloy on post plasma electrolytic oxidation. Appl. Surf. Sci. 2014, 317, 962–969. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, X.; Bian, Z.J. Paving the way for surface modification in one-dimensional channels of mesoporous materials via plasma treatment. Microporous Mesoporous Mater. 2015, 202, 16–21. [Google Scholar] [CrossRef]
- Chong, D.S.; Turner, L.A.; Gadegaard, N. Nanotopography and plasma treatment: Redesigning the surface for vascular graft endothelialisation. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Daudt, N.F.; Bram, M.; Cysne Barbosa, A.P. Surface modification of highly porous titanium by plasma treatment. Mater. Lett. 2015, 141, 194–197. [Google Scholar] [CrossRef]
- Gui, W.Y.; Lin, J.P.; Hao, G.J.; Qu, Y.; Liang, Y.; Zhang, H. Electrolytic plasma processing-an innovative treatment for surface modification of 304 stainless steel. Sci. Rep. 2017, 7, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, E.V.; Yerokhinb, A.; Nevyantseva, R.R. Towards smart electrolytic plasma technologies: An overview of methodological approaches to process modelling. Surf. Coat. Technol. 2015, 269, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Rakoch, A.G.; Gladkova, A.A.; Zayar, L. The evidence of cathodic micro-discharges during plasma electrolytic oxidation of light metallic alloys and micro-discharge intensity depending on pH of the electrolyte. Surf. Coat. Technol. 2015, 269, 138–144. [Google Scholar] [CrossRef]
- Gui, W.Y.; Lin, J.P.; Hao, G.J.; Qu, Y.; Jiang, Z.; Cheng, F.; Liang, Y.; Zhang, H. Effect of Electrolytic Plasma Processing on the Removal of Surface Scale for Fe6.5Si Alloy. ChemistrySelect 2017, 2, 1158–1162. [Google Scholar] [CrossRef]
- Jones, H.; Kunhardt, E. Development of pulsed dielectric breakdown in liquids. J. Phys. D Appl. Phys. 1995, 28, 178–188. [Google Scholar] [CrossRef]
- Yamada, H.; Chayahara, A.; Mokuno, Y. Numerical analysis of power absorption and gas pressure dependence of microwave plasma using a tractable plasma description. Diam. Relat. Mater. 2006, 15, 1395–1399. [Google Scholar] [CrossRef]
- He, J.; Luo, Q.; Cai, Q.Z. Microstructure and photocatalytic properties of WO3/TiO2 composite films by plasma electrolytic oxidation. Mater. Chem. Phys. 2011, 129, 242–248. [Google Scholar] [CrossRef]
- Van, T.B.; Brown, S.D.; Wirtz, G.P. Mechanism of Anodic Spark Deposition. Am. Ceram. Soc. Bull. 1976, 56, 6. [Google Scholar]
- Yerokhin, A.L.; Lyubimov, V.V.; Ashitkov, R.V. Phase formation in ceramic coatings during plasma electrolytic oxidation of aluminium alloys. Ceram. Int. 1998, 24, 1–6. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Meletis, E.I.; Nie, X.; Wang, F.L.; Jiang, J.C. Electrolytic plasma processing for cleaning and metal-coating of steel surfaces. Surf. Coat. Technol. 2002, 150, 246–256. [Google Scholar] [CrossRef]
- Gui, W.Y.; Hao, G.J.; Liang, Y.F.; Li, F.; Lin, J. Surface modification by electrolytic plasma processing for high Nb-TiAl alloys. Appl. Surf. Sci. 2016, 389, 1161–1168. [Google Scholar] [CrossRef]



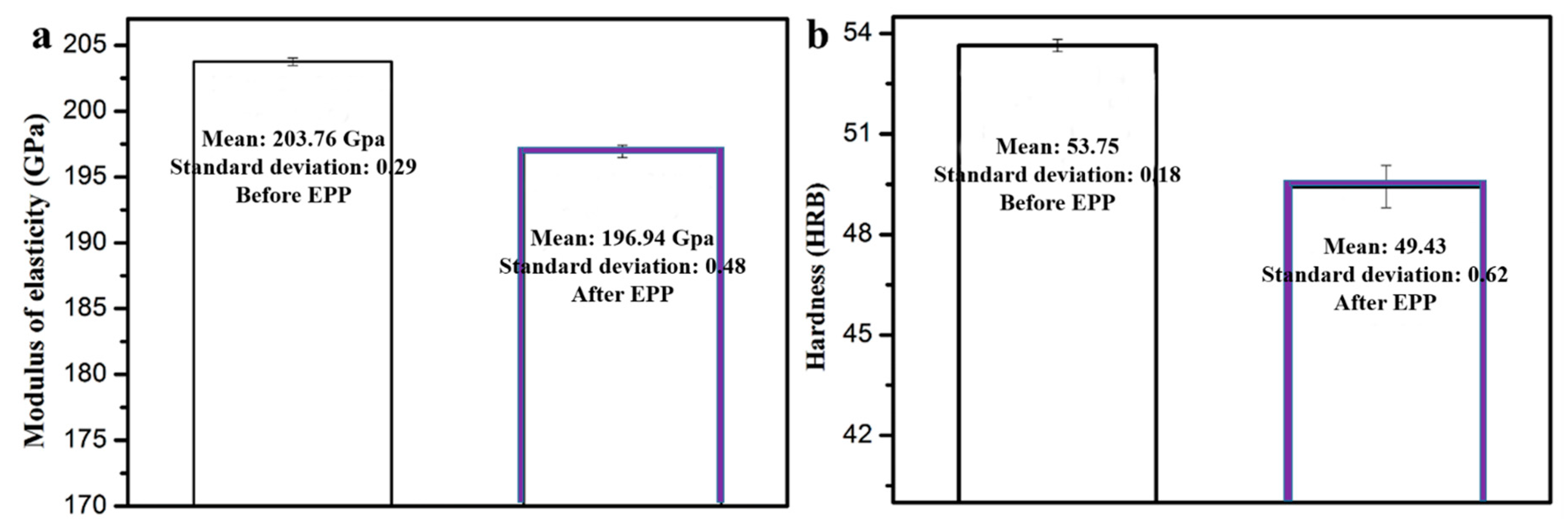


| Sample | EPP Treatment | Elastic Modulus/GPa | Hardness/GPa |
|---|---|---|---|
| Q195 carbon structural steel | 0 s | 203.76 | 53.75 |
| Q195 carbon structural steel | 40 s | 196.94 | 49.43 |
| Sample | Zn (at. %) | Fe (at. %) | C (at. %) | Si (at. %) | O (at. %) |
|---|---|---|---|---|---|
| a | 68.42 | 26.97 | 0.51 | 0.05 | 4.05 |
| b | 96.93 | 1.23 | 0.00 | 0.00 | 1.84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, F.; Gui, W.; Lin, J. Surface Modification of Q195 Structure Carbon Steel by Electrolytic Plasma Processing. Metals 2018, 8, 831. https://doi.org/10.3390/met8100831
Cheng F, Gui W, Lin J. Surface Modification of Q195 Structure Carbon Steel by Electrolytic Plasma Processing. Metals. 2018; 8(10):831. https://doi.org/10.3390/met8100831
Chicago/Turabian StyleCheng, Fang, Wanyuan Gui, and Junpin Lin. 2018. "Surface Modification of Q195 Structure Carbon Steel by Electrolytic Plasma Processing" Metals 8, no. 10: 831. https://doi.org/10.3390/met8100831





