Initial Stages of AZ31B Magnesium Alloy Degradation in Ringer’s Solution: Interpretation of EIS, Mass Loss, Hydrogen Evolution Data and Scanning Electron Microscopy Observations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Immersion Test
2.3. Microstructure Characterization
2.4. Hydrogen Evolution Measurement
2.5. Electrochemical Measurements
3. Results
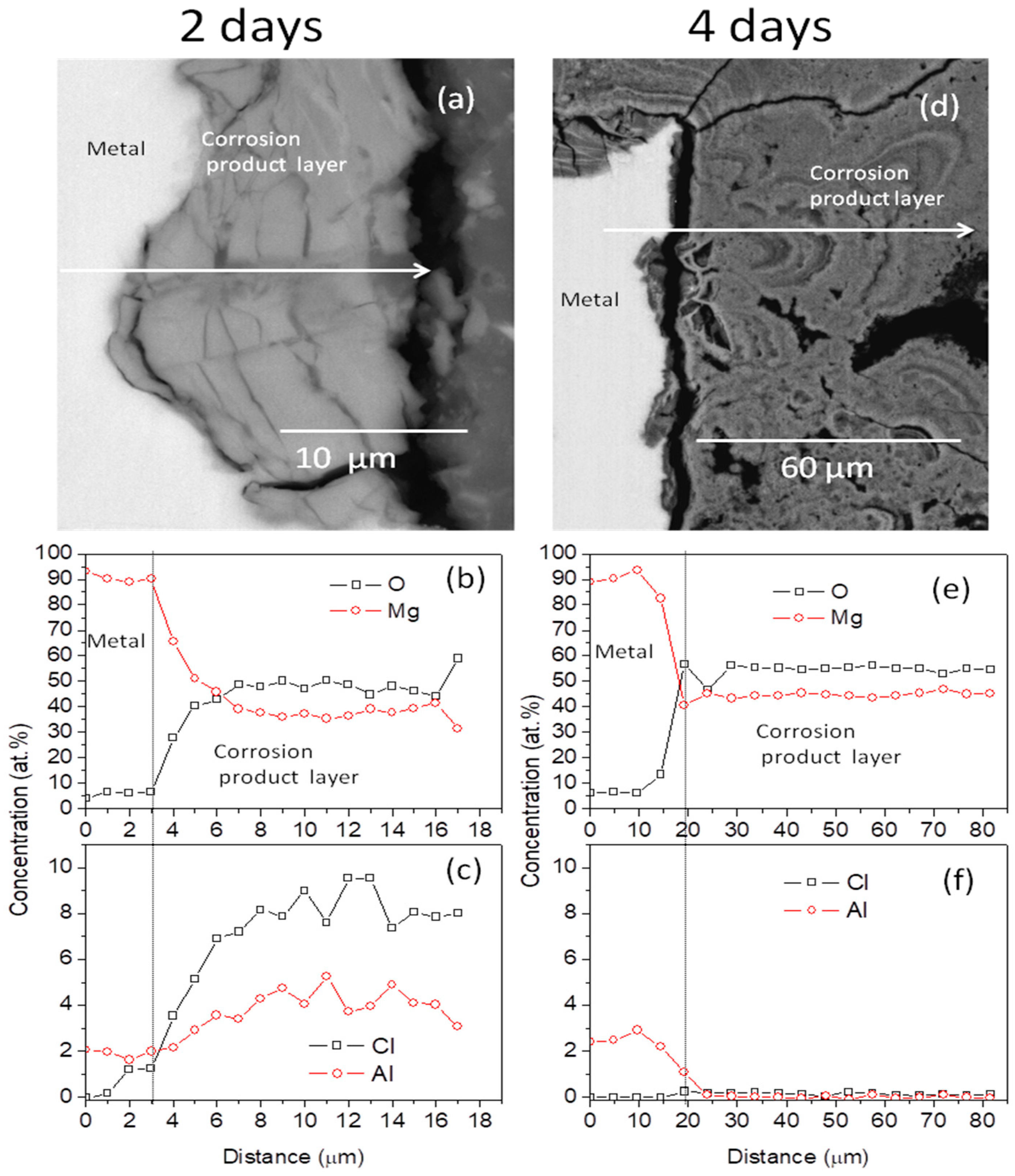
3.1. Surface Morphology and Cross-Sectional Analysis of Corrosion Layers
3.2. XRD Analysis of Corrosion Layers
3.3. Hydrogen Evolution Measurement
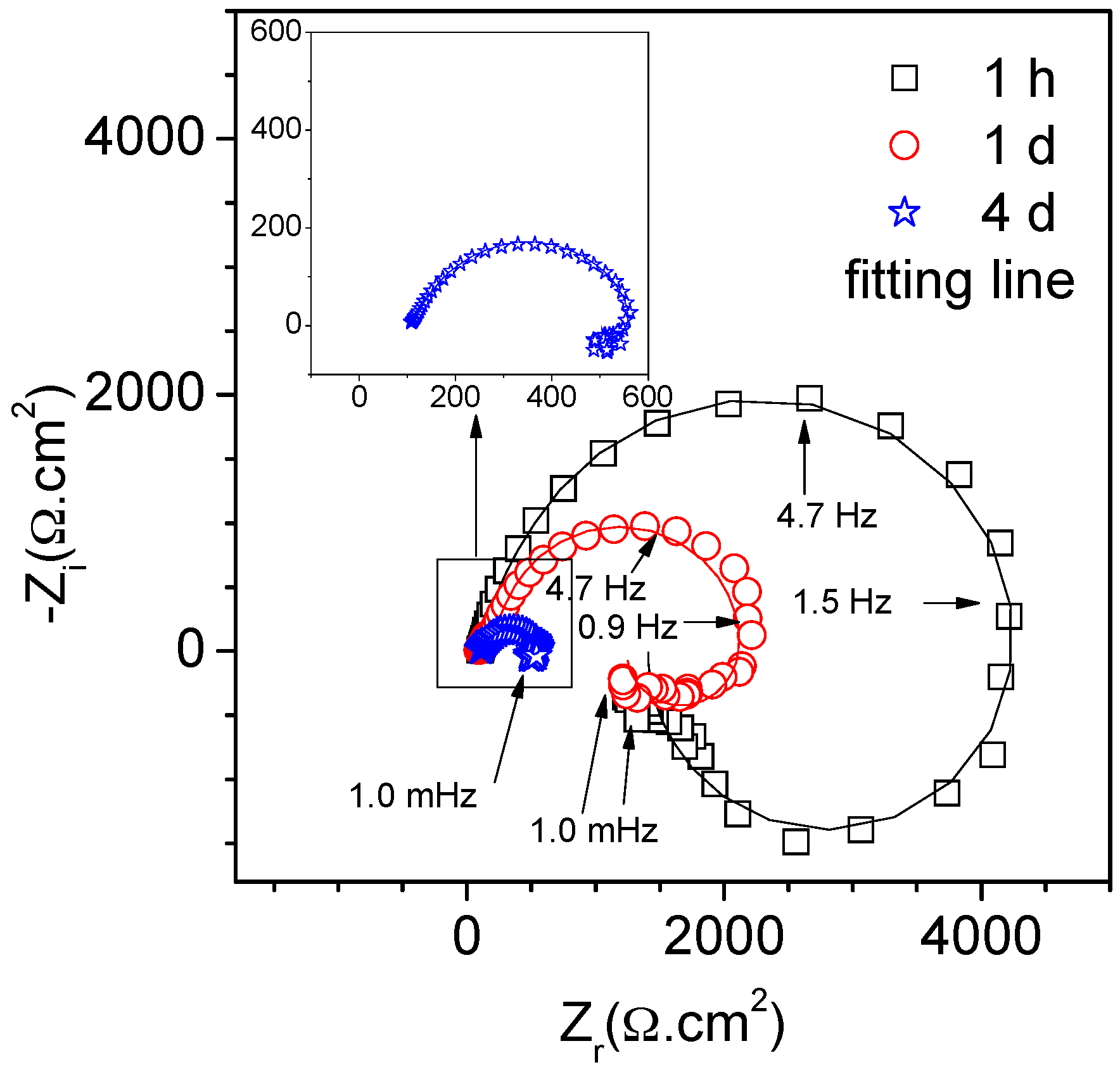
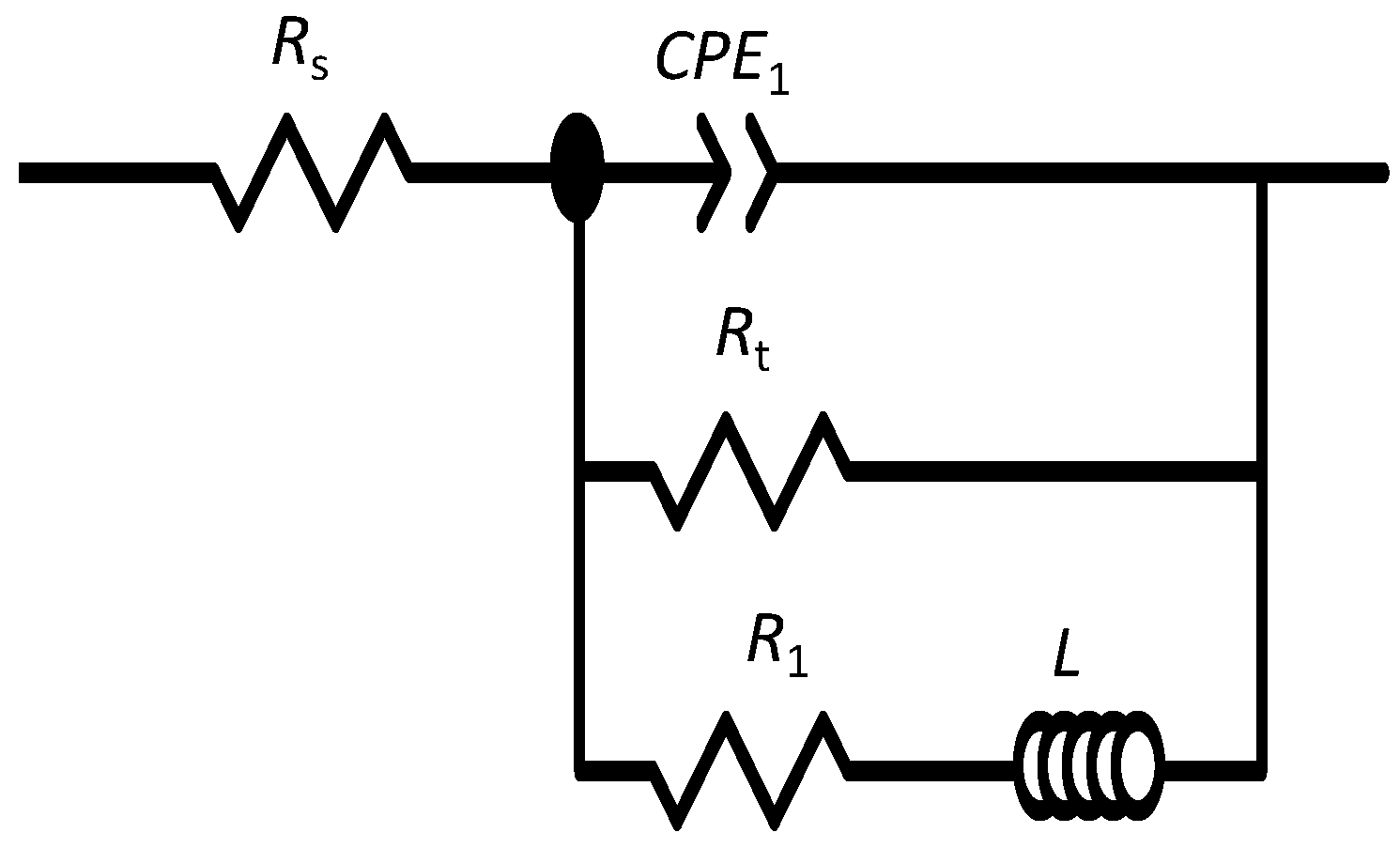
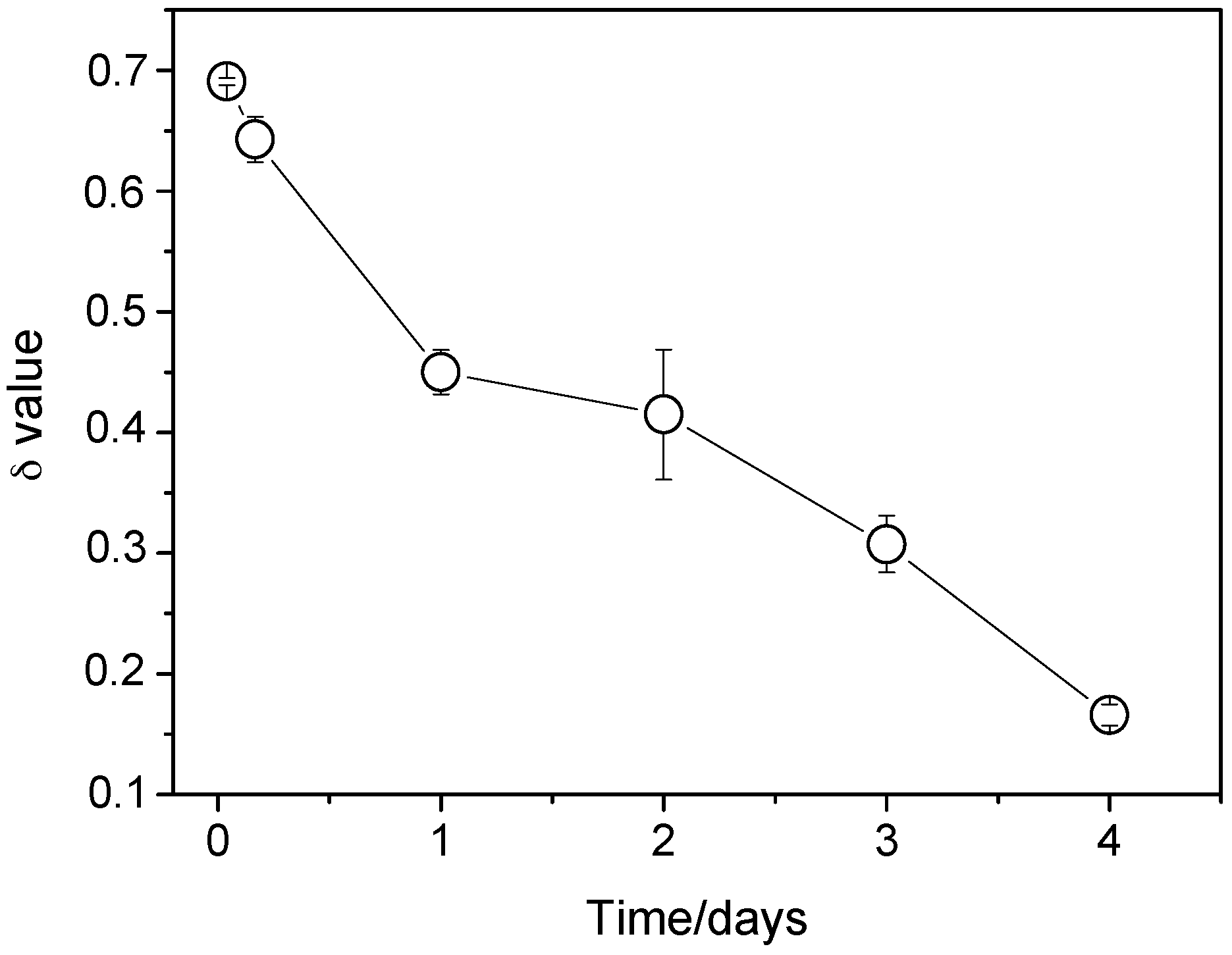
3.4. EIS Diagrams
4. Discussion
4.1. Changes in the Chemistry of the Uniform Corrosion Layer Formed on AZ31B Mg-Alloy Surface in Ringer’s Solution and Correlation with EIS Results
4.2. Correlation between Corrosion Current Densities Estimated by EIS and Hydrogen Evolution Volume
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, G.; Song, S. A possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 2, 63–72. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The influence of immersion time. Corros. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The effect of electrolyte renewal. Corros. Sci. 2015, 91, 297–310. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Chen, J.H.; Zhang, X.B.; Kwak, M.; Wu, Y.; Fan, R.; Zhang, L.; Pei, J.; Yuan, G.Y.; et al. A promising biodegradable magnesium alloy suitable for clinical vascular stent application. Sci. Rep. 2017, 7, 46343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiler, H.G.; Sigel, H.; Sigel, A. Handbook on Toxicity of Inorganic Compounds; Marcel Dekker Inc.: New York, NY, USA, 1988. [Google Scholar]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xu, L.P.; Yang, K. Formation by ion plating of Ti-coating on pure Mg for biomedical applications. Scr. Mater. 2005, 53, 523–527. [Google Scholar] [CrossRef]
- Xiong, H.Q.; Liang, Z.F.; Wang, Z.F.; Qin, C.L.; Zhao, W.M.; Yu, H. Mechanical properties and degradation behavior of Mg(100-7x)Zn6xYx(x = 0.2, 0.4, 0.6, 0.8) alloys. Metals 2018, 8, 261. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.-A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Keim, S.; Brunner, J.G.; Fabry, B.; Virtanen, S. Control of magnesium corrosion and biocompatibility with biomimetic coatings. J. Biomed. Mater. Res. B 2011, 96, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Waizy, H.; Seitz, J.M.; Reifenrath, J.; Weizbauer, A.; Bach, F.W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable magnesium implants for orthopedic applications. J. Mater. Sci. 2013, 48, 39–50. [Google Scholar] [CrossRef]
- Hiromoto, S.; Inoue, M.; Taguchi, T.; Yamane, M.; Ohtsu, N. In vitro and in vivo biocompatibility and corrosion behavior of a bioabsorbable magnesium alloy coated with octacalcium phosphate and hydroxyapatite. Acta Biomater. 2015, 11, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Gray-Munro, J.E.; Seguin, C.; Strong, M. Influence of surface modification on the in vitro corrosion rate of magnesium alloy AZ31. J. Biomed. Mater. Res. A 2009, 91, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Duygulu, O.; Kaya, R.A.; Oktay, G.; Kaya, A.A. Investigation on the potential of Magnesium alloy AZ31 as a bone implant. Mater. Sci. Forum 2007, 546, 421–424. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Chen, C.F.; Gu, Y. Advances in microarc oxidation coated AZ31 Mg alloys for biomedical applications. Corros. Sci. 2015, 91, 7–28. [Google Scholar] [CrossRef]
- Srinivasan, A.; Shin, K.S.; Rajendran, N. Influence of bicarbonate concentration on the conversion layer formation onto AZ31 magnesium alloy and its electrochemical corrosion behavior in simulated body fluid. RSC Adv. 2016, 6, 49910–49922. [Google Scholar] [CrossRef]
- Witte, F.; Abeln, I.; Switzer, E.; Kaese, V.; Meyer-Lindenberg, A.; Windhagen, H. Evaluation of the skin sensitizing potential of biodegradable magnesium alloys. J. Biomed. Mater. Res. A 2008, 86, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.C.; Yun, Y.H.; Tan, Z.Q.; Dong, Z.Y.; Schulz, M.J. In Vivo and in vitro degradation behavior of Magnesium alloys as biomaterials. J. Mater. Sci. Technol. 2012, 28, 261–267. [Google Scholar] [CrossRef]
- Wei, L.Y.; Westengen, H.; Aune, T.K.; Albright, D. Characterisation of manganese-containing intermetallic particles and corrosion behavior of die cast Mg-Al-based alloys. In Magnesium Technology 2000; Kaplan, H.L., Hryn, J.N., Eds.; The Minerals, Metals and Materials Society (TMS): Nashville, TN, USA, 2000; pp. 153–160. [Google Scholar]
- Cao, P.; StJohn, D.H.; Qian, M. The effect of manganese on the grain size of commercial AZ31 alloy. Mater. Sci. Forum 2005, 488, 139–142. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Qin, T.W.; Wang, H.M.; Zhang, Z. Comparison of corrosion behaviors of AZ31, AZ91, AM60 and ZK60 magnesium alloys. Trans. Nonferr. Met. Soc. China 2009, 19, 517–524. [Google Scholar] [CrossRef]
- Liu, F.; Song, Y.; Shan, D.; Han, E. Corrosion behavior of AZ31 magnesium alloy in simulated acid rain solution. Trans. Nonferr. Met. Soc. China 2010, 20, 638–642. [Google Scholar] [CrossRef]
- Pawar, S.; Zhou, X.; Thompson, G.E.; Scamans, G.; Fan, Z. The role of intermetallics on the corrosion initiation of twin roll cast AZ31 Mg alloy. J. Electrochem. Soc. 2015, 162, C442–C448. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Eliezer, D.; Uzan, P.; Aghion, E. Effect of second phases on the corrosion behavior of magnesium alloys. Mater. Sci. Forum 2003, 419, 857–866. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Zhang, J.; Huang, W.-J.; Dietzel, W.; Kainer, K.; Blawert, C.; Wei, K. Review of studies on corrosion of magnesium alloys. Trans. Nonferr. Met. Soc. China 2006, 16, s763–s771. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Mena-Morcillo, E.; Veleva, L.; Wipf, D.O. In situ investigation of the initial stages of AZ91D Magnesium alloy biodegradation in simulated body fluid. Int. J. Electrochem. Sci. 2018, 13, 5141–5150. [Google Scholar] [CrossRef]
- Mena-Morcillo, E.; Veleva, L.; Wipf, D.O. Multi-scale monitoring the first stages of electrochemical behavior of AZ31B magnesium alloy in simulated body fluid. J. Electrochem. Soc. 2018, 165, C749–C755. [Google Scholar] [CrossRef]
- Saikrishna, N.; Reddy, G.P.K.; Munirathinam, B.; Sunil, B.R. Influence of bimodal grain size distribution on the corrosion behavior of friction stir processed biodegradable AZ31 magnesium alloy. J. Magn. Alloys 2016, 4, 68–76. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef] [PubMed]
- King, A.D.; Birbilis, N.; Scully, J.R. Accurate electrochemical measurement of magnesium corrosion rates; a combined impedance, mass-loss and hydrogen collection study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Tkacz, J.; Minda, J.; Fintová, S.; Wasserbauer, J. Comparison of electrochemical methods for the evaluation of cast AZ91 Magnesium alloy. Materials 2016, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Scenini, F.; Curioni, M. A study on magnesium corrosion by real-time imaging and electrochemical methods: Relationship between local processes and hydrogen evolution. Electrochim. Acta 2016, 198, 174–184. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Hofstetter, J.; Uggowitzed, P.J.; Song, G.; Liu, M.; Atrens, A. Corrosion of ultra-high-purity Mg in 3.5% NaCl solution saturated with Mg(OH)2. Corros. Sci. 2013, 75, 78–99. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Sankara Narayanan, T.S.N. Corrosion behavior of commercially pure Mg and ZM21 Mg alloy in Ringer’s solution—Long term evaluation by EIS. Corros. Sci. 2011, 53, 645–654. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Wu, G.S.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Effects of zirconium and oxygen plasma ion implantation on the corrosion behavior of ZK60 Mg alloy in simulated body fluids. Corros. Sci. 2014, 82, 7–26. [Google Scholar] [CrossRef]
- Tkacz, J.; Sloukova, K.; Minda, J.; Drabikova, J.; Fintova, S.; Dolezal, P.; Wasserbauer, J. Influence of the composition of the Hank’s balanced salt solution on the corrosion behavior of AZ31 and AZ61 magnesium alloys. Metals 2017, 7, 465. [Google Scholar] [CrossRef]
- Lorenz, W.J.; Mansfeld, F. Determination of corrosion rates by electrochemical DC and AC methods. Corros. Sci. 1981, 21, 647–672. [Google Scholar] [CrossRef]
- Song, G.; Shi, Z. Corrosion mechanism and evaluation of anodized magnesium alloys. Corros. Sci. 2014, 85, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Feliu, S., Jr.; Galván, J.C.; Pardo, A.; Merino, M.C. Estimation of the corrosion rate in circumstances of difficult implementation of the common methods for electrochemical measurements. In Applied Electrochemistry (Chemistry Research and Applications) 2009; Singh, V.G., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009; pp. 387–403. [Google Scholar]
- Feliu, S., Jr.; Garcia-Galvan, F.R.; Llorente, I.; Diaz, L.; Simancas, J. Influence of hydrogen bubbles adhering to the exposed surface on the corrosion rate of magnesium alloys AZ31 and AZ61 in sodium chloride solution. Mater. Corros. 2017, 68, 651–663. [Google Scholar] [CrossRef]
- Makar, G.L.; Kruger, J. Corrosion studies of rapidly solidified Magnesium alloys. J. Electrochem. Soc. 1990, 137, 414–421. [Google Scholar] [CrossRef]
- Bland, L.G.; King, A.D.; Birbilis, N.; Scully, J.R. Assessing the corrosion of commercially pure magnesium and commercial AZ31B by electrochemical impedance, mass-loss, hydrogen collection and inductively coupled plasma optical emission spectrometry solution analysis. Corrosion 2015, 71, 128–145. [Google Scholar] [CrossRef]
- Bland, L.G.; Scully, L.C.; Scully, J.R. Assessing the corrosion of multi-phase Mg-Al alloys with high Al content by electrochemical impedance, mass loss, hydrogen collection and inductively coupled plasma optical emission spectrometry solution analysis. Corrosion 2017, 73, 526–543. [Google Scholar] [CrossRef]
- Delgado, M.C.; Garcia-Galvan, F.R.; Barranco, V.; Feliu, S., Jr. A measuring approach to assess the corrosion rate of magnesium alloys using electrochemical impedance measurements. In Magnesium Alloys; Aliofkhazraei, M., Ed.; Intech: Rijeka, Croatia, 2017; pp. 129–159. [Google Scholar]
- Liu, Y.X.; Curioni, M.; Liu, Z. Correlation between electrochemical impedance measurements and corrosion rates of Mg-1Ca alloy in simulated body fluid. Electrochim. Acta 2018, 264, 101–108. [Google Scholar] [CrossRef]
- Pebere, N.; Riera, C.; Dabosi, F. Investigation of magnesium corrosion in aerated sodium-sulfate solution by electrochemical impedance spectroscopy. Electrochim. Acta 1990, 35, 555–561. [Google Scholar] [CrossRef]
- Curioni, M.; Scenini, F.; Monetta, T.; Bellucci, F. Correlation between electrochemical impedance measurements and corrosion rate of magnesium investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2015, 166, 372–384. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Hu, T.; Chu, P.K. Degradation behavior of pure magnesium in simulated body fluids with different concentrations of HCO3−. Corros. Sci. 2011, 53, 1522–1528. [Google Scholar] [CrossRef]
- Mathieu, S.; Rapin, C.; Hazan, J.; Steinmetz, P. Corrosion behavior of high pressure die-cast and semi-solid cast AZ91D alloys. Corros. Sci. 2002, 44, 2737–2756. [Google Scholar] [CrossRef]
- Baril, G.; Blanc, C.; Keddam, M.; Pebere, N. Local electrochemical impedance spectroscopy applied to the corrosion behavior of an AZ91 magnesium alloy. J. Electrochem. Soc. 2003, 150, B488–B493. [Google Scholar] [CrossRef]
- Baril, G.; Blanc, C.; Pebere, N. AC impedance spectroscopy in characterizing time-dependent corrosion of AZ91 and AM50 magnesium alloys—Characterization with respect to their microstructures. J. Electrochem. Soc. 2001, 148, B489–B496. [Google Scholar] [CrossRef]
- Xin, Y.; Huo, K.; Tao, H.; Tang, G.; Chu, P.K. Influence of aggressive ions on the degradation behavior of biomedical magnesium alloy in physiological environment. Acta Biomater. 2008, 4, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- ISO 16428, Implants for Surgery—Test Solutions and Environmental Conditions for Static and Dynamic Corrosion Tests on Implantable Materials and Medical Devices. 2005. Available online: https://www.iso.org/standard/30280.html (accessed on 9 November 2018).
- Song, G.; Atrens, A.; Stjohn, D. An hydrogen evolution method for the estimation of the corrosion rate of Magnesium alloys. In Essential Readings in Magnesium Technology; Suveen, N.M., Alan, A.L., Neale, N.R., Eric, A.N., Wim, H.S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 565–572. [Google Scholar]
- Wang, B.; Zhang, L.W.; Su, Y.; Xiao, Y.; Liu, J. Corrosion behavior of 5A05 aluminum alloy in NaCl solution. Acta Metall. Sin. (Engl. Lett.) 2013, 26, 581–587. [Google Scholar] [CrossRef]
- Lucuta, P.G.; Halliday, J.D.; Christian, B. Phase evolution in Al2O3 fibre prepared from an oxychloride precursor. J. Mater. Sci. 1992, 27, 6053–6061. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, D.H.; Ou, X.B. Microstructures and properties of aluminum film and its effect on corrosion resistance of AZ31B substrate. Trans. Nonferr. Metals Soc. China 2008, 18, s312–s317. [Google Scholar] [CrossRef]
- Li, Z.C.; Song, G.L.; Song, S.Z. Effect of bicarbonate on biodegradation behavior of pure magnesium in a simulated body fluid. Electrochim. Acta 2014, 115, 56–65. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization-1. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Maffiotte, C.; Samaniego, A.; Galván, J.C.; Barranco, V. Effect of the chemistry and structure of the native oxide surface film on the corrosion properties of commercial AZ31 and AZ61 alloys. Appl. Surf. Sci. 2011, 257, 8558–8568. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, M.K.; Dzombak, D.A.; Vidie, R.D. Bridging gravimetric and electrochemical approaches to determine the corrosion rate of metals and metal alloys in cooling systems: Bench scale evaluation method. Ind. Eng. Chem. Res. 2010, 49, 9117–9123. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Maffiotte, C.; Samaniego, A.; Galvan, J.C.; Barranco, V. Effect of naturally formed oxide films and other variables in the early stages of Mg-alloy corrosion in NaCl solution. Electrochim. Acta 2011, 56, 4454–4565. [Google Scholar] [CrossRef]
- Beldjoudi, T.; Fiaud, C.; Robbiola, L. Influence of homogenization and artificial aging heat-treatments on corrosion behavior of Mg–Al alloys. Corrosion 1993, 49, 738–745. [Google Scholar] [CrossRef]
- Wong, K.P.; Alkire, R.C. Local chemistry and growth of single corrosion pits in aluminum. J. Electrochem. Soc. 1990, 137, 3010–3015. [Google Scholar] [CrossRef]
- Silva, F.S.D.; Bedoya, J.; Dosta, S.; Cinca, N.; Cano, I.G.; Guilemany, J.M.; Benedetti, A.V. Corrosion characteristics of cold gas spray coatings of reinforced aluminum deposited onto carbon steel. Corros. Sci. 2017, 114, 57–71. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C.; Souto, R.M. The effect of temperature on the nucleation of corrosion pits on titanium in Ringer’s physiological solution. Biomaterials 2005, 26, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, L.; Pebere, N.; Dabosi, F. An investigation of the corrosion inhibition of pure aluminum in neutral and acidic chloride solutions. Electrochim. Acta 1996, 41, 1209–1215. [Google Scholar] [CrossRef]
- Lee, E.J.; Pyun, S.I. The effect of oxide chemistry on the passivity of aluminum surfaces. Corros. Sci. 1995, 37, 157–168. [Google Scholar] [CrossRef]






| Element | Mg | Al | Zn | Mn |
|---|---|---|---|---|
| Wt% | 95.8 | 3.0 | 1.0 | 0.2 |
| Exposure Time (days) | O (at%) | Mg (at%) | Al (at%) | Cl (at%) | Zn (at%) | Cl/Al at.ratio |
|---|---|---|---|---|---|---|
| 2 | 45 ± 6 | 43 ± 8 | 3.5 ± 1 | 7.0 ± 2 | 1.5 ± 0.5 | 2.0 ± 0.4 |
| 4 | 55 ± 1 | 45 ± 1 | 0.0 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.1 |
| Immersion Time | RS (KΩ·cm2) | Q1 (sn/Ω·cm2) | n1 | Rt (KΩ·cm2) | R1 (KΩ·cm2) | L (H·cm2) | Rp (KΩ·cm2) |
|---|---|---|---|---|---|---|---|
| 1h | 0.1 | 9.1 × 10−6 | 0.92 | 4.3 | 1.9 | 3.0 | 1.3 |
| 1d | 0.1 | 2.2 × 10−5 | 0.87 | 2.2 | 2.6 | 4.2 | 1.2 |
| 4d | 0.1 | 3.8 × 10−5 | 0.78 | 0.5 | 2.2 | 0.5 | 0.4 |
| Exposure Time (days) | Mass Loss (mg·cm−2·day) | B′ Estimated Using Rt Values (mV) | B′ Estimated Using Rp Values (mV) | |||
|---|---|---|---|---|---|---|
| 4 | 3.4 | 1.24 | 3.92 × 10−3 | 317 | 6.07 × 10−3 | 204 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veleva, L.; Fernández-Olaya, M.G.; Feliu, S., Jr. Initial Stages of AZ31B Magnesium Alloy Degradation in Ringer’s Solution: Interpretation of EIS, Mass Loss, Hydrogen Evolution Data and Scanning Electron Microscopy Observations. Metals 2018, 8, 933. https://doi.org/10.3390/met8110933
Veleva L, Fernández-Olaya MG, Feliu S Jr. Initial Stages of AZ31B Magnesium Alloy Degradation in Ringer’s Solution: Interpretation of EIS, Mass Loss, Hydrogen Evolution Data and Scanning Electron Microscopy Observations. Metals. 2018; 8(11):933. https://doi.org/10.3390/met8110933
Chicago/Turabian StyleVeleva, Lucien, Mareny Guadalupe Fernández-Olaya, and Sebastián Feliu, Jr. 2018. "Initial Stages of AZ31B Magnesium Alloy Degradation in Ringer’s Solution: Interpretation of EIS, Mass Loss, Hydrogen Evolution Data and Scanning Electron Microscopy Observations" Metals 8, no. 11: 933. https://doi.org/10.3390/met8110933





