Effect of Sub-Zero Treatment Temperatures on Hardness, Flexural Strength, and Fracture Toughness of Vanadis 6 Ledeburitic Die Steel
Abstract
:1. Introduction
2. Material and Experimental Methods
2.1. Material and Processing
2.2. Experimental Methods
3. Results and Discussion
- -
- -
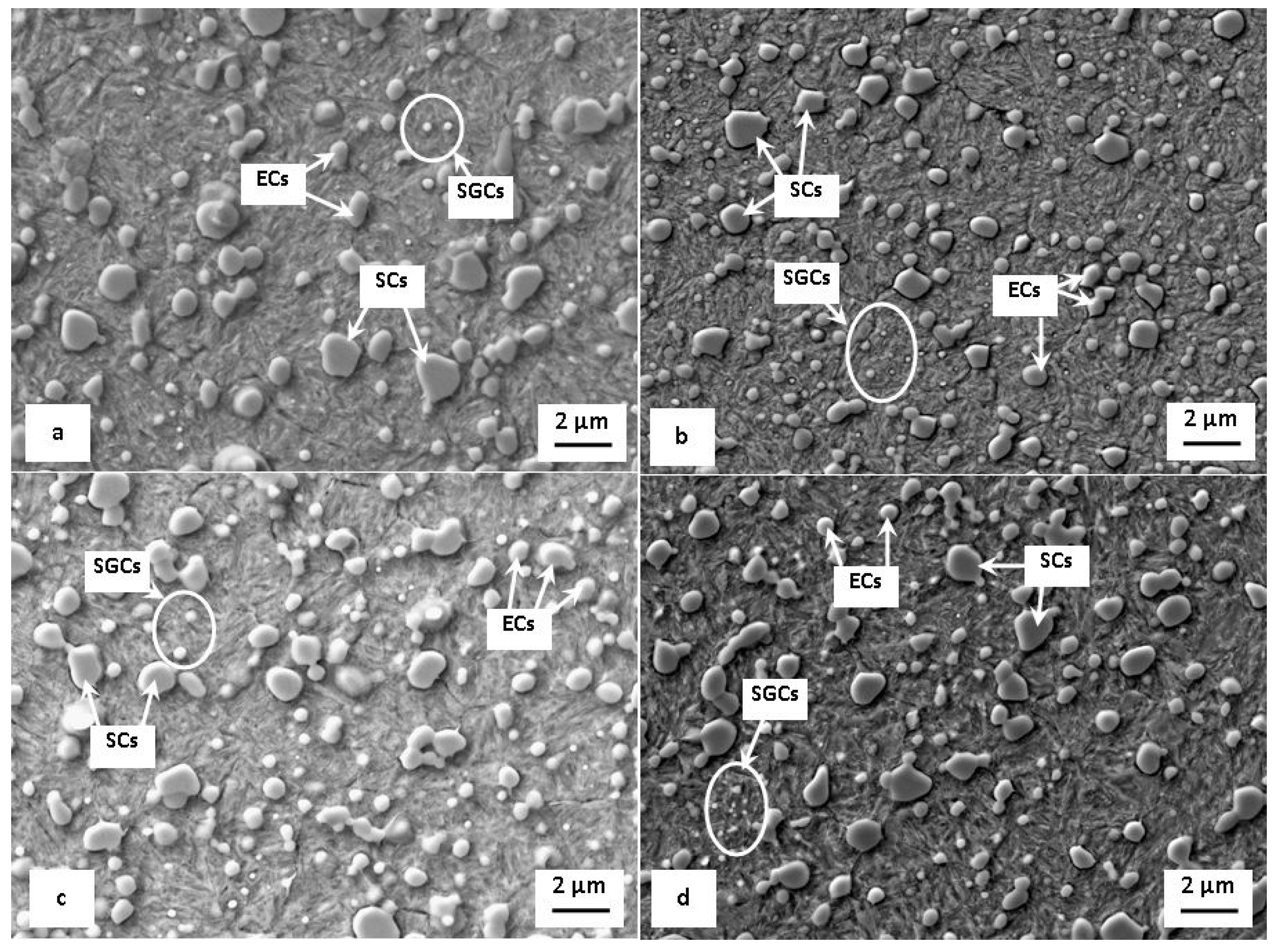
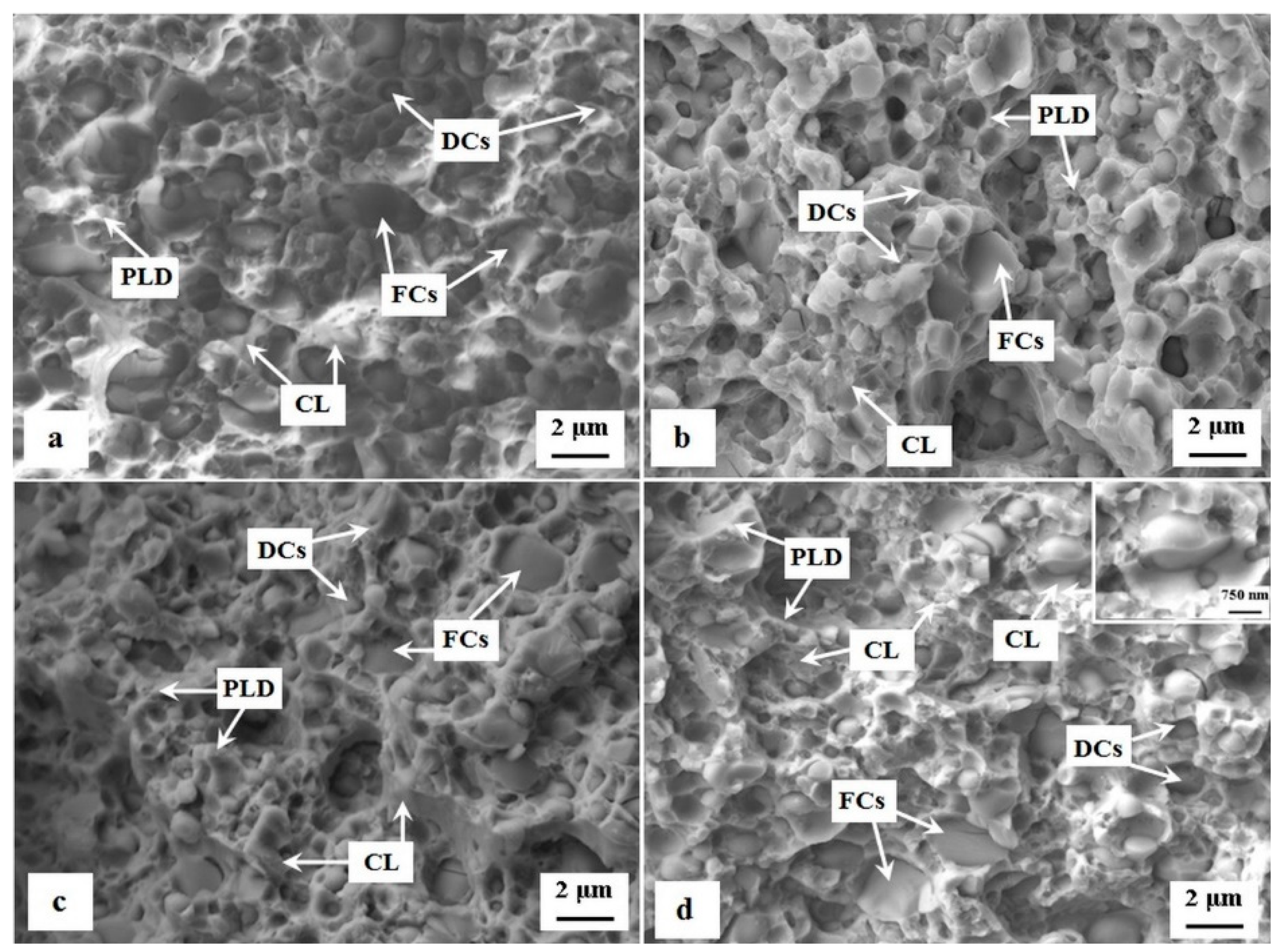
- The application of SZT produces a considerably higher amount and population density of cementite particles (of size 100–500 nm, small globular carbides (SGCs)), whereas the SZT at −140 °C acts more effectively in this way [11,14,15,18,40]. Tempering always reduces the amount of these carbides. Despite that, however, it remains much higher than what can be obtained by CHT (Figure 5). The crack propagates by a decohesive mechanism at the carbide/matrix interfaces [15,22], which is connected with micro-plastic deformation. The more carbides, the higher is the probability to form the microvoids at the interfaces, and the higher the deformation energy is that is needed for the fracture propagation.
- -
4. Conclusions
- (i)
- The hardness is generally improved by the SZTs applied; this improvement is the most pronounced for SZT at −140 °C, while other SZT conditions gave a less remarkable hardness increase. The observed improvement has been assigned to the increased volume fraction of small globular cementite carbides, because of the microstructural changes that occurred at subzero temperatures.
- (ii)
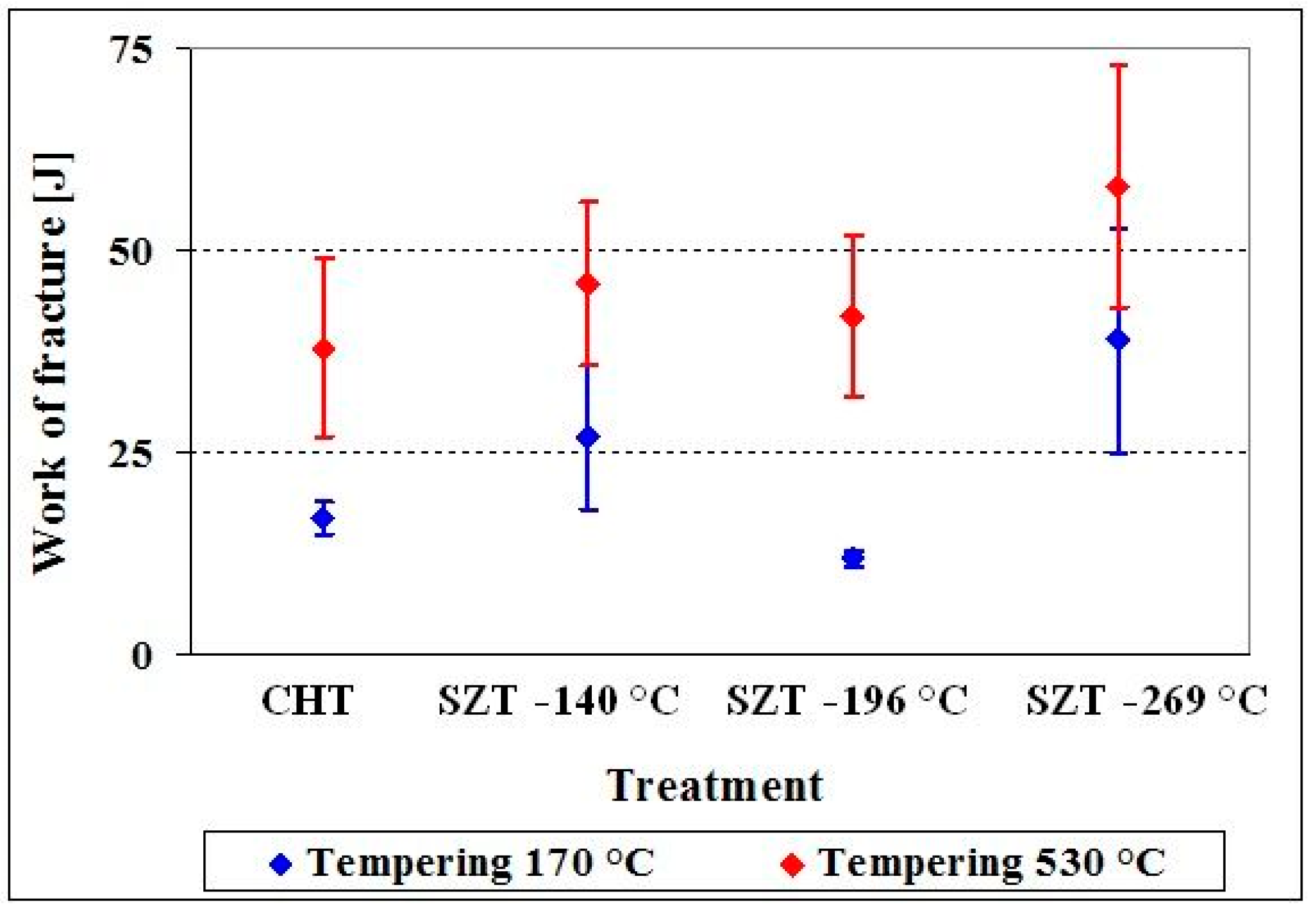
- The fracture toughness level is generally unaffected or somewhat worsened for the material after low-temperature tempering. In high-temperature tempered samples (in secondary hardening range), an improvement of the fracture toughness has been recorded. A higher number of small globular cementite particles increases the number of nucleation sites, by decohesion particles to the matrix interface boundary. On the other hand, the microstructure refinement contributes to the higher fracture resistance of the matrix. As a consequence, the potential for fracture toughness enhancement is not too high, but, under improvement of the hardness, there is no risk for a toughness decrease.
- (iii)
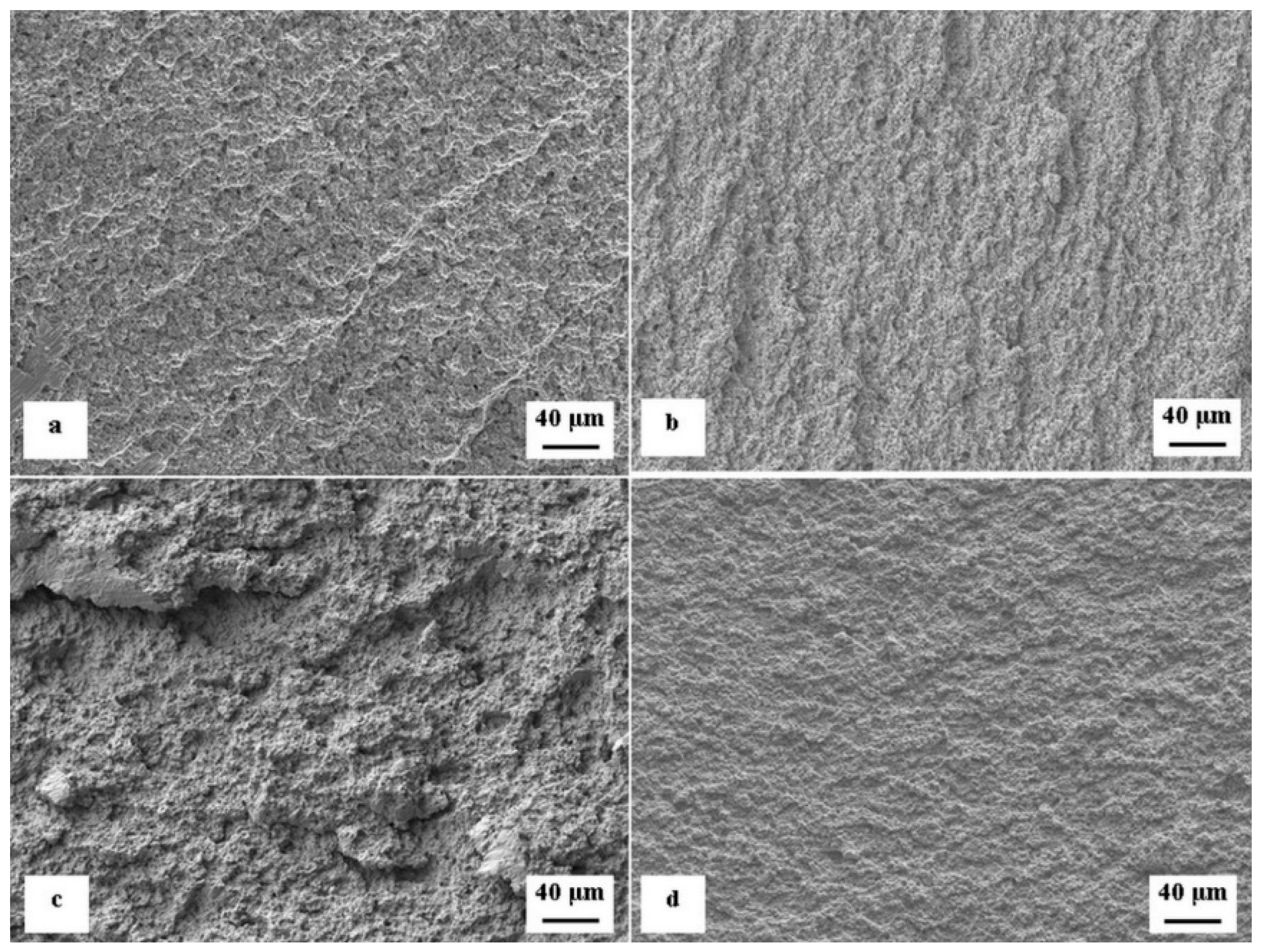
- The flexural strength is slightly improved by SZTs, except for the case of SZT in liquid nitrogen, where no effect or a slight worsening has been recorded. Although changes in the flexural strength with applied SZTs conditions are analogous to the hardness changes, there is another aspect going against the better improvement. These are associated with increased number of decohesion sites at the carbide-matrix interface. contributing to a low energy ductile fracture.
- (iv)
- The SZTs at −140 and −269 °C make it possible to obtain the simultaneous improvement of both the hardness and toughness in a much greater extent than the SZTs carried out at other treatment temperatures. However, the use of the boiling temperature of liquid helium may be associated with excessive production costs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stratton, P.F. Optimising nano-carbide precipitation in tool steels. Mater. Sci. Eng. 2007, A449–451, 809–812. [Google Scholar] [CrossRef]
- Reitz, W.; Pendray, J. Cryoprocessing of Materials: A Review of Current Status. Mater. Manuf. Proc. 2001, 16, 829–840. [Google Scholar] [CrossRef]
- Sweeney, T.P. Deep cryogenics: the great cold debate. J. Heat. Treat. 1986, 2, 28–33. [Google Scholar]
- Yugandhar, T.; Krishnan, P.K.; Bhaskar Rao, C.V.; Kalidas, R. Cryogenic Treatment and It’s Effect on Tool Steel. In Proceedings of the 6th International Tooling Conference, Karlstad, Sweden, 10–13 September 2002; Bergstrom, J., Fredriksson, G., Johansson, M., Kotik, O., Thuvander, F., Eds.; Karlstad University: Karlstad, Sweden, 2012; pp. 671–684. [Google Scholar]
- Das, D.; Dutta, A.K.; Ray, K.K. On the enhancement of wear resistance of tool steels by cryogenic treatment. Philos. Mag. Lett. 2008, 88, 801–811. [Google Scholar] [CrossRef]
- Das, D.; Ray, K.K. Structure-property correlation of sub-zero treated AISI D2 steel. Mater. Sci. Eng. A 2012, 541, 45–60. [Google Scholar] [CrossRef]
- Akhbarizadeh, A.; Golozar, M.A.; Shafeie, A.; Kholghy, M. Effects of austenitizing time on wear behaviour of D6 tool steel after deep cryogenic treatment. J. Iron Steel Res. 2009, 16, 29–32. [Google Scholar] [CrossRef]
- Collins, D.N. Deep cryogenic treatment of tool steels—A review. Heat Treat. Met. 1996, 23, 40–42. [Google Scholar]
- Das, D.; Dutta, A.K.; Ray, K.K. Sub-zero treatments of AISI D2 steel: Part I. Microstructure and hardness. Mater. Sci. Eng. A 2010, 527, 2182–2193. [Google Scholar] [CrossRef]
- Jurči, P.; Kusý, M.; Ptačinová, J.; Kuracina, V.; Priknerová, P. Long-term Sub-zero Treatment of P/M Vanadis 6 Ledeburitic Tool Steel—A Preliminary Study. Manuf. Technol. 2015, 15, 41–47. [Google Scholar]
- Jurči, P.; Dománková, M.; Čaplovič, L.; Ptačinová, J.; Sobotová, J.; Salabová, P.; Prikner, O.; Šuštaršič, B.; Jenko, D. Microstructure and hardness of sub-zero treated and no tempered P/M Vanadis 6 ledeburitic tool steel. Vacuum 2015, 111, 92–101. [Google Scholar] [CrossRef]
- Tyshchenko, A.I.; Theisen, W.; Oppenkowski, A.; Siebert, S.; Razumov, O.N.; Skoblik, A.P.; Sirosh, V.A.; Petrov, J.N.; Gavriljuk, V.G. Low-temperature martensitic transformation and deep cryogenic treatment of a tool steel. Mater. Sci. Eng. A 2010, 527, 7027–7039. [Google Scholar] [CrossRef]
- Meng, F.; Tagashira, K.; Azuma, R.; Sohma, H. Role of Eta-carbide Precipitation’s in the Wear Resistance Improvements of Fe-12Cr-Mo-V-1.4C Tool Steel by Cryogenic Treatment. ISIJ Int. 1994, 34, 205–210. [Google Scholar] [CrossRef]
- Jurči, P.; Dománková, M.; Hudáková, M.; Ptačinová, J.; Pašák, M.; Palček, P. Characterization of microstructure and tempering response of conventionally quenched, short- and long-time sub-zero treated PM Vanadis 6 ledeburitic tool steel. Mater. Charact. 2017, 134, 398–415. [Google Scholar] [CrossRef]
- Sobotová, J.; Jurči, P.; Dlouhý, I. The effect of sub-zero treatment on microstructure, fracture toughness, and wear resistance of Vanadis 6 tool steel. Mater. Sci. Eng. A 2016, 652, 192–204. [Google Scholar] [CrossRef]
- Collins, D.N.; Dormer, J. Deep Cryogenic Treatment of a D2 Cold-Work Tool Steel. Heat Treat. Met. 1997, 24, 71–74. [Google Scholar]
- Das, D.; Sarkar, R.; Dutta, A.K.; Ray, K.K. Influence of sub-zero treatments on fracture toughness of AISI D2 steel. Mater. Sci. Eng. A 2010, 528, 589–603. [Google Scholar] [CrossRef]
- Jurči, P.; Dománková, M.; Ptačinová, J.; Pašák, M.; Kusý, M.; Priknerová, P. Investigation of the Microstructural Changes and Hardness Variations of Sub-Zero Treated Cr-V Ledeburitic Tool Steel Due to the Tempering Treatment. J. Mater. Eng. Perform. 2018, 27, 1514–1529. [Google Scholar] [CrossRef]
- Surberg, C.H.; Stratton, P.; Lingenhole, K. The effect of some heat treatment parameters on the dimensional stability of AISI D2. Cryogenics 2008, 48, 42–47. [Google Scholar] [CrossRef]
- Molinari, A.; Pellizzari, M.; Gialanella, S.; Straffelini, G.; Stiasny, K.H. Effect of deep cryogenic treatment on mechanical properties of tool steels. J. Mater. Proc. Technol. 2001, 118, 350–355. [Google Scholar] [CrossRef]
- Rhyim, Y.M.; Han, S.H.; Na, Y.S.; Lee, J.H. Effect of deep cryogenic treatment on carbide precipitation and mechanical properties of tool steel. Solid State Phenom. 2006, 118, 9–14. [Google Scholar] [CrossRef]
- Ptačinová, J.; Sedlická, V.; Hudáková, M.; Dlouhý, I.; Jurči, P. Microstructure—Toughness relationships in sub-zero treated and tempered Vanadis 6 steel compared to conventional treatment. Mater. Sci. Eng. A 2017, 702, 241–258. [Google Scholar] [CrossRef]
- Ptačinová, J.; Jurči, P.; Dlouhý, I. Fracture toughness of ledeburitic Vanadis 6 steel after sub-zero treatment for 17 H and double tempering. Mater. Technol. 2017, 51, 729–733. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Theisen, W.; Sirosh, V.V.; Polshin, E.V.; Kortmann, A.; Mogilny, G.S.; Petrov, Yu.N.; Tarusin, Y.V. Low-temperature martensitic transformation in tool steels in relation to their deep cryogenic treatment. Acta Mater. 2013, 61, 1705–1715. [Google Scholar] [CrossRef]
- Nemec, M.; Jurči, P.; Kosnáčová, P.; Kučerová, M. Evaluation of structural isotropy of Cr-V ledeburitic steel made by powder metallurgy of rapidly solidified particles. Kovove Mater. 2016, 54, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Jurči, P.; Dlouhý, I. Fracture Behaviour of P/M Cr-V Ledeburitic Steel with Different Surface Roughness. Mater. Eng.-Materiálové inžinierstvo 2011, 18, 36–43. [Google Scholar]
- ISO 12135. Metallic Materials-Unified Method of Test for the Determination of Quasistatic Fracture Toughness. Available online: https://www.iso.org/standard/60891.html (accessed on 15 November 2016).
- Jurči, P. Cr-V Ledeburitic cold-work tool steels. Mater. Technol. 2011, 45, 383–394. [Google Scholar]
- ASTM E975-13. Standard Practice for X-ray Determination of Retained Austenite in Steel with Near Random Crystallographic Orientation; ASTM Book of Standards: West Conshohocken, PA, USA, 2004; Volume 3.01. [Google Scholar]
- Bílek, P.; Sobotová, J.; Jurči, P. Evaluation of the microstructural changes in Cr-V ledeburitic tool steels depending on the austenitization temperature. Mater. Technol. 2011, 44, 489–493. [Google Scholar]
- Pirtovšek, T.V.; Kugler, G.; Terčelj, M. The behaviour of the carbides of ledeburitic AISI D2 tool steel during multiple hot deformation cycles. Mater. Charact. 2013, 83, 97–108. [Google Scholar] [CrossRef]
- Mostafari, S.; Fotouhi, M.; Motasemi, A.; Ahmadi, M.; Sindi, C.T. Acoustic emission methodology to evaluate the fracture toughness in heat treated AISI D2 tool steel. J. Mater. Eng. Perform. 2012, 21, 2106–2116. [Google Scholar] [CrossRef]
- Wang, J.; Guo, W.; Sun, H.; Li, H.; Gou, H.; Zhang, J. Plastic deformation behaviors and hardening mechanism of M7C3 carbide. Mater. Sci. Eng. A 2016, 662, 88–94. [Google Scholar] [CrossRef]
- Fukaura, K.; Yokoyama, Y.; Yokoi, D.; Tsuji, N.; Ono, K. Fatigue of cold-work tool steels: effect of heat treatment and carbide morphology on fatigue crack formation, life, and fracture surface observations. Metall. Mater. Trans. A 2004, 35, 1289–1300. [Google Scholar] [CrossRef]
- Sohar, C.R.; Betzwar-Kotas, A.; Gierl, C.; Weiss, B.; Danninger, H. Fractographic evaluation of gigacycle fatigue crack nucleation and propagation of a high Cr alloyed cold work tool steel. Int. J. Fatigue 2008, 30, 2191–2199. [Google Scholar] [CrossRef]
- Casellas, D.; Caro, J.; Molas, S.; Prado, J.M.; Valls, I. Fracture toughness of carbides in tool steels evaluated by nanoindentation. Acta Mater. 2007, 55, 4277–4286. [Google Scholar] [CrossRef]
- Lin, C.M.; Chang, C.M.; Chen, J.-H.; Wu, W. Hardness, toughness and cracking systems of primary (Cr, Fe)23C6 and (Cr, Fe)7C3 carbides in high-carbon Cr-based alloys by indentation. Mater. Sci. Eng. A 2010, 527, 5038–5043. [Google Scholar] [CrossRef]
- Hansen, N. Hall-Petch relation and boundary strengthening. Scr. Mater. 2004, 51, 801–806. [Google Scholar] [CrossRef]
- Jurči, P.; Ptačinová, J.; Sahul, M.; Dománková, M.; Dlouhý, I. Metallurgical principles of microstructure formation in sub-zero treated cold-work tool steels—A review. Matériaux Tech. 2018, 106, 104–112. [Google Scholar] [CrossRef]
- Ďurica, J.; Ptačinová, J.; Hudáková, M.; Kusý, M.; Jurči, P. Microstructure and Hardness of Cold Work Vanadis 6 Steel after Subzero Treatment at −140 degrees C. Adv. Mater. Sci. Eng. 2018, 2018, 6537509. [Google Scholar] [CrossRef]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurči, P.; Dlouhý, I.; Priknerová, P.; Mrštný, Z. Effect of Sub-Zero Treatment Temperatures on Hardness, Flexural Strength, and Fracture Toughness of Vanadis 6 Ledeburitic Die Steel. Metals 2018, 8, 1047. https://doi.org/10.3390/met8121047
Jurči P, Dlouhý I, Priknerová P, Mrštný Z. Effect of Sub-Zero Treatment Temperatures on Hardness, Flexural Strength, and Fracture Toughness of Vanadis 6 Ledeburitic Die Steel. Metals. 2018; 8(12):1047. https://doi.org/10.3390/met8121047
Chicago/Turabian StyleJurči, Peter, Ivo Dlouhý, Petra Priknerová, and Zdeněk Mrštný. 2018. "Effect of Sub-Zero Treatment Temperatures on Hardness, Flexural Strength, and Fracture Toughness of Vanadis 6 Ledeburitic Die Steel" Metals 8, no. 12: 1047. https://doi.org/10.3390/met8121047




