Study of the Effect of Carbon on the Contraction of Hypo-Peritectic Steels during Initial Solidification by Surface Roughness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
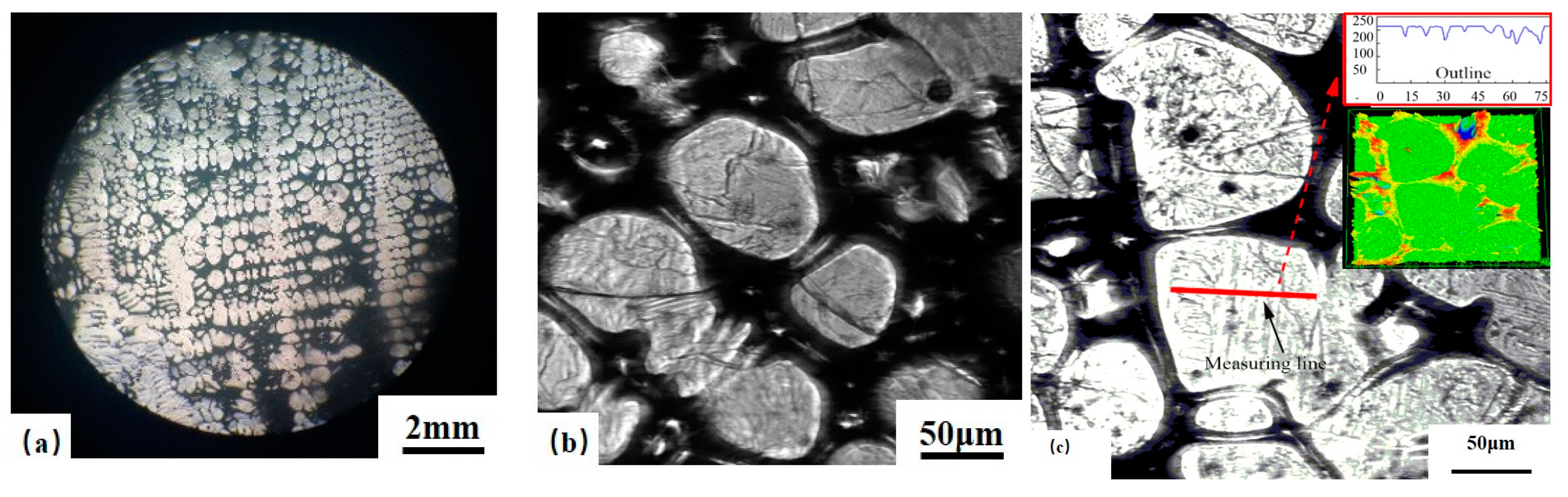
2.2. Confocal Scanning Laser Microscope (CSLM)
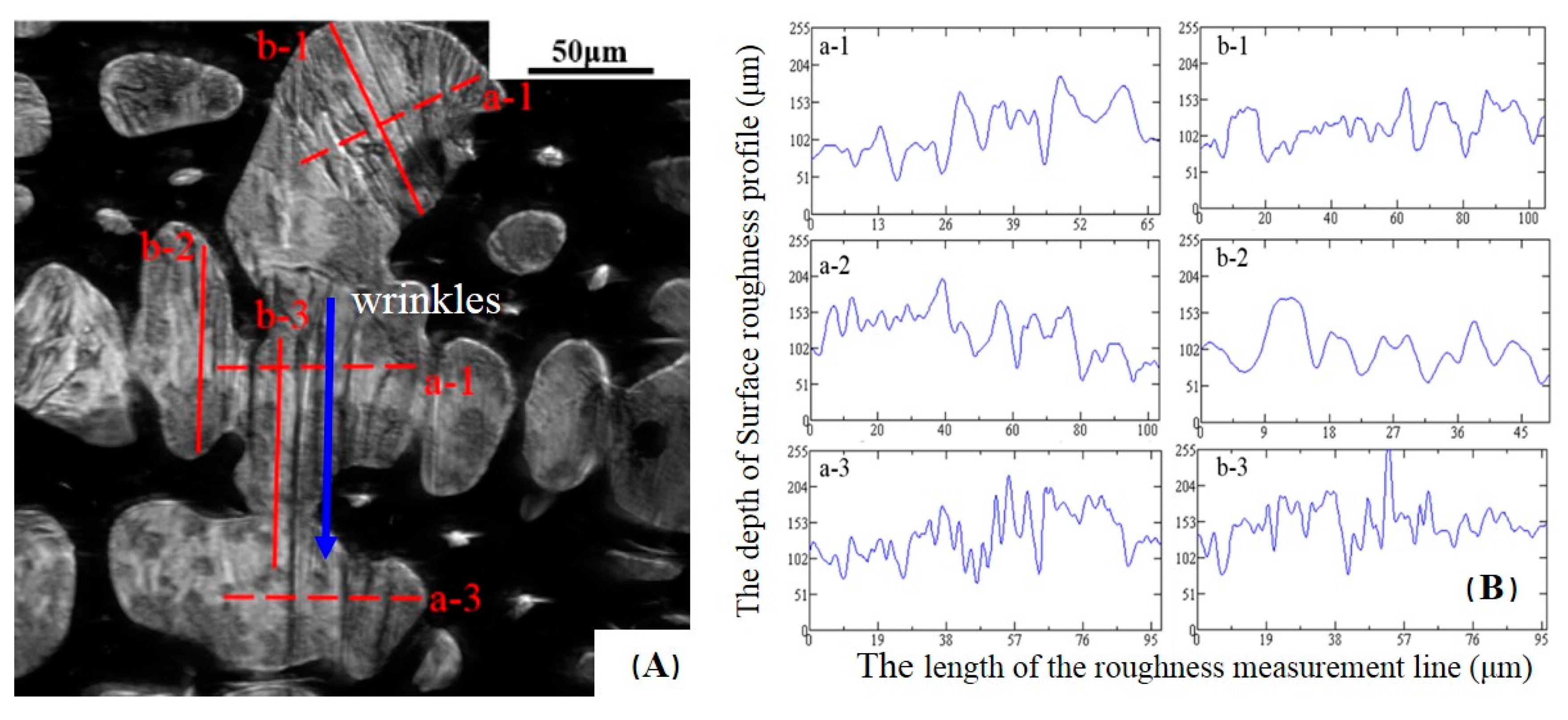
2.3. Measurement of Surface Roughness Ra(δ/γ)
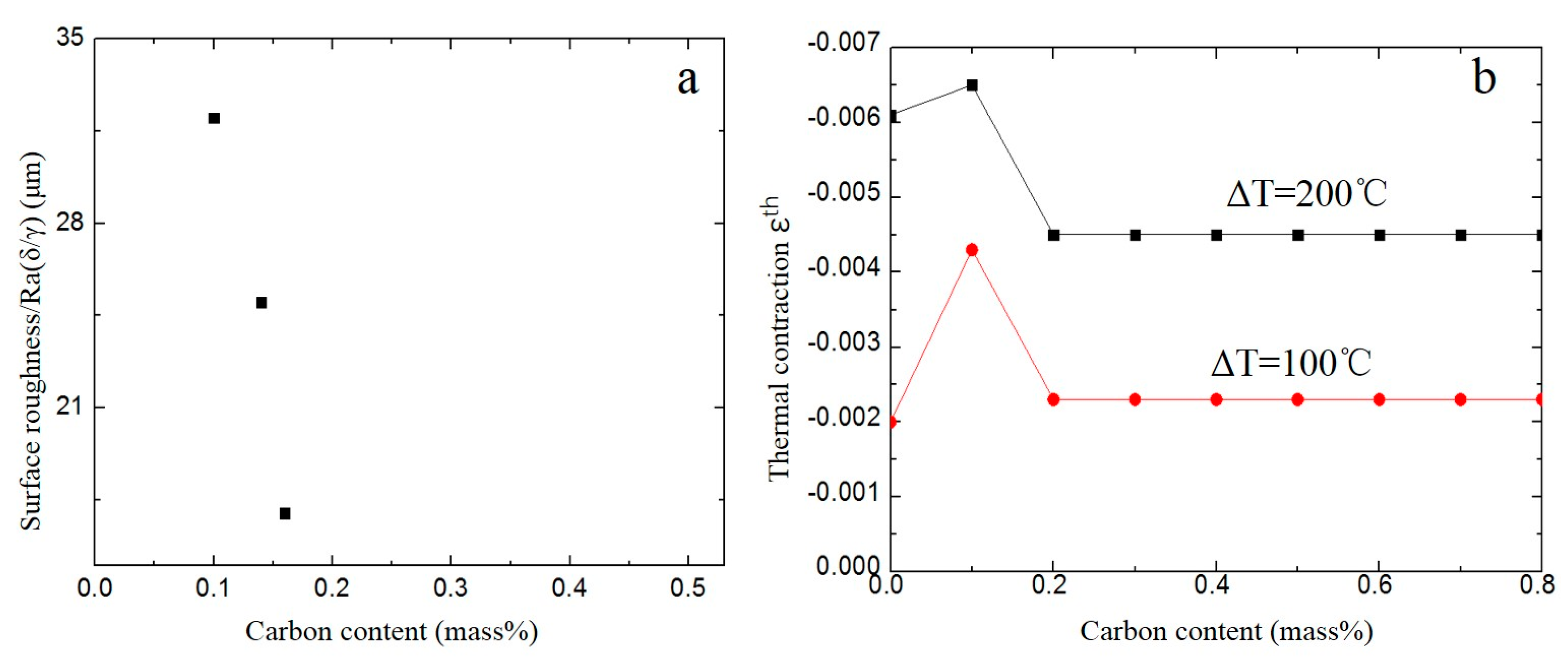
3. Results and Discussion
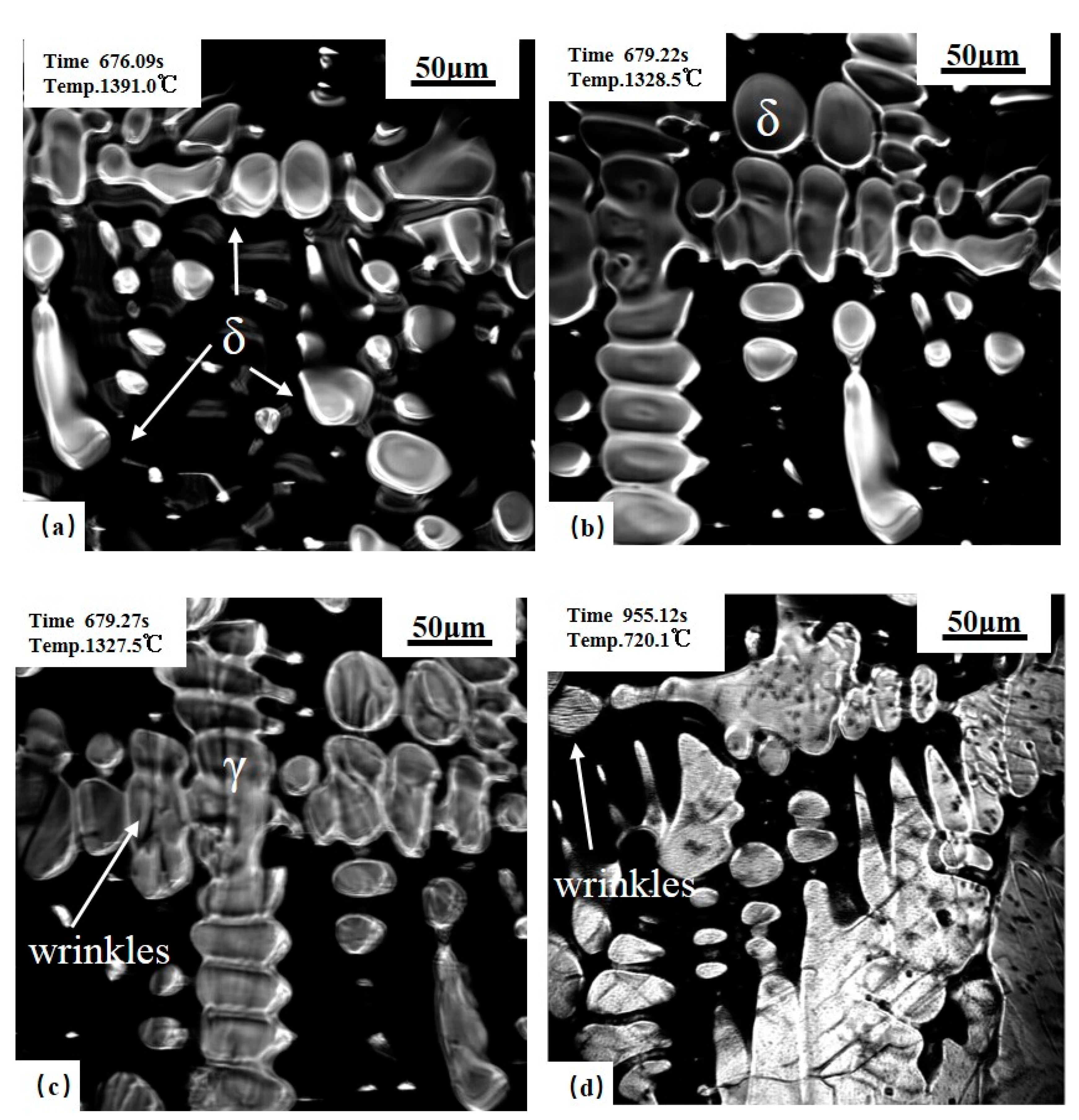
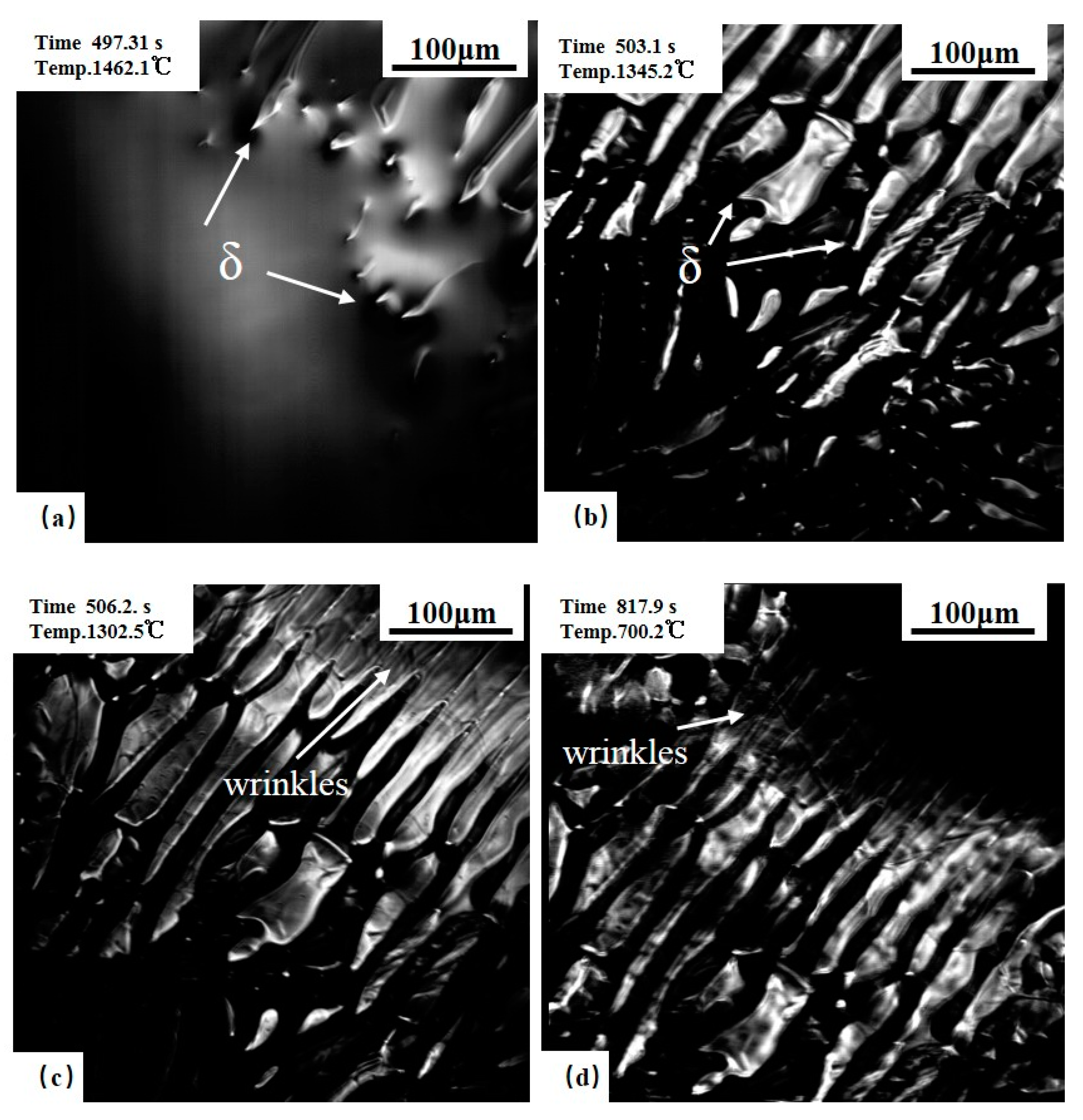
3.1. Solidification Process of Steels during Cooling
3.1.1. 0.10C% Hypo-Peritectic Steel
3.1.2. 0.14C% Hypo-Peritectic Steel
3.1.3. 0.16C% Hypo-Peritectic Steel
3.2. Surface Roughness Ra(δ/γ)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, G.; Bernhard, C.; Ilie, S.; Fuerst, C. A study about the influence of carbon content in the steel on the casting behavior. Steel Res. Int. 2011, 82, 230–236. [Google Scholar] [CrossRef]
- Andrés, C.G.; Caballero, F.G.; Capdevila, C.; Álvarez, L.F. Application of dilatometric analysis to the study of solid–solid phase transformations in steels. Mater. Charact. 2002, 48, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Yamaoka, Y. Influence of carbon content on solidifying shell growth of carbon steels at the initial stage of solidification. Mater. Trans. 2003, 44, 836–844. [Google Scholar] [CrossRef]
- Konishi, J.; Militzer, M.; Samarasekera, I. Modeling the formation of longitudinal facial cracks during continuous casting of hypoperitectic steel. Metall. Mater. Trans. B 2002, 33, 413–423. [Google Scholar] [CrossRef]
- Du, F.; Wang, X.; Yu, G.; Yan, Z.; Zhu, X.; Xu, J. Study on the non-uniform slab shrinkage of special steel during slab continuous casting. Ironmak. Steelmak. 2017, 41, 1–6. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Shen, M. Numerical analysis of the influence of carbon content on the initial shell of continuous casting slab. Ironmak. Steelmak. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Tsal, H.T.; Yin, H.; Lowry, M. Analysis of transverse corner cracks on slabs and countermeasures. Iron Steel Technol. 2006, 7, 23–31. [Google Scholar]
- Pradhan, N.; Banerjee, N.; Reddy, B.B.; Sahay, S.K.; Viswanathan, C.S.; Bhor, P.K.; Basu, S.D.; Mazumdar, S. Control of transverse cracking in special quality slabs. Ironmak. Steelmak. 2001, 28, 305–311. [Google Scholar] [CrossRef]
- Emi, T.; Fredriksson, H. High-speed continuous casting of peritectic carbon steels. Mater. Sci. Eng. 2005, 413–414, 2–9. [Google Scholar] [CrossRef]
- Kim, K.; Yeo, T.J.; Oh, K.H.; Lee, D.N. Effect of carbon and sulfur in continuously cast strand on longitudinal surface cracks. ISIJ Int. 1996, 36, 284–289. [Google Scholar] [CrossRef]
- Jablonka, A.; Harste, K.; Schwerdtfeger, K. Thermomechanical properties of iron and iron-carbon alloys: Density and thermal contraction. Steel Res. 1991, 62, 24–33. [Google Scholar] [CrossRef]
- Mizukami, H.; Yamanaka, A.; Watanabe, T. High Temperature Deformation Behavior of Peritectic Carbon Steel during Solidification. Trans. Iron Steel Inst. Jpn. 2002, 42, 964–973. [Google Scholar] [CrossRef]
- Dong, S.X.; Niyama, E.; Anzai, K. Free deformation of initial solid shell of Fe-C alloys. ISIJ Int. 1995, 35, 730–736. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, J.; Cheng, C.; Zeng, Z. Deformation and structure difference of steel droplets during initial solidification. High Temp. Mater. Process. 2017, 36, 347–357. [Google Scholar] [CrossRef]
- Wielgosz, E.; Kargul, T. Differential scanning calorimetry study of peritectic steel grades. J. Therm. Anal. Calorim. 2015, 119, 1547–1553. [Google Scholar] [CrossRef]
- Bernhard, C.; Xia, G. Influence of alloying elements on the thermal contraction of peritectic steels during initial solidification. Ironmak. Steelmak. 2006, 33, 52–56. [Google Scholar] [CrossRef]
- Shibata, H.; Arai, Y.; Suzuki, M.; Emi, T. Kinetics of peritectic reaction and transformationin Fe-C alloys. Metall. Mater. Trans. B 2000, 31, 981–991. [Google Scholar] [CrossRef]
- Dippenaar, R. In-situ analysis of the peritectic phase transition-relevant to the continuous casting of steel. In Proceedings of the 5th International Congress on the Science and Technology of the Steelmaking, Dresden, Germany, 1–3 October 2012. [Google Scholar]
- Griesser, S.; Bernhard, C.; Dippenaar, R. Effect of nucleation undercooling on the kinetics and mechanism of the peritectic phase transition in steel. Acta Mater. 2014, 81, 111–120. [Google Scholar] [CrossRef]
- Guo, J.; Wen, G.; Pu, D.; Tang, P. A Novel Approach for Evaluating the Contraction of Hypo-Peritectic Steels during Initial Solidification by Surface Roughness. Materials 2018, 11, 571. [Google Scholar] [Green Version]
- Yonekura MFG. HT-CSLM, Confocal Scanning Laser Microscope. Available online: http://yonekuramfg.wixsite.com/ht-cslm/untitled-cv4z (accessed on 30 January 2018).
- Yuan, C. Numerical characterization of 3D Worn surface topography using confocal laser scanning microscopy. Lubr. Eng. 2006, 728, 33–36. [Google Scholar]
- Hanlon, D.N.; Todd, I.; Peekstok, E.; Rainforth, W.M.; van der Zwaag, S. The application of laser scanning confocal microscopy to tribological research. Wear 2001, 251, 1159–1168. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Nagasaka, T.; Hino, M. Surface Roughness of Solidified Mold Flux in Continuous Casting Process. Trans. Iron Steel Inst. Jpn. 1999, 39, 1150–1159. [Google Scholar] [CrossRef]
- Dobashi, Y.; Ijiri, T.; Todo, H.; Iwasaki, K.; Okabe, M.; Nishimura, S. Measuring microstructures using confocal laser scanning microscopy for estimating surface roughness. Presented at the ACM Siggraph 2016, Anaheim, CA, USA, 24–28 July 2016; p. 28. [Google Scholar]
- Trejo, M.H.; Lopez, E.A.; Mondragon, J.J.R.; de Jesus Castro Roman, M.; Tovar, H.S. Effect of solidification path and contraction on the cracking susceptibility of carbon peritectic steels. Met. Mater. Int. 2010, 16, 731–737. [Google Scholar] [CrossRef]
- Cao, L.F.; Guang, X.; Peng, D.; Wang, G.X.; Hu, D.J. Study on thermal expansion properties of steels. J. Univ. Sci. Technol. Beijing 2014, 5, 639–643. [Google Scholar]
- Liu, J.; Wen, G.; Li, Y. Effect of γ→α Phase Transformation on Refining Austenite Grains of Microalloyed Steel in Continuous Casting by Simulation. High Temp. Mater. Process. 2016, 35, 653–659. [Google Scholar] [CrossRef]
- Park, J.Y.; Sohn, I. Evaluating the heat transfer phenomena and the interfacial thermal resistance of mold flux using a copper disc mold simulator. Int. J. Heat Mass Transf. 2017, 109, 1014–1025. [Google Scholar] [CrossRef]
- Long, X.; He, S.; Wang, Q.; Chris Pistorius, P. Structure of Solidified Films of Mold Flux for Peritectic Steel. Metall. Mater. Trans. B 2017, 48, 1652–1658. [Google Scholar] [CrossRef]
- Abe, T.; Nagaki, S.; Nishiyama, T. Inhomegeneous deformation of grains and roughening of free surface of polycrystalline iron during compressive plastic deformation. Mech. Behav. Mater. V 1988, 325–332. [Google Scholar]



| Steel | Chemical Composition (mass%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | Al | S | P | Ni | Cr | Ti | Fe | |
| S1 | 0.10 | 0.250 | 1.22 | 0.049 | 0.001 | 0.011 | 0.120 | 0.179 | 0.017 | bal. |
| S2 | 0.14 | 0.250 | 1.25 | 0.035 | 0.002 | 0.015 | 0.116 | 0.014 | bal. | |
| S3 | 0.16 | 0.250 | 1.17 | 0.033 | 0.002 | 0.012 | 0.120 | 0.120 | 0.016 | bal. |
| Line | Ra (μm) | Rq (μm) | Rp (μm) | Rv (μm) |
|---|---|---|---|---|
| a-1 | 39.3 | 46.1 | 156.6 | 71.5 |
| a-2 | 38.4 | 45.7 | 150.0 | 70.1 |
| a-3 | 40.8 | 46.5 | 158.8 | 71.2 |
| Average value of Ra is 39.5 μm | ||||
| b-1 | 32.6 | 37.7 | 80.2 | 62.0 |
| b-2 | 31.3 | 37.0 | 78.6 | 60.1 |
| b-3 | 32.1 | 37.5 | 78.8 | 61.3 |
| Average value of Ra is 32.0 μm | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, D.; Wen, G.; Fu, D.; Tang, P.; Guo, J. Study of the Effect of Carbon on the Contraction of Hypo-Peritectic Steels during Initial Solidification by Surface Roughness. Metals 2018, 8, 982. https://doi.org/10.3390/met8120982
Pu D, Wen G, Fu D, Tang P, Guo J. Study of the Effect of Carbon on the Contraction of Hypo-Peritectic Steels during Initial Solidification by Surface Roughness. Metals. 2018; 8(12):982. https://doi.org/10.3390/met8120982
Chicago/Turabian StylePu, Dazhi, Guanghua Wen, Dachao Fu, Ping Tang, and Junli Guo. 2018. "Study of the Effect of Carbon on the Contraction of Hypo-Peritectic Steels during Initial Solidification by Surface Roughness" Metals 8, no. 12: 982. https://doi.org/10.3390/met8120982




