Evaluation of Workability on the Microstructure and Mechanical Property of Modified 9Cr-2W Steel for Fuel Cladding by Cold Drawing Process and Intermediate Heat Treatment Condition
Abstract
:1. Introduction
2. Materials and Methods
3. Results
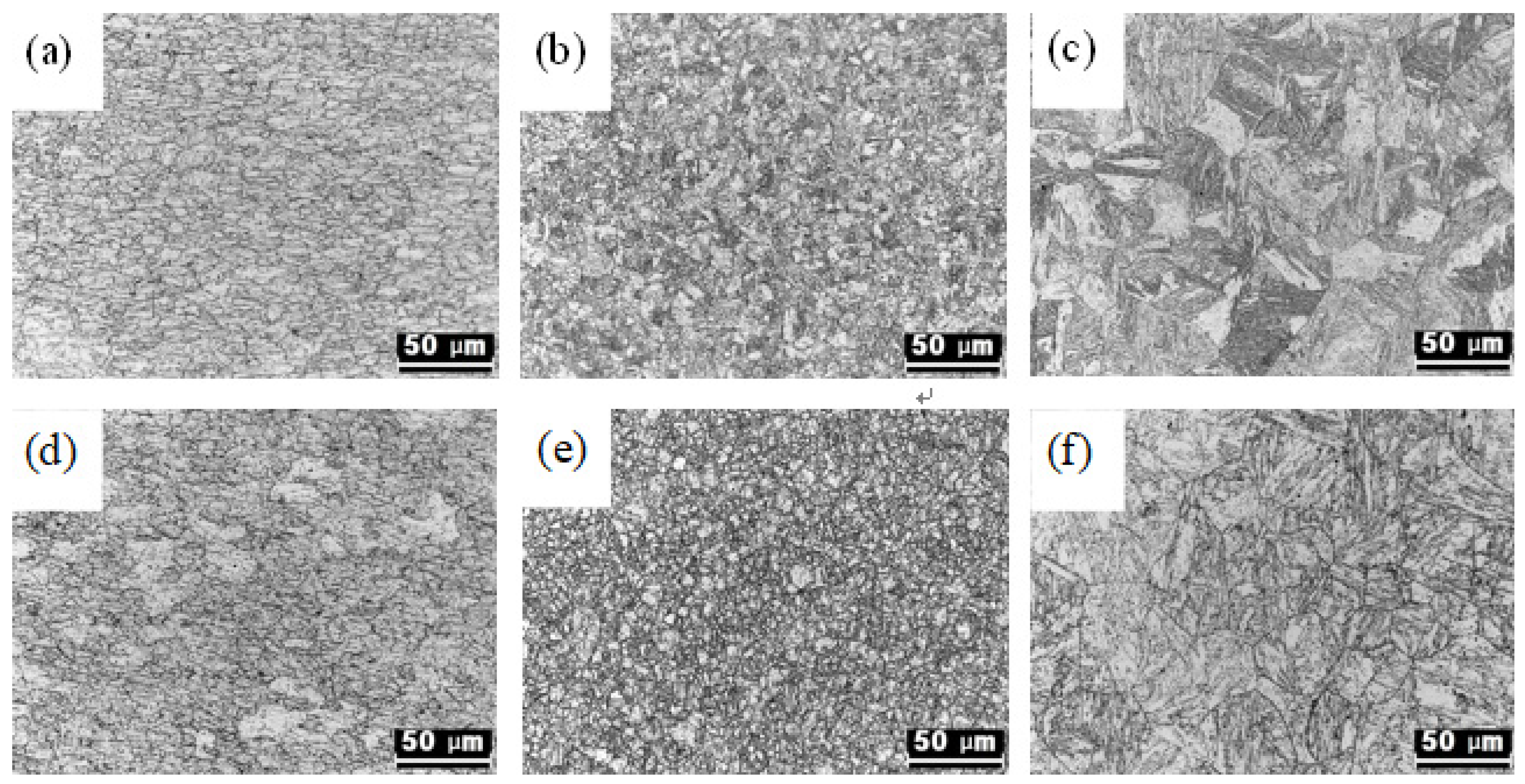
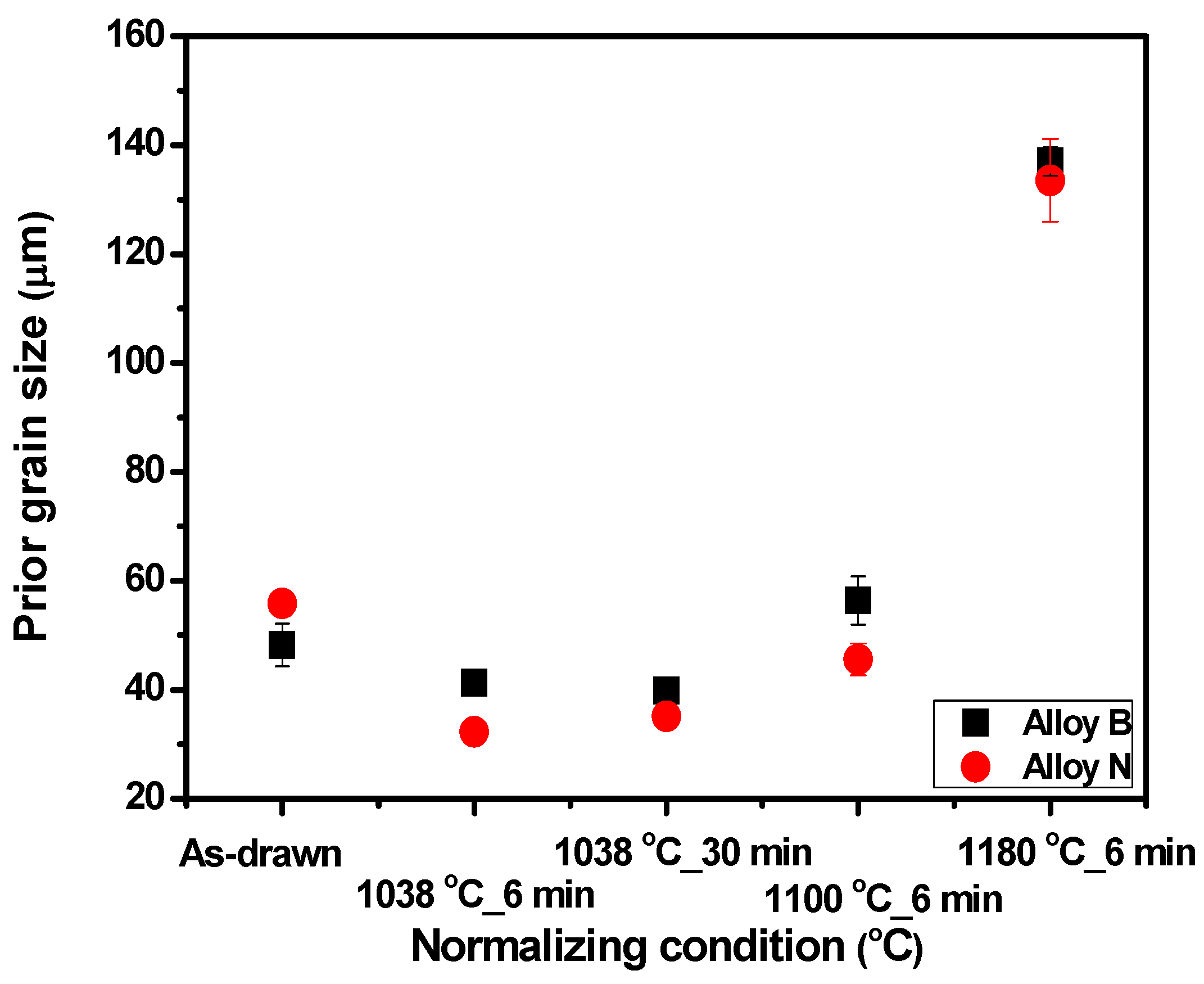
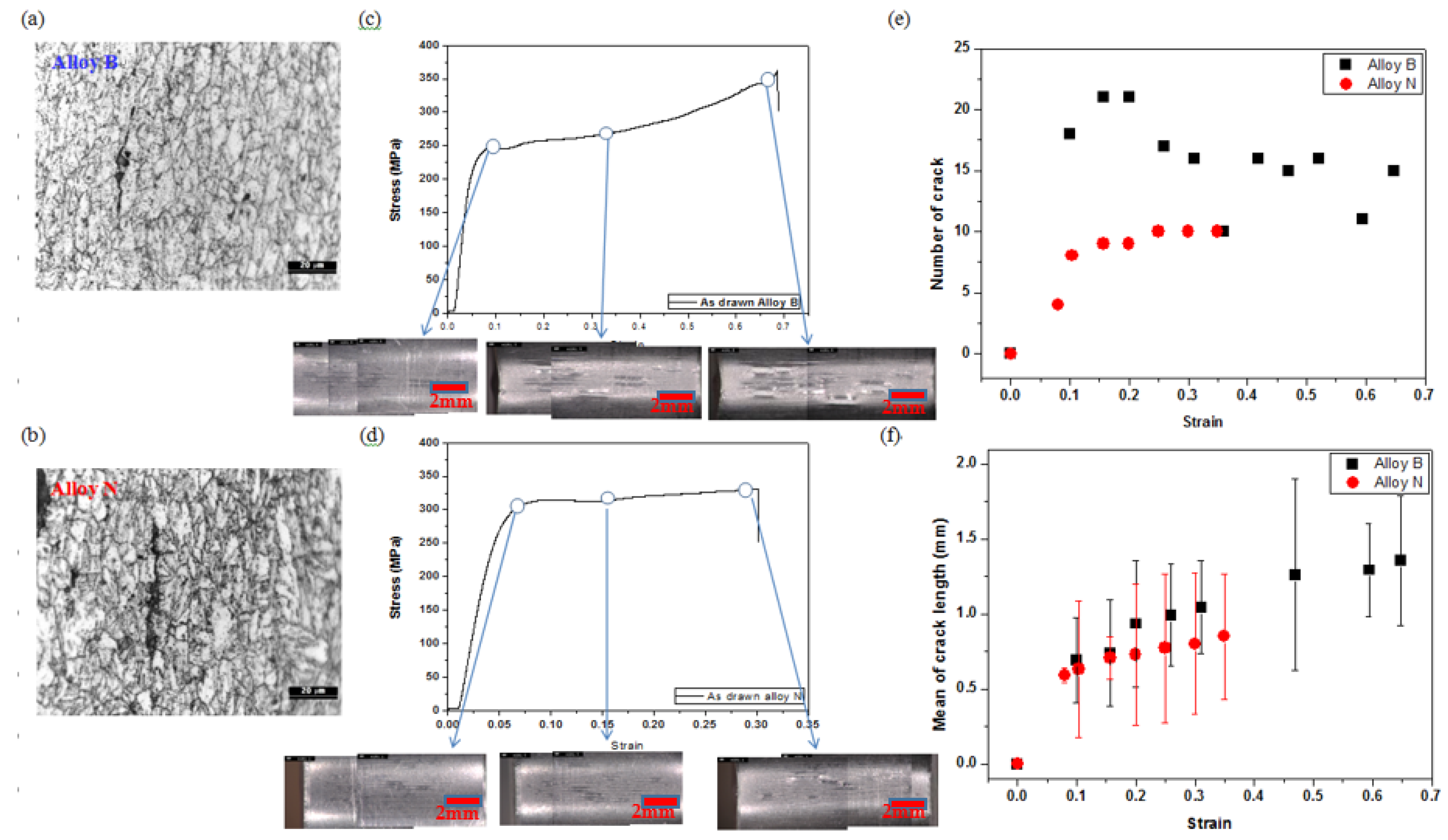
3.1. Changes in Microstructure According to Heat Treatment Conditions
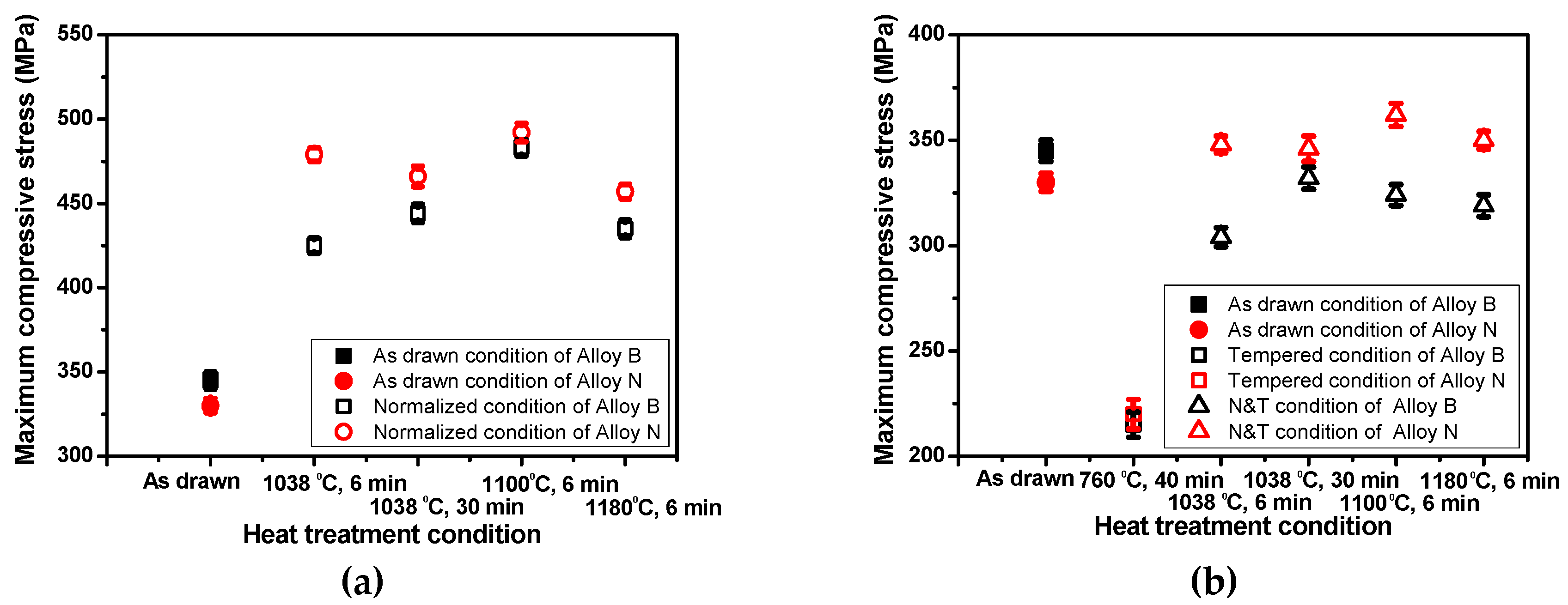
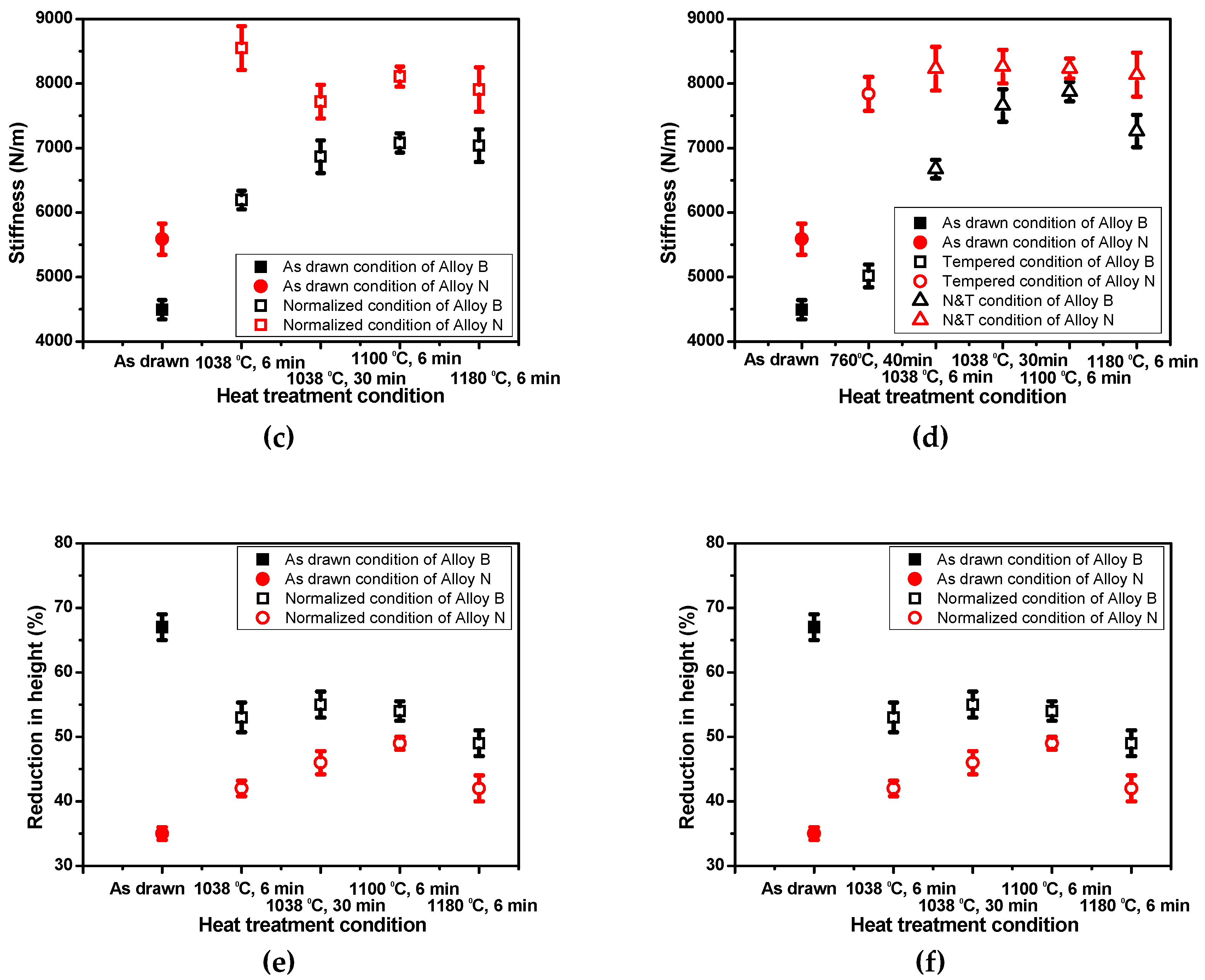
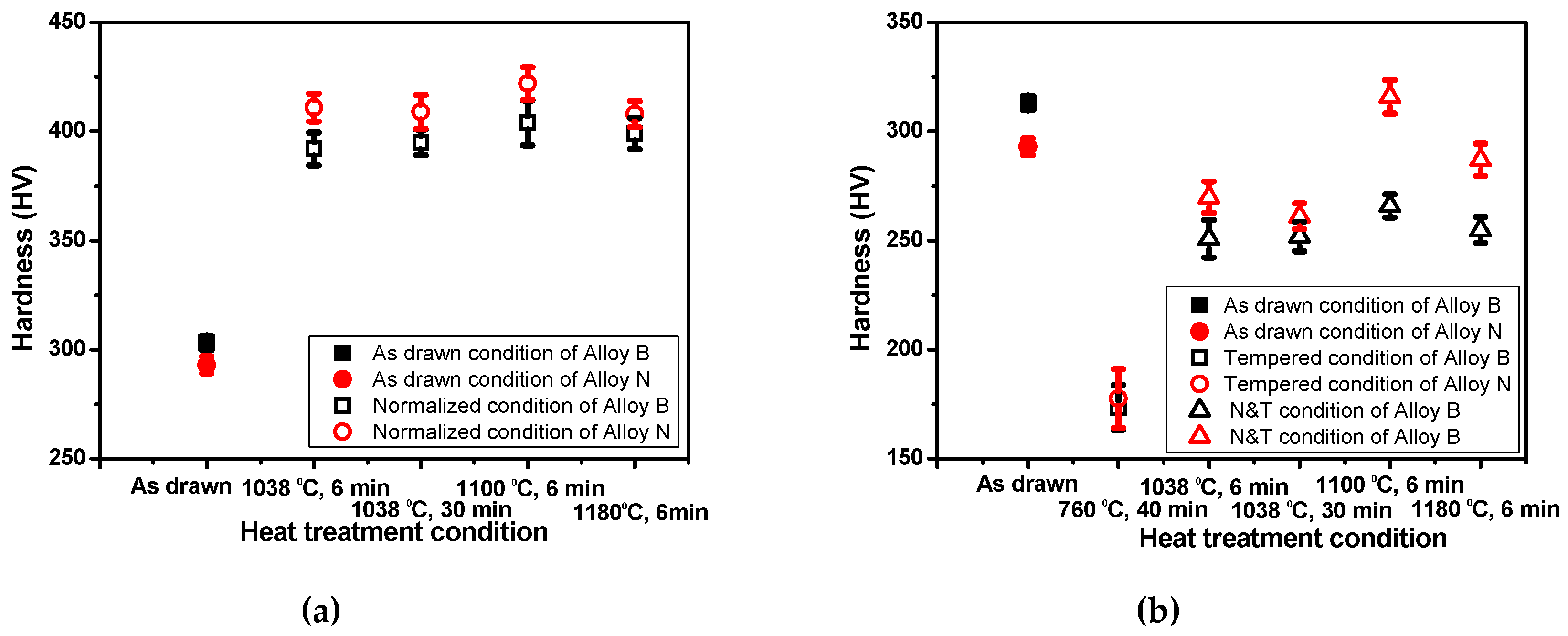
3.2. Effect of Heat Treatment Conditions on Mechanical Properties
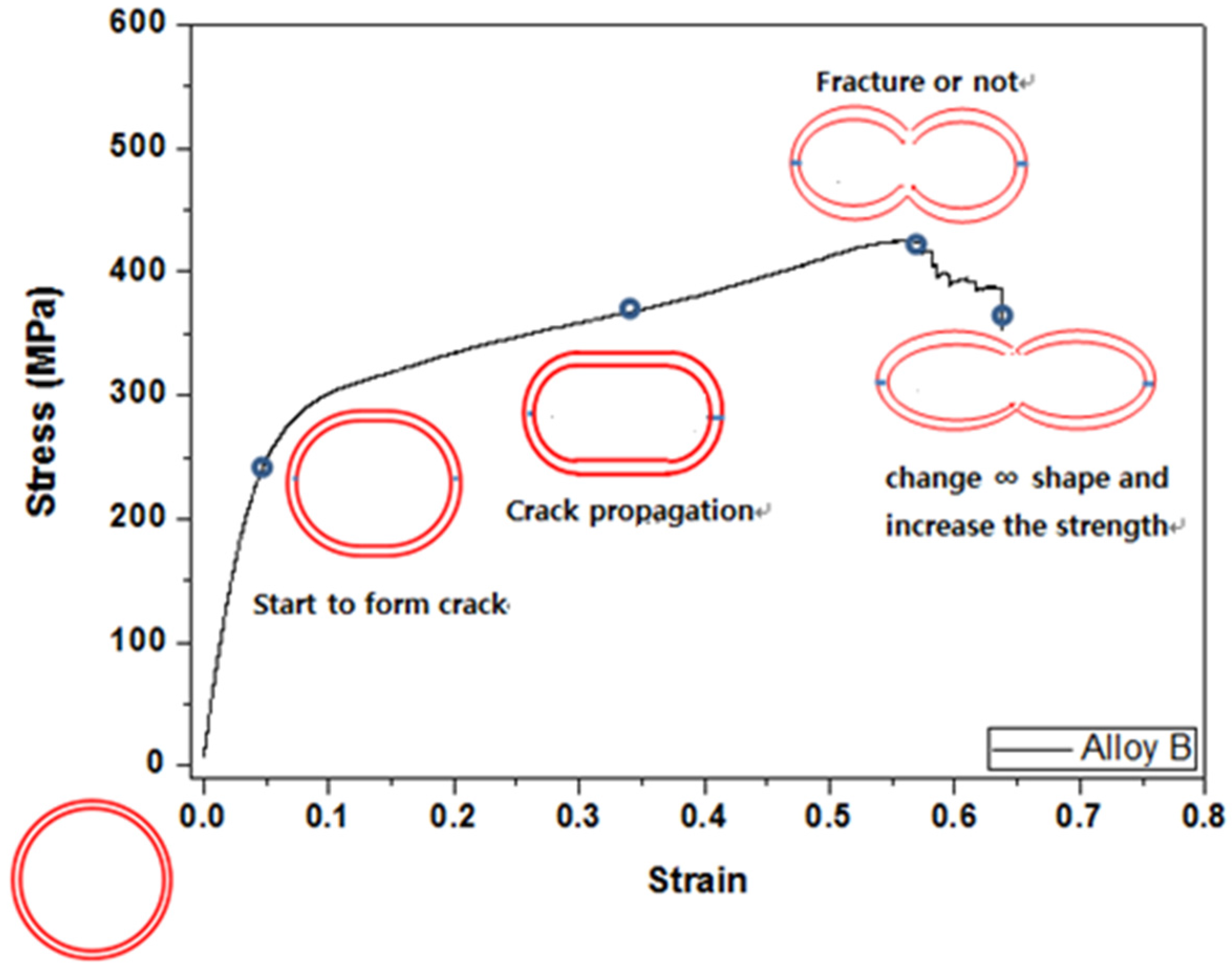
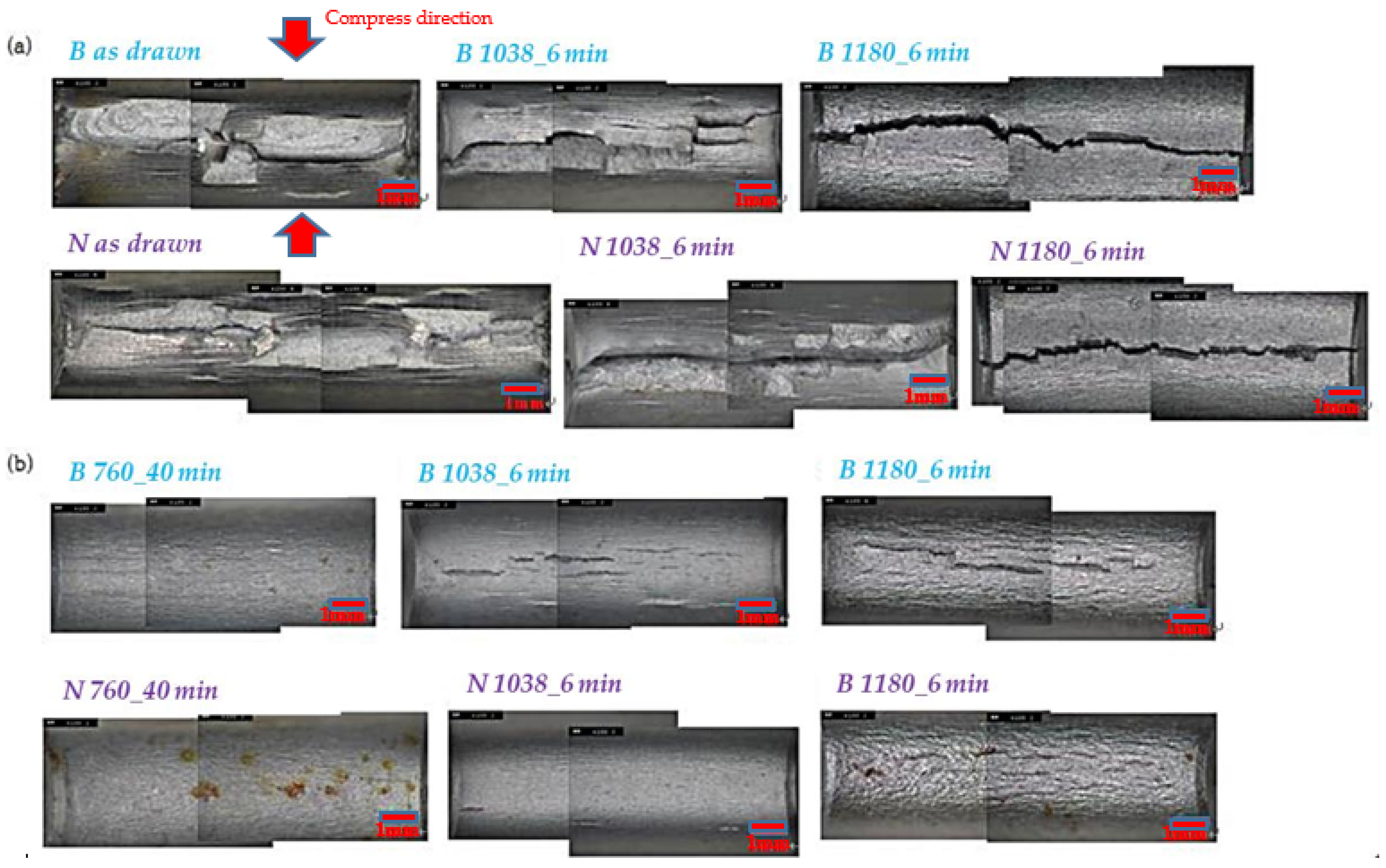
3.3. Crack Formation and Observation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, J.H.; Heo, H.M.; Baek, J.H.; Kim, S.H. Evaluation of the mechanical properties of a HT9 fuel cladding tube for a sodium-cooled fast reactor. J. Korean Inst. Met. Mater. 2013, 51, 25–31. [Google Scholar] [CrossRef]
- Klueh, R.L. Elevated-temperature Ferritic and Martensitic Steels and Their Application to Future. Int. Mater. Rev. 2005, 50, 287–310. [Google Scholar] [CrossRef]
- Paul, V.T.; Saroja, S.; Vijayalakshmi, M. Microstructural stability of modified 9Cr-1Mo steel during long term exposures at elevated temperatures. J. Nucl. Mater. 2008, 378, 273–281. [Google Scholar] [CrossRef]
- Abe, F. Effect of Boron on Microstructure and Creep Strength of Advanced Ferritic Power Plant Steels. Procedia Eng. 2011, 10, 94–99. [Google Scholar] [CrossRef]
- Ryu, W.S.; Kim, S.H. Thermal treatment improving creep properties of nitrogen-added Mod. 9Cr-1Mo steels. Trans. Indian Inst. Met. 2010, 63, 111–115. [Google Scholar] [CrossRef]
- Lu, Y. Effect of Boron on Microstructure and Mechanical Properties of Low Carbon Microalloyed Steels. Master’s Thesis, McGil University, Montreal, QC, Canada, 2007. [Google Scholar]
- Kim, Y.H.; Kim, K.Y.; Lee, Y.D. Nitrogen-Alloyed, Metastable Austenitic Stainless Steel for Automotive Structural Applications. Mater. Manuf. Process. 2004, 19, 51–59. [Google Scholar] [CrossRef]
- Liu, F.; Fors, D.H.R.; Golpayegani, A.; Andrén, H.O.; Wahnström, G. Effect of boron on carbide coarsening at 873 K in 9 to 12 pct chromium steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 4053–4062. [Google Scholar] [CrossRef]
- Cho, K.C.; Koo, Y.M.; Park, J.K. Effect of cooling rate on the hot ductility of boron bearing steel during continuous casting. J. Korean Inst. Met. Mater. 2008, 46, 329–337. [Google Scholar]
- Lim, J.H.; Kim, J.S.; Park, B.H.; Lee, J.H.; Choi, J.M. Effects of Heat Treatment on the Micro-structures and the Mechanical Properties of 0.002% Boron-added Low Carbon Steel. Korean J. Mater. Res. 2011, 21, 303–308. [Google Scholar] [CrossRef]
- Yamamoto, K.; Susuki, H.G.; Oono, Y.; Noda, N.; Inoue, T. Formation mechanism and prevention method of facial cracks of continuously cast steel slabs containing boron. Tetsu-to-Hagane 1987, 73, 115–122. [Google Scholar] [CrossRef]
- Abe, H.; Furugen, M. Evaluation Method of Workability in Cold Pilgering of Zirconium-based Alloy Tube. Mater. Trans. 2010, 51, 1200–1205. [Google Scholar] [CrossRef]
- Klueh, R.L.; Harries, D.R. High-Chromium Ferritic and Martensitic Steels for Nuclear Applications; ASTM International: West Conshohocken, PA, USA, 2001; ISBN 978-0-8031-2090-7. [Google Scholar]
- Saroja, S.; Vijayalakshmi, M.; Raghunathan, V.S. Influence of cooling rates on the transformation behaviour of 9Cr-1 Mo-O.07C steel. J. Mater. Sci. 1992, 27, 2389–2396. [Google Scholar] [CrossRef]
- Morito, S.; Huang, X.; Furuhara, T.; Maki, T.; Hansen, N. The morphology and crystallography of lath martensite in Fe-C alloy steels. Acta Mater. 2006, 54, 5323–5331. [Google Scholar] [CrossRef]
- Maki, T. Morphology and substructure of martensite in steels. Anal. Tech. 2012, 2, 34–58. [Google Scholar] [CrossRef]
- Barbadikar, D.R.; Deshmukh, G.S.; Maddi, L.; Laha, K.; Parameswaran, P.; Ballal, A.R.; Peshwe, D.R.; Paretkar, R.K.; Nandagopal, M.; Mathew, M.D. Effect of normalizing and tempering temperatures on microstructure and mechanical properties of P92 steel. Int. J. Press. Vessel. Pip. 2015, 132–133, 97–105. [Google Scholar] [CrossRef]
- Wang, S.S.; Peng, D.L.; Chang, L.; Hui, X.D. Enhanced mechanical properties induced by refined heat treatment for 9Cr-0.5Mo-1.8W martensitic heat resistant steel. Mater. Des. 2013, 50, 174–180. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.; Bao, H.; Weng, Y.; Liu, W. Effect of tempering temperature on the toughness of 9Cr-3W-3Co martensitic heat resistant steel. Mater. Des. 2014, 54, 874–879. [Google Scholar] [CrossRef]
- Saini, N.; Pandey, C.; Mahapatra, M.M. Characterization and evaluation of mechanical properties of CSEF P92 steel for varying normalizing temperature. Mater. Sci. Eng. A 2017, 688, 250–261. [Google Scholar] [CrossRef]
- Miller, M.K. Atom probe tomography characterization of solute segregation to dislocations. Microsc. Res. Tech. 2006, 69, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, S. Cu precipitation on dislocation and interface in quench-aged steel. MRS Commun. 2012, 2, 127–132. [Google Scholar] [CrossRef]
- Li, Y.J.; Ponge, D.; Choi, P.; Raabe, D. Atomic scale investigation of non-equilibrium segregation of boron in a quenched Mo-free martensitic steel. Ultramicroscopy 2015, 159, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Priester, L. Grain Boundaries: From Theory to Engineering; Wang, Z.M., Ed.; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-4968-9. [Google Scholar]
- Love, G.R. Dislocation Pipe Diffusion. Acta Metall. 1964, 12, 731–737. [Google Scholar] [CrossRef]
- Heo, H.M.; Jeong, E.H.; Kim, S.H.; Kim, J.R. Comparison between effect of B and N on the microstructure of modified 9Cr-2W steel during aging and creep. Mater. Sci. Eng. A 2016, 670, 106–111. [Google Scholar] [CrossRef]
- Du, C. Micro-Plasticity Characterization of Martensite, Ferrite, and Dual-Phase Steel. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2016. [Google Scholar]
- Yamada, R.; Suzuki, M.; Harayama, Y. Application of finite element method to ring compression test. Nucl. Eng. Des. 1977, 44, 75–85. [Google Scholar] [CrossRef]
- Liu, A.F. Mechanics and Mechanisms of Fracture: An Introduction; ASM International: West Conshohocken, PA, USA, 2005; ISBN 0871708027. [Google Scholar]
- Mun, D.J.; Shin, E.J.; Koo, Y.M. A study on the behavior of boron distribution in low carbon steel by particle tracking autoradiography. Nucl. Eng. Technol. 2011, 43, 1–6. [Google Scholar] [CrossRef]
- Williams, T.M.; Stoneham, A.M.; Harries, D.R. The segregation of boron to grain boundaries in solution-treated Type 316 austenitic stainless steel. Met. Sci. 1976, 10, 14–19. [Google Scholar] [CrossRef]
- Park, S.G.; Lee, K.H.; Kim, M.C.; Lee, B.S. Effects of boundary characteristics on resistance to temper embrittlement and segregation behavior of Ni–Cr–Mo low alloy steel. Mater. Sci. Eng. A 2013, 561, 277–284. [Google Scholar] [CrossRef]
- Militzer, M.; Wieting, J. Segregation mechanisms of temper embrittlement. Acta Metall. 1989, 37, 2585–2593. [Google Scholar] [CrossRef]

| Alloy | C | Cr | W | N | B | Fe |
|---|---|---|---|---|---|---|
| Alloy B | 0.07 | 8.89 | 1.934 | 0.02 | 0.013 | Bal. |
| Alloy N | 0.063 | 9.09 | 2.019 | 0.0767 | 0.004 | Bal. |
| Normalizing | Tempering |
|---|---|
| 1038 °C, 6 min | 760 °C, 40 min |
| 1038 °C, 30 min | |
| 1100 °C, 6 min | |
| 1180 °C, 6 min | |
| No heat treatment | 760 °C, 40 min |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, H.-M.; Kim, J.-H.; Kim, S.-H.; Kim, J.-R. Evaluation of Workability on the Microstructure and Mechanical Property of Modified 9Cr-2W Steel for Fuel Cladding by Cold Drawing Process and Intermediate Heat Treatment Condition. Metals 2018, 8, 193. https://doi.org/10.3390/met8030193
Heo H-M, Kim J-H, Kim S-H, Kim J-R. Evaluation of Workability on the Microstructure and Mechanical Property of Modified 9Cr-2W Steel for Fuel Cladding by Cold Drawing Process and Intermediate Heat Treatment Condition. Metals. 2018; 8(3):193. https://doi.org/10.3390/met8030193
Chicago/Turabian StyleHeo, Hyeong-Min, Jun-Hwhan Kim, Sung-Ho Kim, and Jong-Ryoul Kim. 2018. "Evaluation of Workability on the Microstructure and Mechanical Property of Modified 9Cr-2W Steel for Fuel Cladding by Cold Drawing Process and Intermediate Heat Treatment Condition" Metals 8, no. 3: 193. https://doi.org/10.3390/met8030193




