Study on the Control of Rare Earth Metals and Their Behaviors in the Industrial Practical Production of Q420q Structural Bridge Steel Plate
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
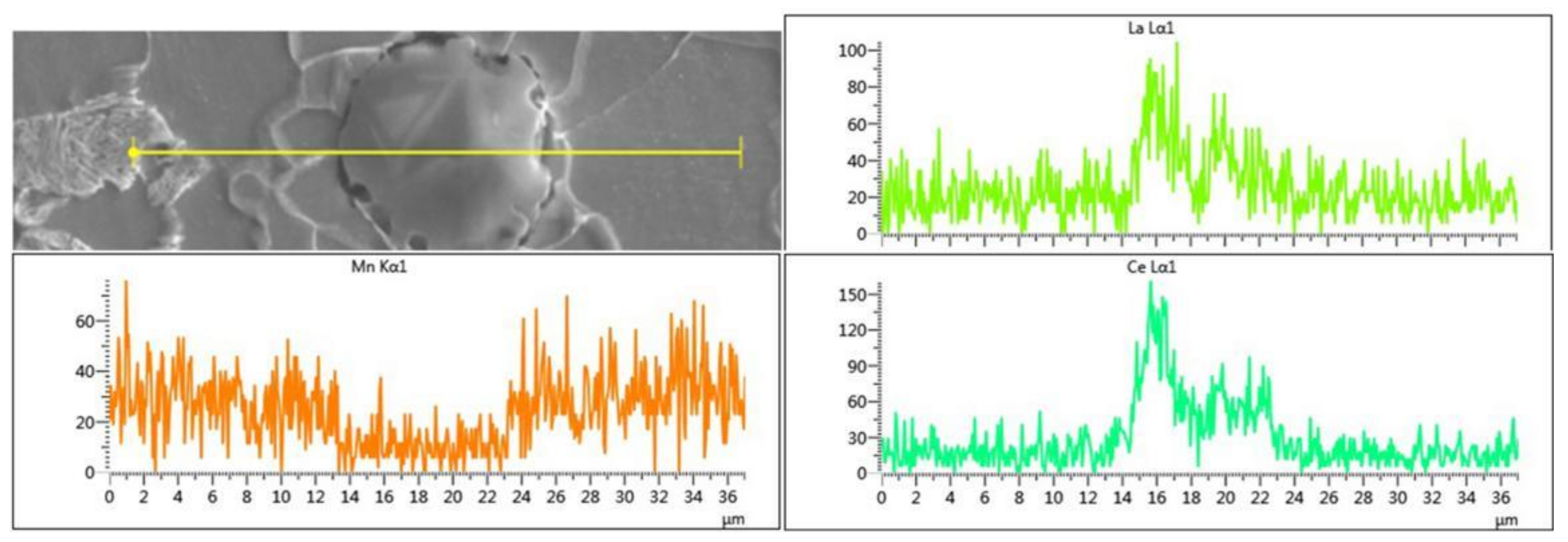
3.1. Comparison of Inclusions Resulted from Ladle Furnace (LF) and Ruhrstahl and Hereaeus (RH) Processes
3.2. Toughness and Welding Performance
4. Conclusions
- (1)
- Regarding the structural steel of 420 MPa plate without RE, most of the inclusions were calcium aluminate with a high melting point >1873 K (1600 °C), and other inclusions were calcium aluminate with a low melting point <1773 K (1500 °C). The most significant inclusions in class B could reach a size of 180 μm. The addition of RE in the LF refining process found that no low melting point calcium aluminate inclusions were precipitated. The addition RE in the RH refining process showed the presence of low melting point inclusion.
- (2)
- The quantity of RE inclusions from the LF process was low. La and Ce inclusions occasionally appeared alone or coexist together. There is no Nb content in the RE inclusions, and only Nb mono-precipitates exist. The La and Ce inclusions from the RH process would always coexist together. Nb precipitation did not appear, and Al was also drastically reduced, while S increased in the inclusions.
- (3)
- RE has a positive effect on the toughness of the steel plate. The impact energy of the plate with RE is approximately 50 J higher than for a regular plate without RE. The toughness of the plate with RE from LF refining is better than that from RH refining.
- (4)
- RE inclusions could induce the intragranular ferrite, and refine the grains to the preferred size. After the welding at a heat input of 200 kJ/cm, the finest grain size at the heat affected zone was found in the plate from LF process with RE addition. The microstructure of ferrite was quasi-polygonal.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Naser, M.Z.; Kodur, V.K.R. Comparative fire behavior of composite girders under flexural and shear loading. Thin-Walled Struct. 2017, 116, 82–90. [Google Scholar] [CrossRef]
- Kodur, V.K.; Aziz, E.M.; Naser, M.Z. Strategies for enhancing fire performance of steel bridges. Eng. Struct. 2017, 131, 446–458. [Google Scholar] [CrossRef]
- Gowda, S.; Hotz, C.; Manigandan, K.; Srivatsan, T.S.; Patnaik, A.; Payer, J. Quasi-Static, Cyclic Fatigue and Fracture Behavior of Alloy Steel for Structural Applications: Influence of Orientation. Mater. Perform. Charact. 2016, 5, 148–163. [Google Scholar] [CrossRef]
- Jiang, Q.C.; Liang, H.Q.; Sui, H.L. Effect of Y-Ce Complex Modification on Thermal Fatigue Behavior of High Cr Cast Hot Working Die Steels. ISIJ Int. 2004, 44, 1762–1766. [Google Scholar] [CrossRef]
- Lan, J.; He, J.; Ding, W.; Wang, Q.; Zhu, Y. Effect of Rare Earth Metals on the Microstructure and Impact Toughness of a Cast 0.4C–5Cr–1.2Mo–1.0V Steel. ISIJ Int. 2000, 40, 1275–1282. [Google Scholar] [CrossRef]
- Guo, M.; Suito, H. Influence of Dissolved Cerium and Primary Inclusion Particles of Ce2O3 and CeS on Solidification Behavior of Fe-0.20 mass%C-0.02 mass%P Alloy. ISIJ Int. 1999, 39, 722–729. [Google Scholar] [CrossRef]
- Waudby, P.E. Rare earth additions to steel. Int. Met. Rev. 1978, 23, 74–98. [Google Scholar] [CrossRef]
- Luyckx, L. Current trends in the use of rare earths in steelmaking. Electr. Furn. Conf. 1973, 31, 5–7. [Google Scholar]
- Wilson, W.G.; Heaslip, L.J.; Sommerville, I.D. Rare Earth Additions in Continuously Cast Steel. JOM 1985, 37, 36–41. [Google Scholar] [CrossRef]
- Song, M.-M.; Song, B.; Xin, W.-B.; Sun, G.-L.; Song, G.-Y.; Hu, C.-L. Effects of rare earth addition on microstructure of C–Mn steel. Ironmak. Steelmak. Process. 2015, 8, 594–599. [Google Scholar] [CrossRef]
- Mills, A.R.; Thewlis, G.; Whiteman, J.A. Nature of inclusions in steel weld metals and their influence on formation of acicular ferrite. Mater. Sci. Tech. 1987, 12, 1051–1061. [Google Scholar] [CrossRef]
- Zhang, Z.; Farrar, R.A. Role of non-metallic inclusions in formation of acicular ferrite in low alloy weld metals. Mater. Sci. Tech. 1996, 12, 237–260. [Google Scholar] [CrossRef]
- Song, M.; Song, B.; Zhang, S.; Xue, Z.; Yang, Z.; Xu, R. Role of Lanthanum Addition on Acicular Ferrite Transformation in C–Mn Steel. ISIJ Int. 2017, 57, 1261–1267. [Google Scholar] [CrossRef]
- Thewlis, G. Effect of cerium sulphide particle dispersions on acicular ferrite microstructure development in steels. Mater. Sci. Technol. 2006, 22, 153–166. [Google Scholar] [CrossRef]
- Wen, B.; Song, B. In Situ Observation of the Evolution of Intragranular Acicular Ferrite at Ce–Containing Inclusions in 16Mn Steel. Steel Res. Int. 2012, 83, 487–495. [Google Scholar]
- Pan, F.; Zhang, J.; Chen, H.-L.; Su, Y.-H.; Kuo, C.-L.; Su, Y.-H.; Chen, S.-H.; Lin, K.-J.; Hsieh, P.-H.; Hwang, W.-S. Effects of Rare Earth Metals on Steel Microstructures. Materials 2016, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Evaluation of the nucleation potential of intragranular acicular ferrite in steel weldments. Acta Metall. Mater. 1994, 42, 3291–3298. [Google Scholar] [CrossRef]
- Hsu, T.Y. Effects of Rare Earth Element on Isothermal and Martensitic Transformations in Low Carbon Steels. ISIJ Int. 1998, 38, 1153–1164. [Google Scholar] [CrossRef]
- Nako, H.; Okazaki, Y.; Speer, J.G. Acicular Ferrite Formation on Ti-Rare Earth Metal-Zr Complex Oxides. ISIJ Int. 2015, 55, 250–256. [Google Scholar] [CrossRef]
- Gao, J.; Fu, P.; Liu, H.; Li, D. Effects of Rare Earth on the Microstructure and Impact Toughness of H13. Steel. Met. 2015, 5, 383–394. [Google Scholar] [CrossRef]
- Ye, G.; Jonsson, P.; Lund, T. Thermodynamics and Kinetics of the Modification of Al2O3 Inclusions. ISIJ Int. 1996, 36, S105–S108. [Google Scholar] [CrossRef]
- Dawson, S.; Mountford, N.D.G.; Sommerville, I.D.; McLean, A. The Evaluation of Metal Cleanliness in the Steel Industry. IV. Preferential Dissolution Techniques. Ironmak. Steelmak. 1988, 15, 54–55. [Google Scholar]
- Zhao, D.W.; Li, H.B.; Bao, C.; Yang, J. Inclusion Evolution during Modification of Alumina Inclusions by Calcium in Liquid Steel and Deformation during Hot Rolling Process. ISIJ Int. 2015, 55, 2125–2134. [Google Scholar] [CrossRef]
- Zhao, D.W.; Li, H.B.; Cui, Y.; Yang, J. Control of Inclusion Composition in Calcium Treated Aluminum Killed Steels. ISIJ Int. 2016, 56, 1181–1187. [Google Scholar] [CrossRef]








| The Position for RE Alloy Added | C | Si | Mn | P | S | (Nb + Ti + etc.) | La | Ce |
|---|---|---|---|---|---|---|---|---|
| LF refining | 0.144 | 0.28 | 1.46 | 0.014 | 0.0019 | <1 | <0.0010 | <0.0020 |
| RH refining | 0.148 | 0.27 | 1.5 | 0.014 | 0.0016 | <1 | <0.0030 | <0.0060 |
| Element | Mg | Al | S | Ca | Nb | La | Ce |
|---|---|---|---|---|---|---|---|
| Average content | 0.4 | 3.14 | 1.33 | 0.75 | 17.84 | 5.54 | 7.9 |
| Element | Mg | Al | S | Ca | Nb | La | Ce |
|---|---|---|---|---|---|---|---|
| Average content | 0.01 | 0.005 | 5.19 | 0.43 | 0.00 | 12.42 | 18.73 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, R.; Fan, Y.; Li, Z.; Liu, J.; Yin, N.; Hao, N. Study on the Control of Rare Earth Metals and Their Behaviors in the Industrial Practical Production of Q420q Structural Bridge Steel Plate. Metals 2018, 8, 240. https://doi.org/10.3390/met8040240
Chu R, Fan Y, Li Z, Liu J, Yin N, Hao N. Study on the Control of Rare Earth Metals and Their Behaviors in the Industrial Practical Production of Q420q Structural Bridge Steel Plate. Metals. 2018; 8(4):240. https://doi.org/10.3390/met8040240
Chicago/Turabian StyleChu, Rensheng, Yong Fan, Zhanjun Li, Jingang Liu, Na Yin, and Ning Hao. 2018. "Study on the Control of Rare Earth Metals and Their Behaviors in the Industrial Practical Production of Q420q Structural Bridge Steel Plate" Metals 8, no. 4: 240. https://doi.org/10.3390/met8040240





