Study on the Interfacial Reactions between an Fe–Mn–Si Alloy and Complex Oxides Containing FeO during Isothermal Heating
Abstract
:1. Introduction
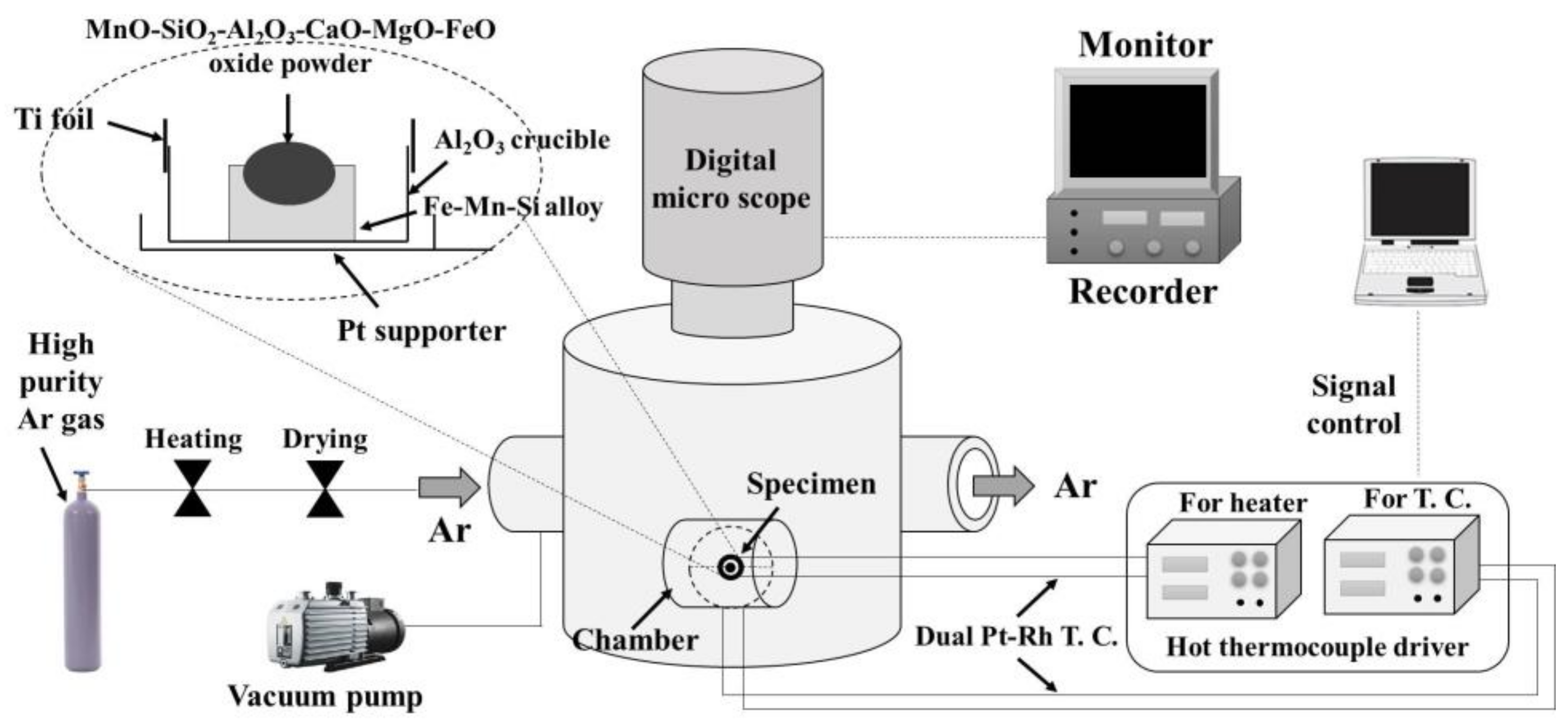
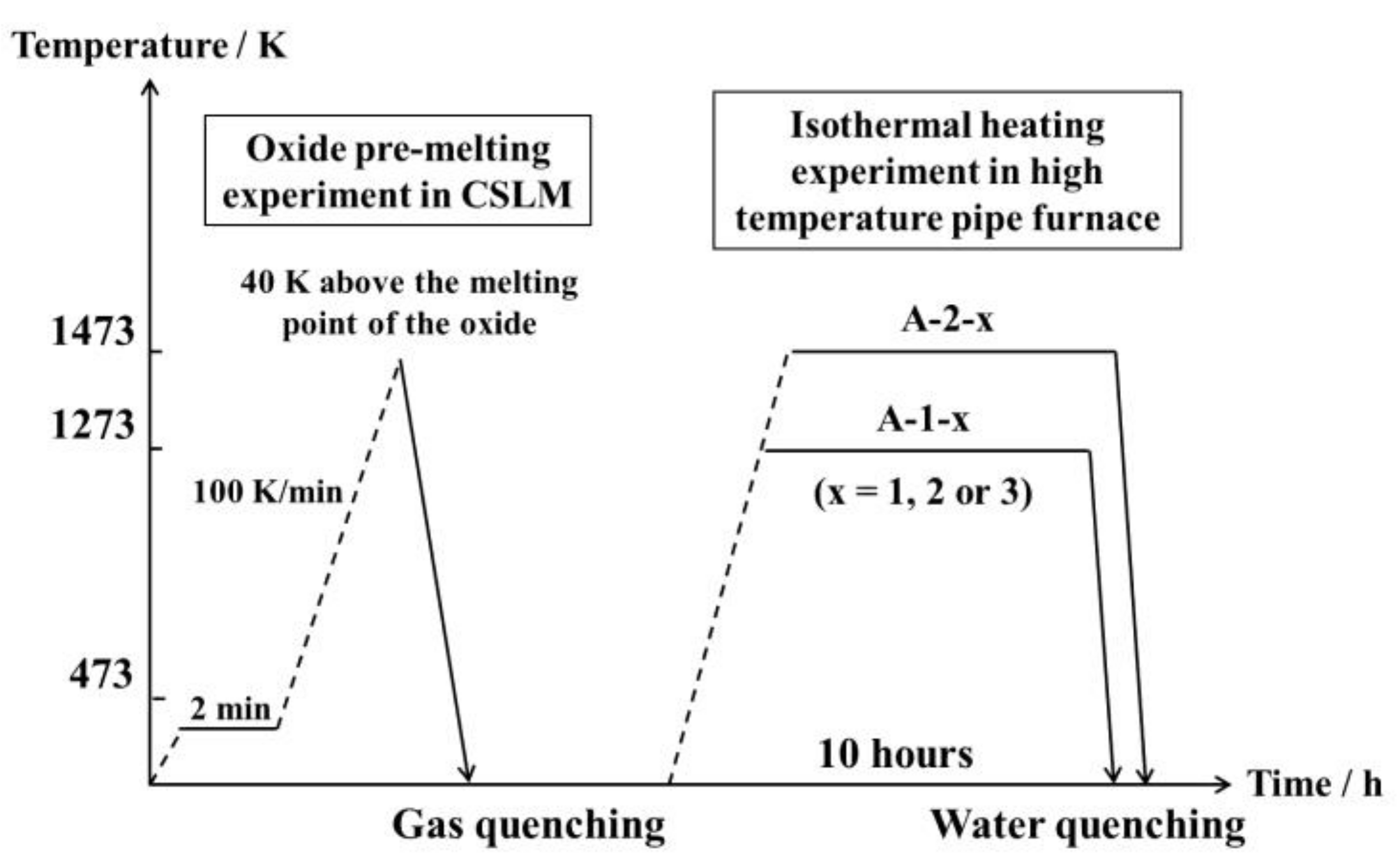
2. Experimental Methods
3. Results
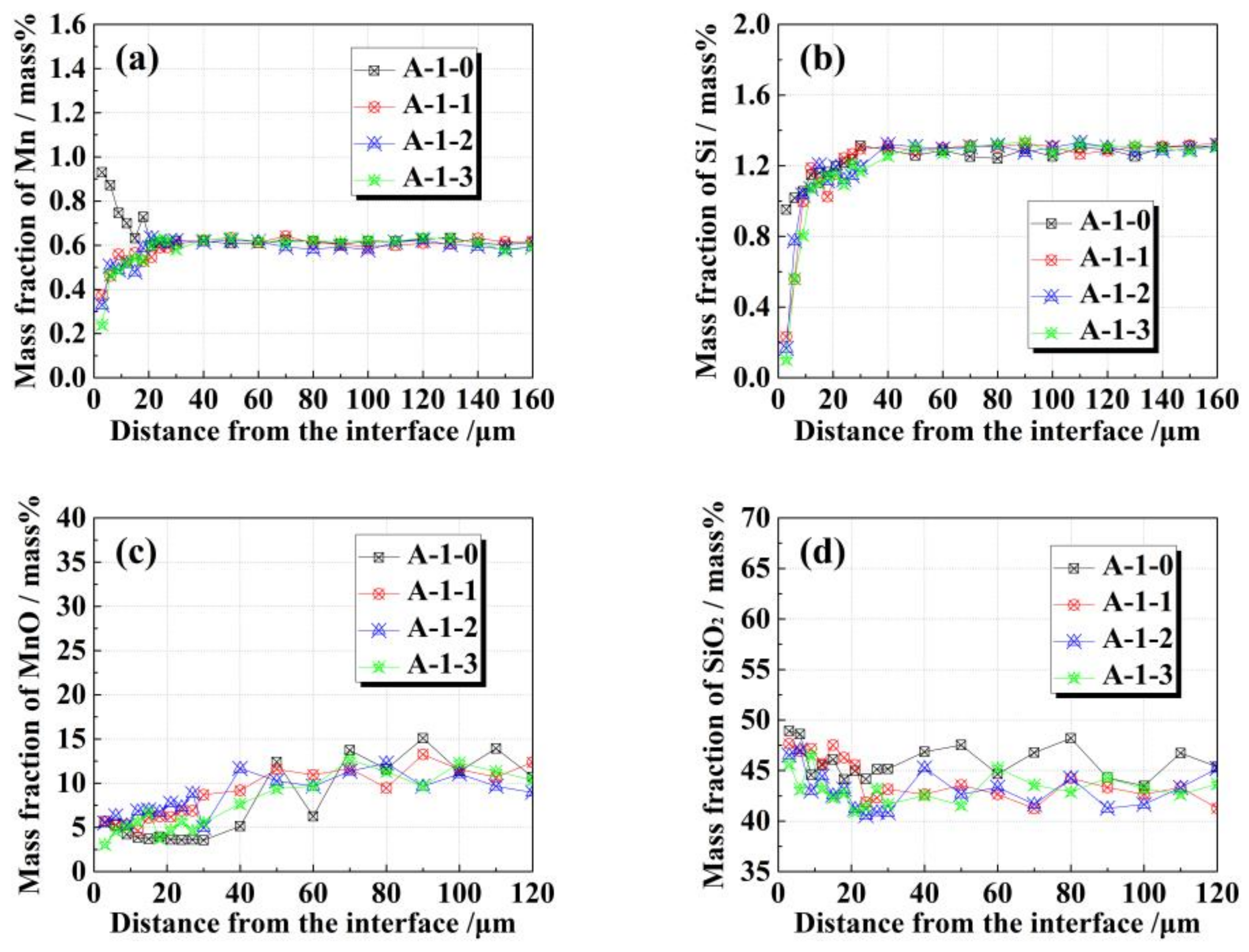
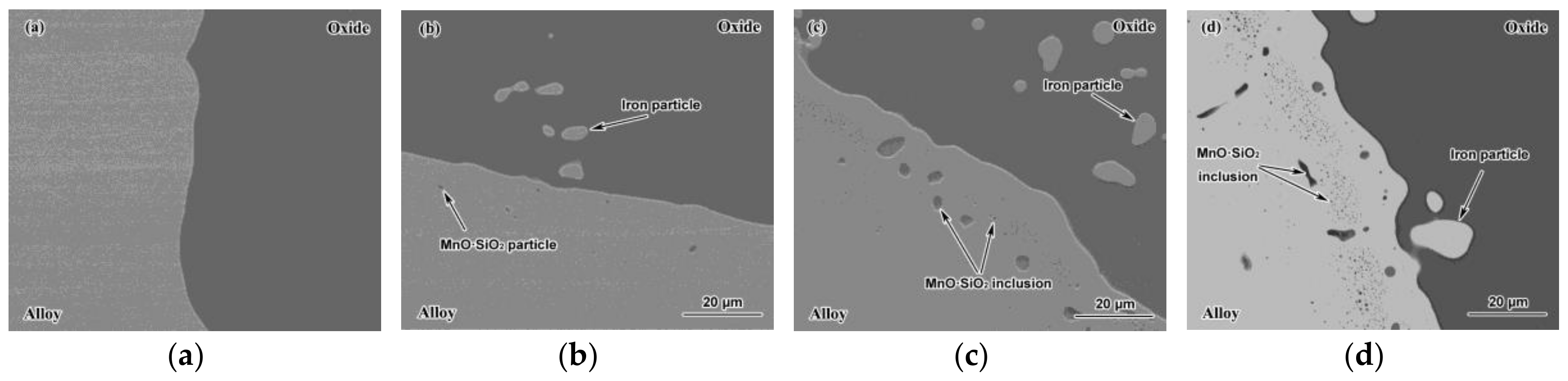
3.1. Effects of FeO on Solid–Solid Interfacial Reactions between the Alloy and Oxide after Isothermal Heating at 1273 K.
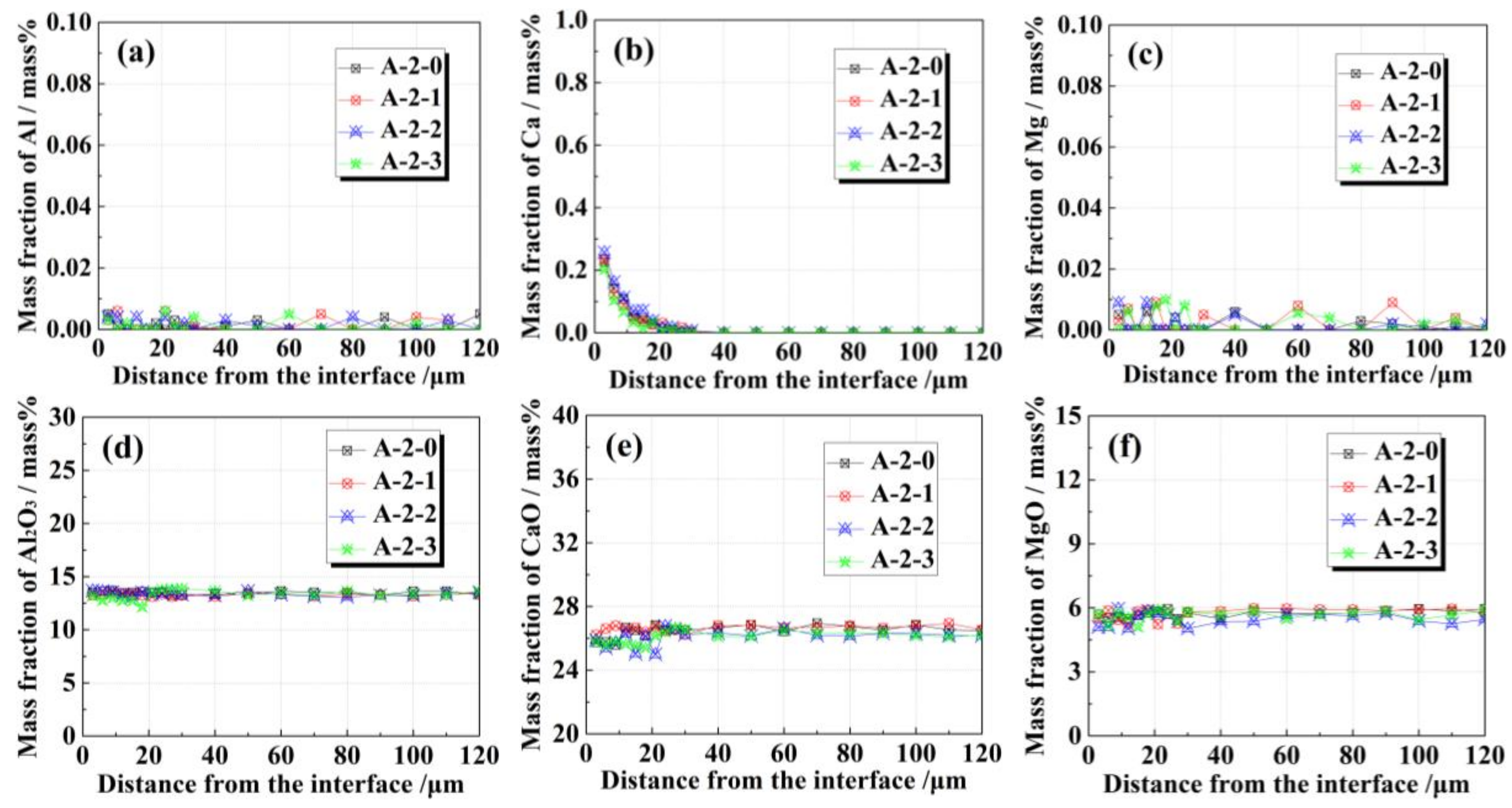
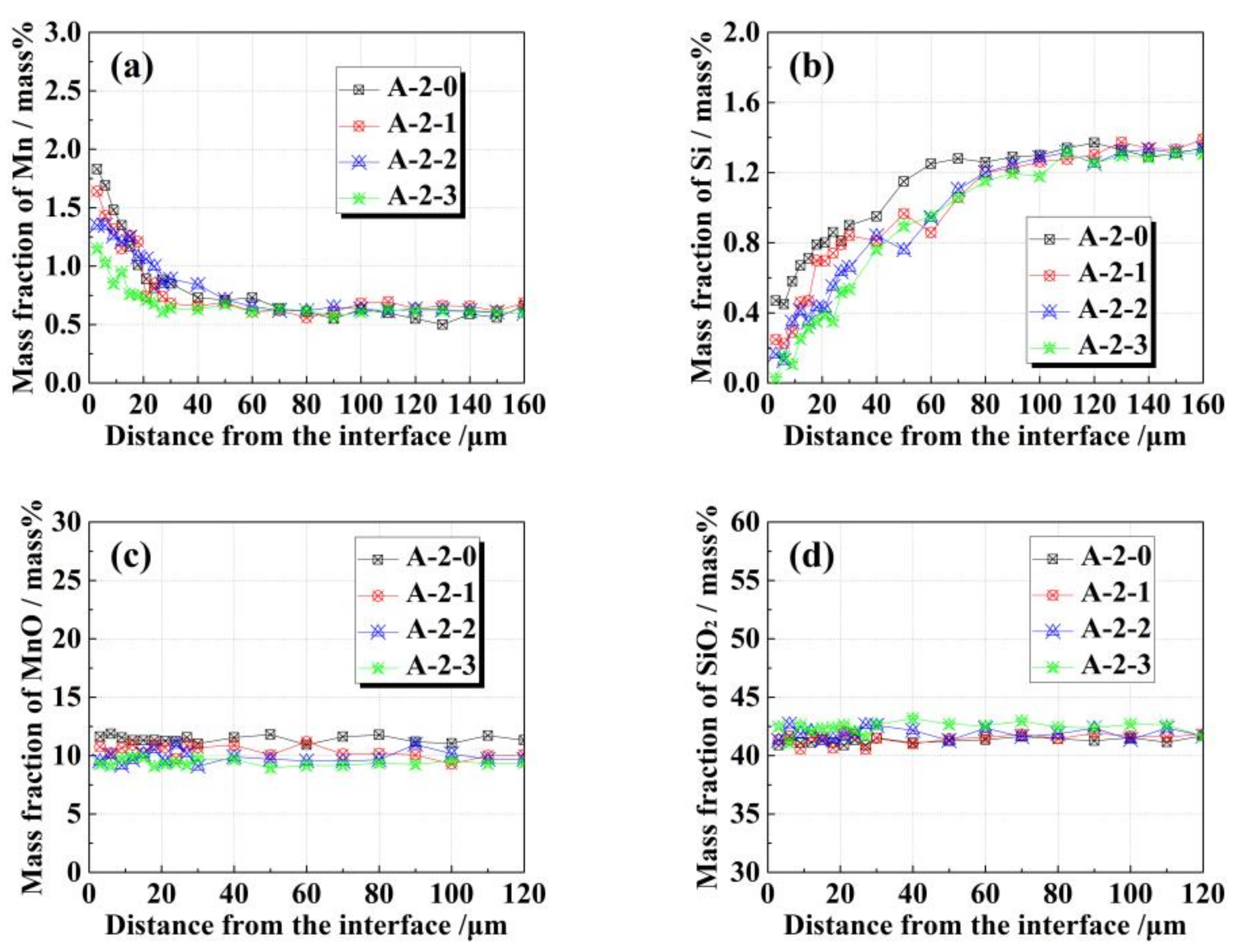
3.2. Effects of FeO on Solid–Liquid Interfacial Reactions between the Alloy and Oxide after Isothermal Heating at 1473 K
4. Discussion
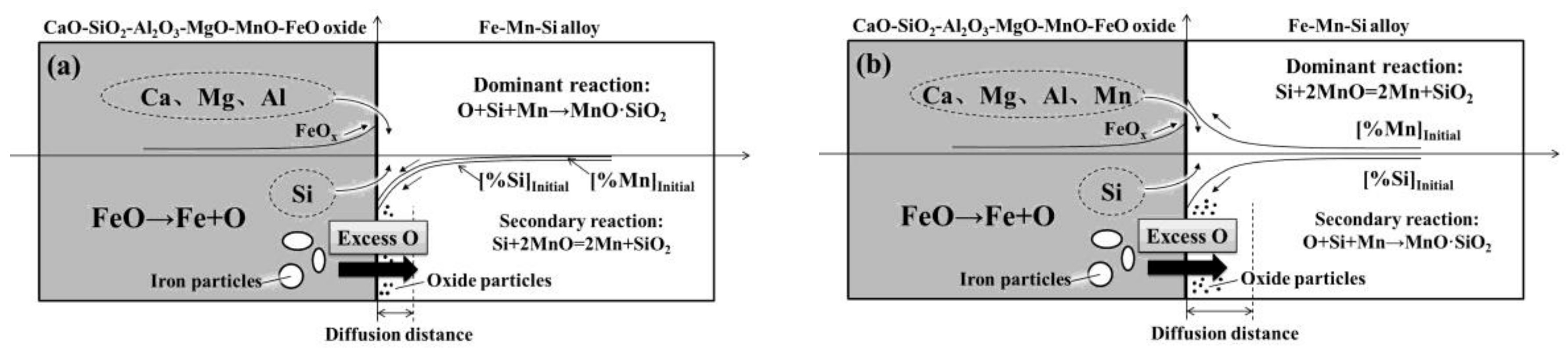
4.1. Interfacial Reaction Mechanism between the Alloy and Oxide
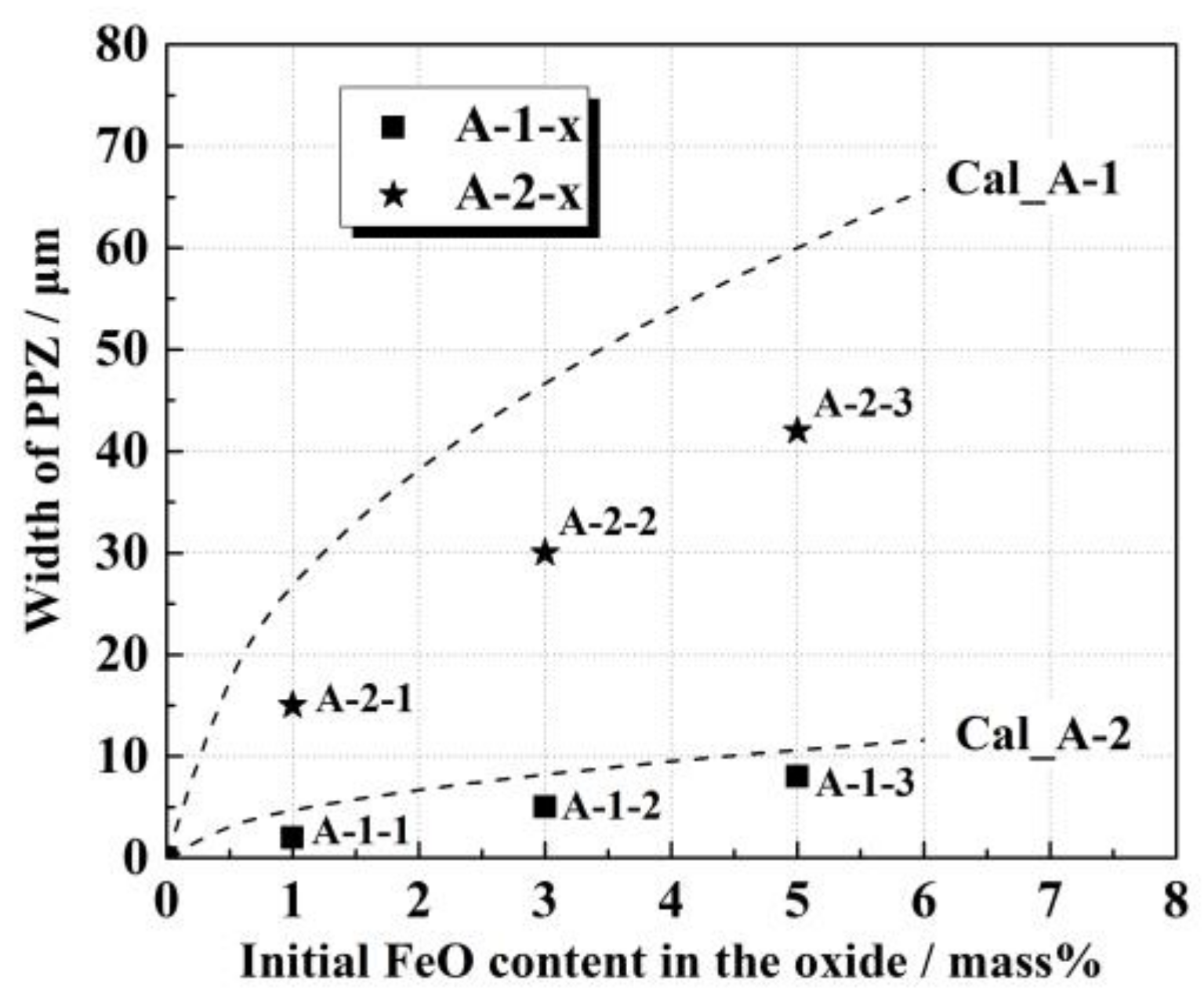
4.2. Dynamic Calculation Model
5. Conclusions
- During isothermal heating at 1273 and 1473 K, “solid–solid” and “solid–liquid” alloy–oxide reactions occurred in A-1-x and A-2-x diffusion couples, respectively.
- With an increase in the initial FeO content in the oxide, the PPZ, MDZ, and SDZ widths increased in A-1-x and A-2-x diffusion couples after heating.
- Diffused oxygen from the oxide reacting with elemental Mn and Si in the alloy plays a dominant role in the A-1-x diffusion couple during heating, whereas in the case of A-2-x, the dominant reaction is elemental Si in the alloy reacting with MnO in the oxide.
- A modified dynamic calculation model was established and verified to achieve better understanding on the interfacial reaction mechanism between the Fe–Mn–Si alloy and the CaO–SiO2–Al2O3–MgO–MnO–FeO oxide with different FeO content during isothermal heating.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cai, X.; Bao, Y.; Lin, L.; Gu, C. Effect of Al Content on the Evolution of Non-metallic Inclusions in Si-Mn Deoxidized Steel. Steel Res. Int. 2016, 87, 1168–1178. [Google Scholar] [CrossRef]
- Xin, X.; Yang, J.; Wang, Y.; Wang, R.; Wang, L.; Zheng, H.; Hu, H. Effects of Al Content on Non-metallic Inclusion Evolution in Fe–16Mn–xAl–0.6C High Mn TWIP Steel. Ironmak. Steelmak. 2016, 43, 234–242. [Google Scholar] [CrossRef]
- Cho, M.; Jung, I. Corrosion of Nozzle Refractories by Liquid Inclusion in High Oxygen Steels. ISIJ Int. 2012, 52, 1281–1288. [Google Scholar] [CrossRef]
- Shibata, H.; Kimura, K.; Tanaka, T.; Kitamura, S. Mechanism of Change in Chemical Composition of Oxide Inclusions in Fe–Cr Alloys Deoxidized with Mn and Si by Heat Treatment at 1473 K. ISIJ Int. 2011, 51, 1944–1950. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.; Shibata, H.; Kitamura, S. Reaction between MnO-SiO2-FeO Oxide and Fe-Mn-Si Solid Alloy during Heat Treatment. ISIJ Int. 2014, 54, 2144–2153. [Google Scholar] [CrossRef]
- Choi, W.; Matsuura, H.; Tsukihashi, F. Changing Behavior of Non-metallic Inclusions in Solid Iron Deoxidized by Al–Ti Addition during Heating at 1473 K. ISIJ Int. 2011, 51, 1951–1956. [Google Scholar] [CrossRef]
- Liu, C.; Yang, S.; Li, J.; Ni, H.; Zhang, X. Solid-state Reaction between Fe-Al-Ca alloy and Al2O3-CaO-FeO Oxide during Heat Treatment at 1473 K (1200 °C). Metall. Matter. Trans. B 2017, 48B, 1348–1357. [Google Scholar] [CrossRef]
- Liu, C.; Yang, S.; Kim, K.; Li, J.; Shibata, H.; Kitamura, S. Influence of FeO and Sulfur on Solid State Reaction between MnO-SiO2-FeO Oxides and an Fe-Mn-Si Solid Alloy during Heat Treatment at 1473 K. Int. J. Min. Met. Mater. 2015, 22, 811–819. [Google Scholar] [CrossRef]
- Li, M.; Matsuura, H.; Tsukihashi, F. Evolution of Al-Ti Oxide Inclusion during Isothermal Heating of Fe-Al-Ti Alloy at 1573 K (1300 °C). Metall. Matter. Trans. B 2017, 48B, 1915–1923. [Google Scholar] [CrossRef]
- Liu, C.; Kim, K.; Kim, S.; Li, J.; Ueda, S.; Gao, X.; Shibata, H.; Kitamura, S. Reaction between MnO-SiO2-FeO Solid Oxide and Solid Steel Deoxidized by Si and Mn during Heat Treatment at 1473 K (1200 °C). Metall. Matter. Trans. B 2015, 46B, 1875–1884. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Wang, X.; Wang, W. Experimental Research of Composition Control of CaO-SiO2-Al2O3-MgO Inclusion by Using Low Basic Top Slag for Tire Cord Steel. J. Univ. Sci. Technol. Beijing 2004, 26, 26–29. [Google Scholar]
- Liu, C.; Yang, S.; Li, J.; Ni, H.; Zhang, X. The Influence of FeO on the Reaction between Fe-Al-Ca Alloy and Al2O3-CaO-FeO Oxide during Heat Treatment at 1473 K. Metals 2017, 7, 129. [Google Scholar] [CrossRef]
- Singh, J.; Lim, W.; Chae, K. Atomic Diffusion Processes in MgO/Fe/MgO Multilayer. Superlattice Microst. 2015, 88, 609–619. [Google Scholar] [CrossRef]
- Zhang, L. Several Iimportant Scientific Research Points of Non-metallic Inclusions in Steel. Steelmaking 2016, 32, 1–16. [Google Scholar]
- Barin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances, 1st ed.; Springer: Berlin, Germany, 1992; pp. 56–59. [Google Scholar]
- Ohba, Y.; Yamashita, Y.; Ohno, K.; Maeda, T.; Nishioka, K.; Shimizu, M. Formation Mechanism of Oxide Particles in Subscale Layer around Surface Cracks of Steel. J. Iron Steel Inst. Japan 2009, 95, 531–540. [Google Scholar] [CrossRef]
- Takada, J.; Yamamoto, S.; Kikuchi, S.; Adachi, M. Determination of Diffusion Coefficient of Oxygen in γ-iron from Measurements of Internal Oxidation in Fe-Al Alloys. Metall. Matter. Trans. A 1986, 17A, 221–229. [Google Scholar] [CrossRef]
- Swisher, J.; Turkdogan, E. Soubility, Permeability, and Diffusivity of Oxygen in Solid Iron. Trans. Met. Soc. AIME 1967, 239, 426–431. [Google Scholar]
- Ban-Ya, S. Mathematical Expression of Slag-Metal Reactions in Steelmaking Process by Quadratic Formalism Based on the Regular Solution Model. ISIJ Int. 1993, 33, 2–11. [Google Scholar] [CrossRef]
- Madelung, O. Diffusion in Solid Metals, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 124–130. [Google Scholar]




| No. | Fe–Mn–Si Alloy/Mass Percent (pct) | |||||
|---|---|---|---|---|---|---|
| Mn | Si | Fe | S | |||
| A | 0.60 | 1.31 | 98.09 | 0.0023 | ||
| No. | CaO–SiO2–Al2O3–MgO–MnO–FeO oxide/mass pct | |||||
| CaO | SiO2 | Al2O3 | MgO | MnO | FeO | |
| 1-0 | 27.10 | 43.00 | 13.70 | 5.80 | 10.40 | 0.00 |
| 1-1 | 26.83 | 42.57 | 13.56 | 5.74 | 10.30 | 1.00 |
| 1-2 | 26.29 | 41.71 | 13.29 | 5.63 | 10.09 | 3.00 |
| 1-3 | 25.75 | 40.85 | 13.02 | 5.51 | 9.88 | 5.00 |
| 2-0 | 26.70 | 39.30 | 12.90 | 5.40 | 15.70 | 0.00 |
| 2-1 | 26.43 | 38.91 | 12.77 | 5.35 | 15.54 | 1.00 |
| 2-2 | 25.90 | 38.12 | 12.51 | 5.24 | 15.23 | 3.00 |
| 2-3 | 25.37 | 37.34 | 12.26 | 5.13 | 14.92 | 5.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ye, F.; Zhang, H.; Liu, X.; Wang, B. Study on the Interfacial Reactions between an Fe–Mn–Si Alloy and Complex Oxides Containing FeO during Isothermal Heating. Metals 2018, 8, 251. https://doi.org/10.3390/met8040251
Liu C, Ye F, Zhang H, Liu X, Wang B. Study on the Interfacial Reactions between an Fe–Mn–Si Alloy and Complex Oxides Containing FeO during Isothermal Heating. Metals. 2018; 8(4):251. https://doi.org/10.3390/met8040251
Chicago/Turabian StyleLiu, Chengsong, Fei Ye, Hua Zhang, Xiaoqin Liu, and Bao Wang. 2018. "Study on the Interfacial Reactions between an Fe–Mn–Si Alloy and Complex Oxides Containing FeO during Isothermal Heating" Metals 8, no. 4: 251. https://doi.org/10.3390/met8040251





