Influence of Solution-Annealing Parameters on the Continuous Cooling Precipitation of Aluminum Alloy 6082
Abstract
:1. Introduction
2. Materials and Methods
2.1. Investigated Aluminum Alloys
2.2. Differential Scanning Calorimetry (DSC)
2.3. Data Processing of Raw Measured Heat Flow Curves
2.4. Microscopy
2.5. Hardness Tests
3. Results and Discussion
3.1. Continuous Heating of the T651 Initial Condition
- Solution-annealing temperatures: 540, 550, and 560 °C;
- Solution-annealing soaking durations: 1 and 20 min;
- cooling rates: 0.01–5 K/s.
3.2. Continuous Cooling Experiments, Starting from Different Solution Treatment Conditions
- According to Figure 2 (and the related discussion), the solvus temperature of the initial T651 condition of this particular batch of EN AW-6082 for slow heating is below 560 °C.
- If cooling is starting from a more-or-less complete solution (e.g., 560 °C solution-annealing temperature and 20 min soaking time), the main precipitation begins after a certain degree of undercooling.
- The quasi-binary phase diagram Al-Mg2Si (Figure 6) indicates that the Mg2Si equilibrium solvus temperature for this alloy is near or slightly above 540 °C.
- →
- i.: The heating rate-specific solvus temperature can drop below 560 °C during very slow heating, as demonstrated in Figure 2. The cooling experiments in Figure 5 were conducted after relatively fast heating (2 K/s). Therefore, according to Figure 2, the dissolution remained incomplete at the end of the heating process. A complete solution can still be obtained with the switch to isothermal soaking, as seen in Figure 5B. Here 20 min of soaking at 550 and 560 °C results in precipitation beginning after a certain degree of undercooling, while a short soaking of 1 min at 560 °C still causes the direct onset of precipitation.
- →
- ii.: Taking the cooling rate of 0.3 K/s as an example, cooling from 560 °C and 20 min of soaking causes the precipitation to start at about 500 °C, after a certain degree of undercooling. However, starting from a condition that we claim to be an incomplete solution (e.g., 540 °C solution-annealing temperature), the same cooling rate of 0.3 K/s causes the immediate onset of precipitation when the temperature drops below 540 °C, with precipitation thus starting 40 K higher than that in the complete solution. Two different types of precipitation onset (1: immediate precipitation onset with the onset of cooling; and 2: onset of precipitation after a certain degree of undercooling) were attributed to the observations from the quasi-binary Al-Mg2Si phase diagram, which depended on whether the cooling starts from the α-Al solid-solution single-phase region or from the α-Al + β-Mg2Si two-phase region [21].
- →
- iii.: The three applied solution temperatures are indicated in the quasi-binary phase diagram Al-Mg2Si and shown in Figure 6, as well as the estimated maximum amount of Mg2Si for the particular batch of EN AW-6082 that was used in the experiments. While the comparison of EN AW-6082 with this phase diagram is a simplification, the precipitation of the β-Mg2Si phase from the α-Al solid solution is dominating the HTRs during cooling from the solution treatment of AlMgSi alloys [5], including two batches of EN AW-6082. It is thus inferred that the most likely phase to be considered in terms of complete solution is β-Mg2Si. The equilibrium solvus of the estimated β-Mg2Si mass fraction is close to 540 °C, which is additionally supported by the DSC experiments shown in Figure 7. The latter used soaking durations ranging from 1 to 120 min at 540 °C that preceded a cooling rate of 1 K/s. A complete solution was still not attained after two hours of soaking at 540 °C, with the Mg2Si precipitation starting concurrently with the onset of cooling. Therefore, it is reasonable to assume that the alloy batch-specific equilibrium β-Mg2Si solvus temperature is close to 540 °C (very likely a few Kelvin above 540 °C).
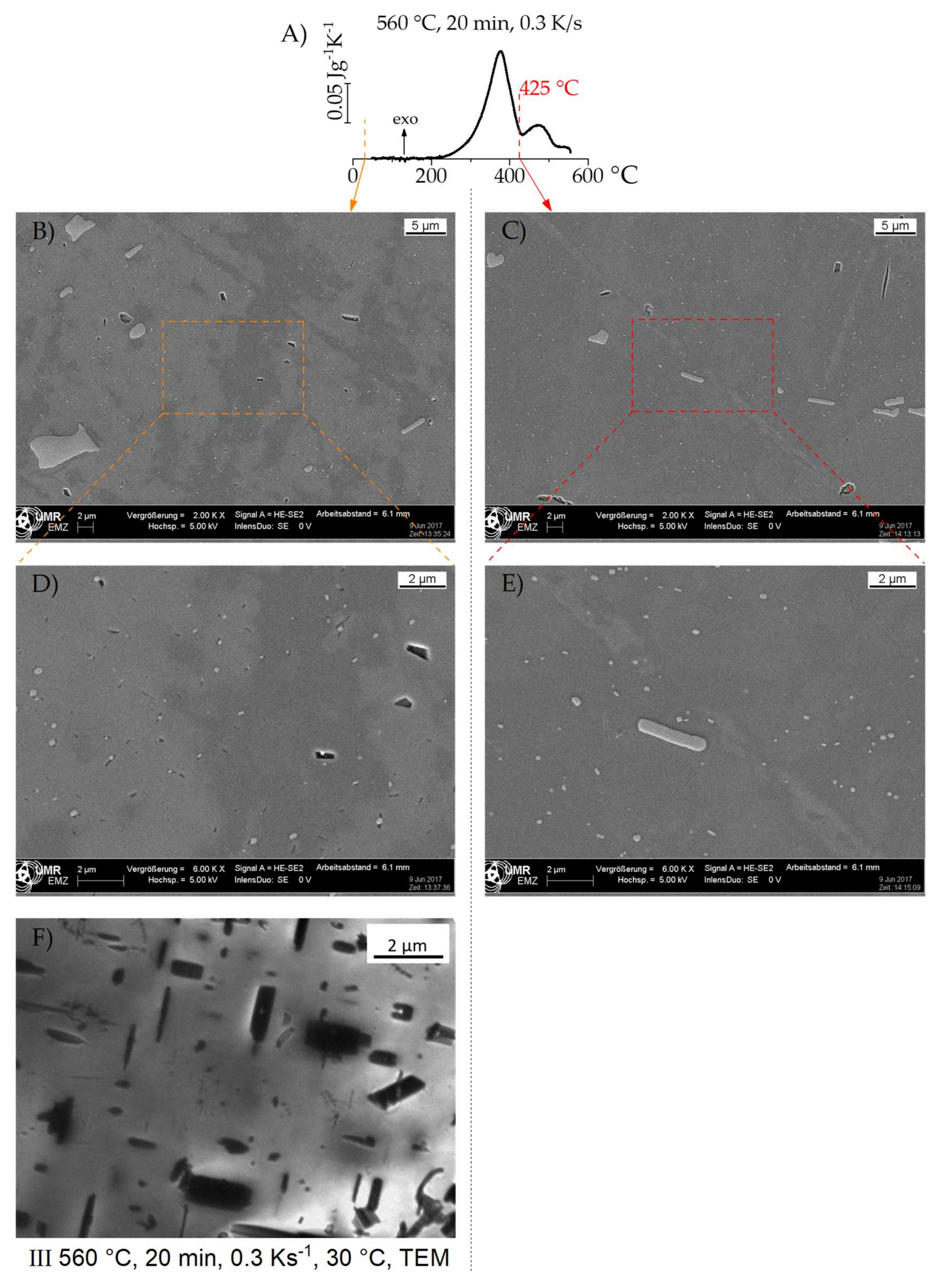
3.3. Analysis of the Quench-Induced Microstructure
3.4. Hardness Development after Cooling at Various Rates and Additional Artificial Aging
4. Conclusions
- The precipitation behavior during cooling after solution-annealing is significantly dependent upon whether complete or incomplete dissolution occurred during the solution treatment, particularly for medium to faster cooling rates (which are technologically relevant).
- The undissolved Mg2Si particles can instantly begin to grow within the solution at the onset of cooling when there is incomplete dissolution. Thus, undissolved particles allow for the immediate start of quench-induced precipitation.
- Quench-induced precipitation requires nucleation when there is complete dissolution. The required nucleation, in combination with dynamic suppression due to cooling, causes a certain degree of undercooling before the onset of precipitation.
- Incomplete dissolution increases the quench sensitivity. The upper critical cooling rate may increase by a factor of three. At medium cooling rates, the hardness is reduced by up to 30 %.
- Complete dissolution is highly recommended to ensure optimal alloy performance that exploits its full age-hardening potential. This can be achieved by using an appropriately high-solution temperature while maintaining a short soaking duration (a few minutes).
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Polmear, I.J. Light Alloys. From Traditional Alloys to Nanocrystals, 4th ed.; Elsevier Butterworth-Heinemann: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Milkereit, B.; Kessler, O.; Schick, C. Recording of continuous cooling precipitation diagrams of aluminium alloys. Thermochim. Acta 2009, 492, 73–78. [Google Scholar] [CrossRef]
- Kammer, C. Aluminium Taschenbuch Band 1; Aluminium-Verlag, Aluminium-Zentrale Düsseldorf: Düsseldorf, Germany, 1998. [Google Scholar]
- Milkereit, B.; Wanderka, N.; Schick, C.; Kessler, O. Continuous cooling precipitation diagrams of Al-Mg-Si alloys. Mater. Sci. Eng. A 2012, 550, 87–96. [Google Scholar] [CrossRef]
- Milkereit, B.; Starink, M.J. Quench sensitivity of Al-Mg-Si alloys: A model for linear cooling and strengthening. Mater. Des. 2015, 76, 117–129. [Google Scholar] [CrossRef]
- Ostermann, F. Anwendungstechnologie Aluminium, 3rd ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Sarmast, A.; Serajzadeh, S.; Kokabi, A.H. A study on thermal responses, microstructural issues, and natural aging in gas tungsten arc welding of AA2024-T4. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2013, 228, 413–421. [Google Scholar] [CrossRef]
- Merklein, M.; Maren, J.; Lechner, M.; Kuppert, A. A review on tailored blanks—Production, applications and evaluation. J. Mater. Process. Technol. 2014, 214, 151–164. [Google Scholar] [CrossRef]
- Fröck, H.; Graser, M.; Milkereit, B.; Reich, M.; Lechner, M.; Merklein, M.; Kessler, O. Precipitation Behaviour and Mechanical Properties during Short-Term Heat Treatment for Tailor Heat Treated Profiles (THTP) of Aluminium Alloy 6060 T4. MSF 2016, 877, 400–406. [Google Scholar] [CrossRef]
- Geiger, M.; Merklein, M.; Vogt, U. Aluminum tailored heat treated blanks. Prod. Eng. Res. Dev. 2009, 3, 401–410. [Google Scholar] [CrossRef]
- Kerausch, M.; Merklein, M.; Geiger, M. Improved material flow for deep drawing of aluminium blanks by local laser heat treatment. In Proceedings of the 10th International Conference on Sheet Metal, SheMet 2003; Kals, H.J.J., Shirvani, B., Sing, U.P., Geiger, M., Eds.; University of Ulster: Jordanstown, UK, 2003; pp. 73–80. [Google Scholar]
- Strobel, K.; Lay, M.D.H.; Easton, M.A.; Sweet, L.; Zhu, S.; Parson, N.C.; Hill, A.J. Effects of quench rate and natural ageing on the age hardening behaviour of aluminium alloy AA6060. Mater. Charact. 2016, 111, 43–52. [Google Scholar] [CrossRef]
- Saito, T.; Marioara, C.D.; Røyset, J.; Marthinsen, K.; Holmestad, R. The effects of quench rate and pre-deformation on precipitation hardening in Al–Mg–Si alloys with different Cu amounts. Mater. Sci. Eng. A 2014, 609, 72–79. [Google Scholar] [CrossRef]
- Kim, J.; Hayashi, M.; Kobayashi, E.; Sato, T. Influence of Si addition on quenching sensitivity and formation of nano-precipitate in Al-Mg-Si alloys. J. Nanosci. Nanotechnol. 2016, 16, 1814–1817. [Google Scholar] [CrossRef] [PubMed]
- Osten, J.; Milkereit, B.; Schick, C.; Kessler, O. Dissolution and precipitation behaviour during continuous heating of Al-Mg-Si alloys in a wide range of heating rates. Materials 2015, 8, 2830–2848. [Google Scholar] [CrossRef]
- Sarge, S.M.; Höhne, G.W.H.; Hemminger, W.F. Calorimetry. Fundamentals Instrumentation and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014. [Google Scholar]
- Milkereit, B.; Kessler, O.; Schick, C. Precipitation and Dissolution Kinetics in Metallic Alloys with Focus on Aluminium Alloys by Calorimetry in a Wide Scanning Rate Range. In Fast Scanning Calorimetry; Schick, C., Mathot, V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 723–773. [Google Scholar]
- Kemsies, R.H.; Milkereit, B.; Wenner, S.; Holmestad, R.; Kessler, O. In situ DSC investigation into the kinetics and microstructure of dispersoid formation in Al-Mn-Fe-Si(-Mg) alloys. Mater. Des. 2018, 146C, 96–107. [Google Scholar] [CrossRef]
- Fröck, H.; Graser, M.; Reich, M.; Lechner, M.; Merklein, M.; Kessler, O. Influence of short-term heat treatment on the microstructure and mechanical properties of EN AW-6060 T4 extrusion profiles: Part A. Prod. Eng. Res. Dev. 2016, 10, 383–389. [Google Scholar] [CrossRef]
- Schumacher, P.; Pogatscher, S.; Starink, M.J.; Schick, C.; Mohles, V.; Milkereit, B. Quench-induced precipitates in Al–Si alloys: Calorimetric determination of solute content and characterisation of microstructure. Thermochim. Acta 2015, 602, 63–73. [Google Scholar] [CrossRef]
- Milkereit, B. Kontinuierliche Zeit-Temperatur-Ausscheidungs-Diagramme von Al-Mg-Si-Legierungen. Ph.D. Thesis, University of Rostock, Rostock, Germany, 2011. [Google Scholar]
- Zhang, Z.; Farrar, R.A. An Atlas of Continuous Cooling Transformation (CCT) Diagrams Applicable to Low Carbon Low Alloy Weld Metals; Bourne Press Ltd.: Southampton, UK, 1995. [Google Scholar]










| EN AW-6082 | Mass Fraction, in % | |||||||
|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | |
| OES | 0.83 | 0.38 | 0.06 | 0.48 | 0.92 | 0.03 | 0.01 | 0.02 |
| DIN EN 573-3 | 0.7–1.3 | ≤0.5 | ≤0.1 | 0.4–1.0 | 0.6–1.2 | ≤0.25 | ≤0.2 | ≤0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröck, H.; Milkereit, B.; Wiechmann, P.; Springer, A.; Sander, M.; Kessler, O.; Reich, M. Influence of Solution-Annealing Parameters on the Continuous Cooling Precipitation of Aluminum Alloy 6082. Metals 2018, 8, 265. https://doi.org/10.3390/met8040265
Fröck H, Milkereit B, Wiechmann P, Springer A, Sander M, Kessler O, Reich M. Influence of Solution-Annealing Parameters on the Continuous Cooling Precipitation of Aluminum Alloy 6082. Metals. 2018; 8(4):265. https://doi.org/10.3390/met8040265
Chicago/Turabian StyleFröck, Hannes, Benjamin Milkereit, Philipp Wiechmann, Armin Springer, Manuela Sander, Olaf Kessler, and Michael Reich. 2018. "Influence of Solution-Annealing Parameters on the Continuous Cooling Precipitation of Aluminum Alloy 6082" Metals 8, no. 4: 265. https://doi.org/10.3390/met8040265
APA StyleFröck, H., Milkereit, B., Wiechmann, P., Springer, A., Sander, M., Kessler, O., & Reich, M. (2018). Influence of Solution-Annealing Parameters on the Continuous Cooling Precipitation of Aluminum Alloy 6082. Metals, 8(4), 265. https://doi.org/10.3390/met8040265






