Effect of Micro-Segregation on Impact Toughness of 2.25Cr-1Mo Steel after Post Weld Heat Treatment
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Cause Analysis of Crack Initiation Point
3.2. Cause Analysis of Crack Propagation
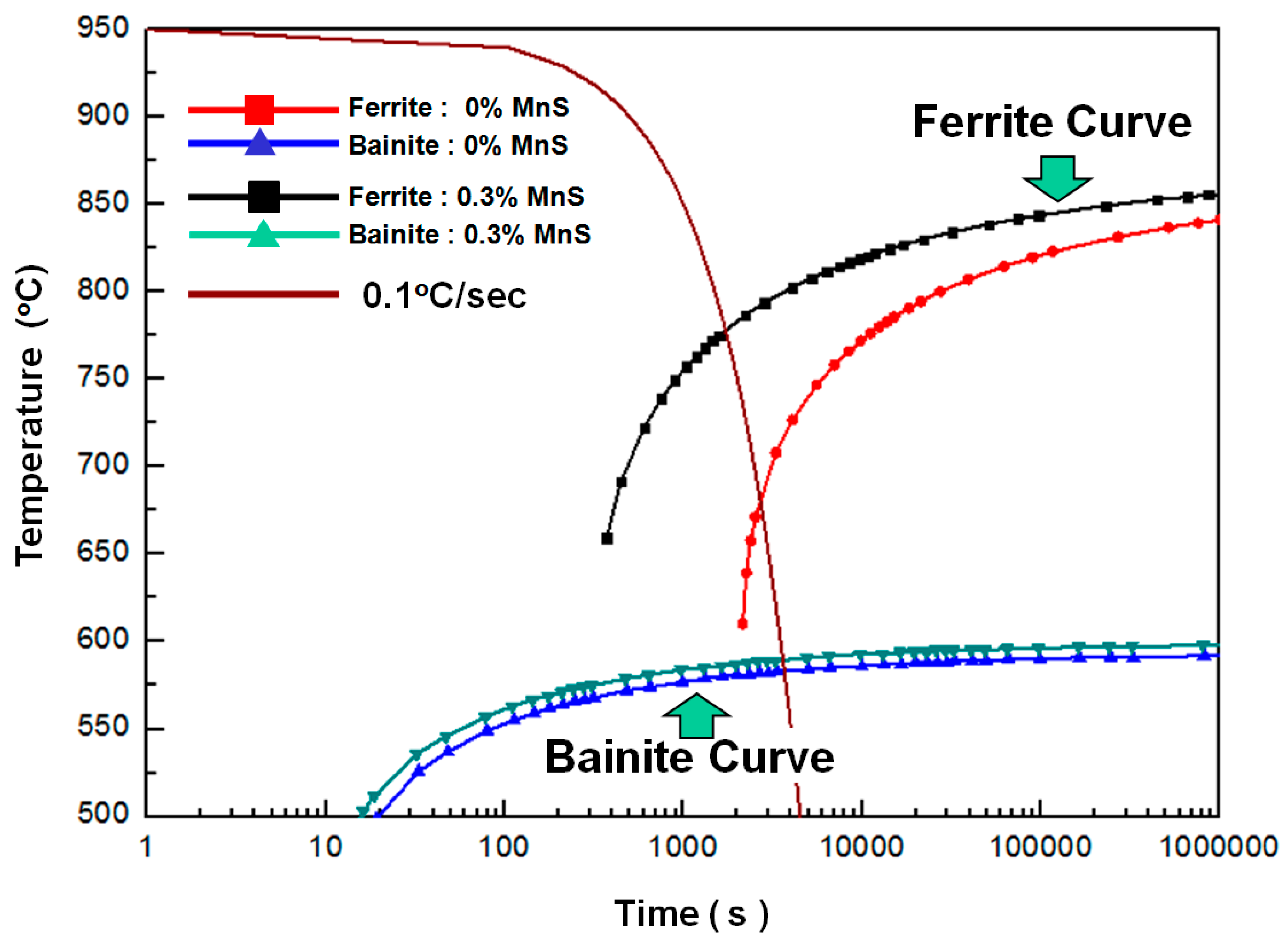
3.2.1. Effect of Ferrite on Impact Value
3.2.2. Effect of Carbide on Impact Value
- -
- 28J: 0.1% C Base Metal Composition = 83.51% Ferrite Volume Fraction X 0.025% C + C content of 16.49% the other constituent ==> C content of 16.49% the other constituent = 0.4798%
- -
- 386J: 0.1% C Base Metal Composition = 64.76% Ferrite Volume Fraction X 0.025% C + C content of 35.24% the other constituent ==> C content of 35.24% the other constituent = 0.2378%
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Viswanathan, R.; Bakker, W. Materials for ultrasupercritical coal power plants—Boiler materials: Part 1. J. Mater. Eng. Perform. 2001, 10, 81–95. [Google Scholar] [CrossRef]
- Masuyama, F. History of power plants and progress in heat resistant steels. ISIJ Int. 2001, 41, 612–625. [Google Scholar] [CrossRef]
- Viswanathan, R.; Coleman, K.; Rao, U. Materials for ultra-supercritical coal-fired power plant boilers. Int. J. Press. Vessels Pip. 2006, 83, 778–783. [Google Scholar] [CrossRef]
- Ray, A.K.; Tiwari, Y.N.; Roy, P.K.; Chaudhuri, S.; Bose, S.C.; Ghosh, R.N.; Whittenberger, J.D. Creep rupture analysis and remaining life assessment of 2.25 Cr–1Mo steel tubes from a thermal power plant. Mater. Sci. Eng. A 2007, 454, 679–684. [Google Scholar] [CrossRef]
- Klueh, R.L.; Nelson, A.T. Ferritic/martensitic steels for next-generation reactors. J. Nucl. Mater. 2007, 371, 37–52. [Google Scholar] [CrossRef]
- Dhooge, A.; Vinckier, A. Reheat cracking—A review of recent studies. Int. J. Press. Vessels Pip. 1987, 27, 239–269. [Google Scholar] [CrossRef]
- Dhooge, A.; Vekeman, J. New generation 2 1/4 Cr steels T/P 23 and T/P 24 weldability and high temperature properties. Weld. World 2005, 49, 75–93. [Google Scholar] [CrossRef]
- Arivazhagan, B.; Vasudevan, M. Studies on A-TIG welding of 2.25 Cr-1Mo (P22) steel. J. Manuf. Process. 2015, 18, 55–59. [Google Scholar] [CrossRef]
- Boiler, A.S.M.E.; Code, P.V. Section VIII Division 1; UG-126 Pressure Relief Valves to UG-129 Marking; ASME International: New York, NY, USA, 2010. [Google Scholar]
- Toshiro, K.; Isamu, Y.; Mitsuo, N. Evaluation of dynamic fracture toughness parameters by instrumented Charpy impact test. Eng. Fract. Mech. 1986, 24, 773–782. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, H.; Lee, Y. Experimental study on variations in charpy impact energies of low carbon steel, depending on welding and specimen cutting method. J. Mech. Sci. Technol. 2016, 30, 2019–2028. [Google Scholar] [CrossRef]
- Lucon, E.; Mccowan, C.; Santoyo, N.; Raymond, L. Instrumented impact tests: Effects of machine variables and specimen position. J. Test. Eval. 2008, 37, 59–68. [Google Scholar]
- Kurishita, H.; Kayano, H.; Narui, M.; Yamazaki, M.; Kano, Y.; Shibahara, I. Effects of V-notch dimensions on Charpy impact test results for differently sized miniature specimens of ferritic steel. Mater. Trans. JIM 1993, 34, 1042–1052. [Google Scholar] [CrossRef]
- Im, Y.R.; Oh, Y.J.; Lee, B.J.; Hong, J.H.; Lee, H.C. Effects of carbide precipitation on the strength and Charpy impact properties of low carbon Mn–Ni–Mo bainitic steels. J. Nucl. Mater. 2001, 297, 138–148. [Google Scholar] [CrossRef]
- Sun, C.; Fu, P.X.; Liu, H.W.; Liu, H.H.; Du, N.Y. Effect of Tempering Temperature on the Low Temperature Impact Toughness of 42CrMo4-V Steel. Metals 2018, 8, 232. [Google Scholar] [CrossRef]
- Shen, D.D.; Song, S.H.; Yuan, Z.X.; Weng, L.Q. Effect of solute grain boundary segregation and hardness on the ductile-to-brittle transition for a Cr–Mo low-alloy steel. Mater. Sci. Eng. A 2005, 394, 53–59. [Google Scholar] [CrossRef]
- Hwang, B.; Kim, Y.G.; Lee, S.; Kim, Y.M.; Kim, N.J.; Yoo, J.Y. Effective grain size and Charpy impact properties of high-toughness X70 pipeline steels. Metall. Mater. Trans. A 2005, 36, 2107–2114. [Google Scholar] [CrossRef]
- Ghosha, A.; Sahoob, S.; Ghoshc, M.; Ghosha, R.N.; Chakrabarti, D. Effect of microstructural parameters, microtexture and matrix strain on the Charpy impact properties of low carbon HSLA steel containing MnS inclusions. Mater. Sci. Eng. A 2014, 613, 37–47. [Google Scholar] [CrossRef]
- Melchers, R.E.; Chaves, I.A.; Jeffrey, R. A Conceptual Model for the Interaction between Carbon Content and Manganese Sulphide Inclusions in the Short-Term Seawater Corrosion of Low Carbon Steel. Metals 2016, 6, 132. [Google Scholar] [CrossRef]
- Speich, G.R.; Spitzig, W.A. Effect of volume fraction and shape of sulfide inclusions on through-thickness ductility and impact energy of high-strength 4340 plate steels. Metall. Trans. A 1982, 13, 2239–2258. [Google Scholar] [CrossRef]
- Tsunekage, N.; Tsubakino, H. Effects of sulfur content and sulfide-forming elements addition on impact properties of ferrite–pearliticmicroalloyed steels. ISIJ Int. 2001, 41, 498–505. [Google Scholar] [CrossRef]
- Cheruvu, N.S. Degradation of mechanical properties of Cr-Mo-V and 2.25 Cr-1Mo steel components after long-term service at elevated temperatures. Metall. Trans. A 1989, 20, 87–97. [Google Scholar] [CrossRef]
- Laha, K.; Rao, K.B.S.; Mannan, S.L. Creep behaviour of post-weld heat-treated 2.25 Cr-1Mo ferritic steel base, weld metal and weldments. Mater. Sci. Eng. A 1990, 129, 183–195. [Google Scholar] [CrossRef]
- Mitra, R.; Srivastava, A.K.; Prasad, N.E.; Kumari, S. Microstructure and mechanical behaviour of reaction hot pressed multiphase Mo–Si–B and Mo–Si–B–Al intermetallic alloys. Intermetallics 2006, 14, 1461–1471. [Google Scholar] [CrossRef]
- Garrison, W.M., Jr.; Wojcieszynski, A.L. A discussion of the effect of inclusion volume fraction on the toughness of steel. Mater. Sci. Eng. A 2007, 464, 321–329. [Google Scholar] [CrossRef]
- Murakami, Y. Material defects as the basis of fatigue design. Int. J. Fatigue 2012, 41, 2–10. [Google Scholar] [CrossRef]
- Yokomizo, T.; Enomoto, M.; Umezawa, O.; Spanos, G.; Rosenberg, R.O. Three-dimensional distribution, morphology, and nucleation site of intragranular ferrite formed in association with inclusions. Mater. Sci. Eng. A 2003, 344, 261–267. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, Z.X.; Yang, Z.G.; Liu, Z.H. Influence of prior austenite deformation and non-metallic inclusions on ferrite formation in low-carbon steels. J. Iron Steel Res. Int. 2010, 17, 36–42. [Google Scholar] [CrossRef]
- Xu, S.; Wang, C.; Wang, W. Failure analysis of stress corrosion cracking in heat exchanger tubes during start-up operation. Eng. Fail. Anal. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Henthorne, M. The Slow Strain Rate Stress Corrosion Cracking Test—A 50 Year Retrospective. Corrosion 2016, 72, 1488–1518. [Google Scholar] [CrossRef]













| Material | Chemical Composition (at. %) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | Cu | Ni | Cr | Mo | V | Nb | Al | P | S | Fe | |
| P22 | 0.102 | 0.218 | 0.433 | 0.120 | 0.1246 | 2.0948 | 0.930 | 0.011 | 0.0023 | 0.0251 | 0.0140 | 0.0048 | Bal |
| 0.097 | 0.227 | 0.443 | 0.124 | 0.1239 | 2.0305 | 0.931 | 0.012 | 0.0009 | 0.0247 | 0.0116 | 0.0039 | Bal | |
| 0.100 | 0.229 | 0.434 | 0.116 | 0.1230 | 2.0051 | 0.931 | 0.0141 | 0 | 0.0246 | 0.0098 | 0.0047 | Bal | |
| Average | 0.100 | 0.225 | 0.436 | 0.120 | 0.1238 | 2.0435 | 0.931 | 0.0124 | 0.0011 | 0.0248 | 0.0118 | 0.0047 | Bal |
| Condition | Actual |
|---|---|
| Loading Temperature | 20 °C |
| Heating Rate | 20 °C/h |
| Holding Temperature | 700–710 °C |
| Holding Time | 11 h |
| Cooling Rate (above 425 °C) | 20–30 °C/h |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, H.; Lee, S.; Kang, C. Effect of Micro-Segregation on Impact Toughness of 2.25Cr-1Mo Steel after Post Weld Heat Treatment. Metals 2018, 8, 373. https://doi.org/10.3390/met8060373
Na H, Lee S, Kang C. Effect of Micro-Segregation on Impact Toughness of 2.25Cr-1Mo Steel after Post Weld Heat Treatment. Metals. 2018; 8(6):373. https://doi.org/10.3390/met8060373
Chicago/Turabian StyleNa, Hyesung, Sanghoon Lee, and Chungyun Kang. 2018. "Effect of Micro-Segregation on Impact Toughness of 2.25Cr-1Mo Steel after Post Weld Heat Treatment" Metals 8, no. 6: 373. https://doi.org/10.3390/met8060373
APA StyleNa, H., Lee, S., & Kang, C. (2018). Effect of Micro-Segregation on Impact Toughness of 2.25Cr-1Mo Steel after Post Weld Heat Treatment. Metals, 8(6), 373. https://doi.org/10.3390/met8060373





