Martens-ite
Abstract
:1. Introduction
2. Kinetics of Martensitic Transformation
3. Crystallography of the Martensitic Transformation
3.1. Homogeneous Shape Strain
3.2. Homogeneous Lattice Strain
3.3. Errors and Variability in Measured Crystallographic Features
3.4. Accommodation of the Martensitic Transformation
4. Nature of the Parent–Martensite Interface
- (a)
- Planar, irrational, and semi-coherent and separating a single crystal parent from a slipped or faulted single crystal of martensite;
- (b)
- Planar, irrational, and separating a single crystal parent from a twinned martensite product;
- (c)
- Curved and macroscopically displaced from the “true” (normally irrational) habit plane.
5. Reversible Martensitic Transformation
- the transformation hysteresis shrinks and the transformation becomes easily reversible [11]
- the martensite plates become much thinner [11]
- the martensite becomes BCT with a high tetragonality [36]
- the transformation twin spacing decreases [36]
- the habit plane normal shifts towards a plane of two-fold symmetry [51]
- elastic softening of the austenite crystal lattice occurs over a temperature range covering Ms, reducing the chemical driving force for nucleation [53].
6. Shape Memory Phenomena
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Osmond, F. Nomenclature in Metallography. J. Iron Steel Inst. 1902, 61, 90. [Google Scholar]
- Bain, E.C. The Sorby Centennial Symposium on the History of Metallurgy; Smith, C.S., Ed.; Gordon and Breach Science Publ.: New York, NY, USA, 1965. [Google Scholar]
- Campbell, W. Twenty-Five Years of Metallography, 3rd Howe Memorial Award, 1926. Trans. AIME 1926, 73, 1135. [Google Scholar]
- Greninger, A.B.; Mooradian, V.G. Strain transformation in metastable beta copper-zinc and beta copper-titanium alloys. Trans. AIME 1938, 128, 337. [Google Scholar]
- Christian, J.W. Military Transformations: An Introductory Survey. In Physical Properties of Martensite and Bainite; Iron and Steel Institute: London, UK, 1965; p. 93. [Google Scholar]
- Wayman, C.M. Martensitic Transformations. In Modern Diffraction and Imaging Techniques in Materials Science; Amelinckx, S., Gevers, R., Remaut, G., Van Lunduyt, J., Eds.; North-Holland Publishing Company: Amsterdam, The Netherland, 1970; pp. 187–232. [Google Scholar]
- Olsen, G.B.; Owen, W.S. Martensite; ASM Press: Washington, DC, USA, 1992. [Google Scholar]
- Petty, E.R. Martensite; Longman Group Ltd.: London, UK, 1970. [Google Scholar]
- Nishiyama, Z. Martensitic Transformations; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Bhattacharya, K. Microstructure of Martensite: Why It Forms and How It Gives Rise to the Shape Memory Effect; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Dunne, D.P.; Wayman, C.M. The effect of austenite ordering on the martensite transformation in Fe-Pt near the composition Fe3Pt: I. Morphology and transformation characteristics; II. Crystallography and general features. Metall. Trans. 1973, 4, 137–145. [Google Scholar] [CrossRef]
- Wayman, C.M. On memory effects related to martensitic transformations and observations in β brass and Fe3Pt. Scr. Metall. 1971, 5, 489–492. [Google Scholar] [CrossRef]
- Raghavan, V. Kinetics of martensitic transformation. In Martensite; Olsen, G.B., Owen, W.S., Eds.; ASM International: Cleveland, OH, USA, 1992; pp. 197–225. [Google Scholar]
- Christian, J.W. Basic crystallography and kinetics. In Martensite; Petty, E.R., Ed.; Longman Group Ltd.: London, UK, 1970; Chapter 2; pp. 11–41. [Google Scholar]
- Bowles, J.S.; Mackenzie, J. The crystallography of martensitic transformations I, II and III. Acta Metall. 1954, 2, 129–137, 138–147, 224–234. [Google Scholar] [CrossRef]
- Wechsler, M.S.; Lieberman, D.S.; Read, T.A. On the theory of formation of martensite. Trans. AIME 1953, 197, 1503–1515. [Google Scholar]
- Kennon, N.F.; Dunne, D.P. Shape strains associated with thermally-induced and stress-induced martensite in a Cu-Al-Ni shape memory alloy. Acta Metall. 1982, 30, 429–435. [Google Scholar] [CrossRef]
- Dunne, D.P.; Bowles, J.S. Measurement of the shape strain for (225) and (259) martensitic transformations. Acta Metall. 1969, 17, 201–212. [Google Scholar] [CrossRef]
- Efsic, E.J.; Wayman, C.M. Crystallography of the fcc-to-bcc martensitic transformation in an Fe-Pt alloy. Trans. AIME 1967, 239, 873–882. [Google Scholar]
- Dunne, D.P.; Wayman, C.M. On the validity of methods for determining the shape strain in deformation twinning and martensitic transformations. Acta Metall. 1979, 18, 981–990. [Google Scholar] [CrossRef]
- Krauklis, P.; Bowles, J.S. Direct measurement of the length change in the (225) martensite habit plane. Acta Metall. 1969, 17, 997. [Google Scholar] [CrossRef]
- McDougall, P.G.; Wayman, C.M. The crystallography and morphology of ferrous martensites. In Martensite; Olsen, G.B., Owen, W.S., Eds.; ASM International: Cleveland, OH, USA, 1992; pp. 59–95. [Google Scholar]
- Otsuka, K.; Wayman, C.M. Martensitic transformations: Crystallography. In Shape Memory Materials; Cambridge University Press: Cambridge, UK, 1998; pp. 5–21. [Google Scholar]
- Otsuka, K.; Wayman, C.M. Mechanism of shape memory and superelasticity. In Shape Memory Materials; Cambridge University Press: Cambridge, UK, 1998; pp. 27–48. [Google Scholar]
- Chang, L. On diffusionless transformation in Au-Cd single crystals containing 47.5 at. % Cd: Characterization of single interface transformation. J. Appl. Phys. 1952, 23, 725–728. [Google Scholar] [CrossRef]
- Bazinski, Z.S.; Christian, J.W. Experiments on martensite transformation in single crystals of indium-thallium alloys. Acta Metall. 1954, 2, 148–166. [Google Scholar] [CrossRef]
- Klostermann, J.A. The Concept of the Habit Plane and the Phenomenological Theories of the Martensite Transformation. J. Less Common Met. 1972, 28, 75–94. [Google Scholar] [CrossRef]
- Umemoto, M.; Tamura, I. The morphology and substructrure of butterfly martensite in ferrous alloys. J. Phys. Colloq. 1982, 43, C4-523–C4-528. [Google Scholar] [CrossRef]
- Maki, T.; Wayman, C.M. Transmission electron microscope studies of thin foil martensite in Fe-Ni and Fe-Ni-C alloys. Acta Metall. 1977, 25, 681–693. [Google Scholar] [CrossRef]
- Tamura, I.; Yoshimura, H.; Ibaraki, M.; Tagaya, M. Hardness and Structure of Ausformed Fe-Ni and Fe-Ni-C Alloys. Trans. Jpn. Inst. Met. 1964, 5, 47–52. [Google Scholar] [CrossRef]
- Acton, A.F.; Bevis, M. A Generalized Martensite Crystallographic Theory. Mater. Sci. Eng. 1969, 5, 19. [Google Scholar] [CrossRef]
- Ross, N.H.D.; Crocker, A.G. A generalized theory of the martensite crystallography and its application to transformations in steels. Acta Metall. 1970, 18, 405. [Google Scholar] [CrossRef]
- Dunne, D.P.; Wayman, C.M. An assessment of the double shear theory as applied to ferrous martensitic transformations. Acta Metall. 1970, 19, 425–438. [Google Scholar] [CrossRef]
- Bowles, J.S.; Dunne, D.P. The role of plastic accommodation in the (225) martensite transformation. Acta Metall. 1969, 17, 677–685. [Google Scholar] [CrossRef]
- Sulaiman, S. Structure and Properties of the Heat Affected Zone of P91 Creep Resistant Steel. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, July 2008. [Google Scholar]
- Tadaki, T.; Shimizu, K. High tetragonality of the thermoelastic Fe3Pt martensite and small volume change during transformation. Scr. Metall. 1973, 9, 771–776. [Google Scholar] [CrossRef]
- Maki, T.; Tamura, I. Shape memory effect in ferrous alloys. In Proceedings of the International Conference on Martensitic Transformations, ICOMAT’86; The Japan Institute of Metals: Sendai, Japan, 1987; pp. 963–970. [Google Scholar]
- Breedis, J.F.; Wayman, C.M. The martensitic transformation in Fe-31 wt. % Ni. Trans. AIME 1962, 24, 1128–1133. [Google Scholar]
- Dunne, D.; Kennon, N. The martensite interface—How regular is the habit? In Proceedings of the Interfaces II Conference, Melbourne, Australia, 2–5 June 1994; pp. 273–278. [Google Scholar]
- Otte, H.M.; Read, T.A. Habit planes of martensite in chrome-carbon steel. J. Met. 1957, 9, 412–417. [Google Scholar] [CrossRef]
- Bell, T.; Bryans, R.G. The Effect of Prior Transformation and Prestrain on the Habit Planes of Acicular Iron-Nickel Martensite. Met. Sci. J. 1971, 5, 135. [Google Scholar] [CrossRef]
- Christian, J.W. The Theory of Phase Transformations in Metals and Alloys; Pergamon Press: Oxford, UK, 1965. [Google Scholar]
- Dunne, D.P. The interface structure of martensite in Fe3Pt. Scr. Metall. 1978, 12, 143–146. [Google Scholar] [CrossRef]
- Kessler, H.; Pitsch, W. On the nature of reverse martensite to austenite transformation. Acta Metall. 1967, 15, 401–405. [Google Scholar] [CrossRef]
- Kurdjumov, G.V.; Khandros, L.G. On the “thermoelastic” equilibrium in martensitic transformations. Dokl. Akad. Nauk SSSR 1949, 66, 211–214. (In Russian) [Google Scholar]
- Chang, L.C.; Read, T.A. Plastic Deformation and Diffusionless Phase Changes in Metals in the Gold-Cadmium Beta Phase. Trans. AIME 1951, 191, 47–52. [Google Scholar] [CrossRef]
- Buehler, W.J.; Gilfrich, J.V.; Wiley, R.C. Effect of Low-Temperature Phase Changes on the Habit Planes of Alloys near the Composition TiNi. J. Appl. Phys. 1963, 34, 1475. [Google Scholar] [CrossRef]
- Firstov, G.S.; Kosorukova, T.A.; Koval, Y.N.; Verhovlyuk, P.A. Directions for High-Temperature Shape Memory Alloy Improvement: Straight Way to High-Entropy Materials? Shape Mem. Superelasticity 2015, 1, 400–407. [Google Scholar] [CrossRef]
- Dunne, D.P.; Stobbs, M.; Kennon, N.F. The effect of order on the martensitic transformation in Fe3Pt. Proc. Mat. Res. Soc. Symp. 1984, 21, 675–680. [Google Scholar] [CrossRef]
- Olson, G.B.; Cohen, M. Reply to “On the equilibrium temperature and thermoelastic martensitic transformation”. Scr. Metall. 1977, 11, 345. [Google Scholar] [CrossRef]
- Dunne, D.P. Martensitic iron-platinum alloys. In Proceedings of the International Conference on Displacive Transformations and Their Applications in Materials Engineering, Urbana, IL, USA; Inoue, K., Mukherjee, K., Otsuka, K., Chen, H., Eds.; TMS: Warrendale, PA, USA, 1996; pp. 133–140. [Google Scholar]
- Davies, R.G.; Magee, C.L. Austenite ferromagnetism and martensite morphology. Metall. Trans. 1971, 2, 1939. [Google Scholar]
- Grujicic, M.; Ling, H.C.; Haezebrouck, D.M.; Owen, W.S. Growth of martensite. In Martensite; Olsen, G.B., Owen, W.S., Eds.; ASM International: Cleveland, OH, USA, 1992; Chapter 10; pp. 175–197. [Google Scholar]
- Magee, C.L.; Davies, R.G. On the volume expansion accompanying f.c.c. to b.c.c. transformation in ferrous alloys. Acta Metall. 1972, 20, 1031–1043. [Google Scholar] [CrossRef]
- Lovey, F.C.; Van Tendeloo, G.; Van Lunduyt, J.; Delaey, L.; Amelinckx, S. On the nature of various stacking defects in 18R martensite in Cu-Al alloys. A study by high resolution microscopy. Phys. Stat. Sol. 1984, 86, 553–564. [Google Scholar] [CrossRef]
- Kajiwara, S.; Owen, W.S. The reversible martensite transformation in iron-platinum alloys near Fe3Pt. Metall. Trans. 1974, 5, 2047–2061. [Google Scholar] [CrossRef]
- Kajiwara, S.; Owen, W.S. The martensite-austenite interface and the thickness of twins in Fe3Pt. Scr. Metall. 1977, 11, 137–142. [Google Scholar] [CrossRef]
- Otsuka, K.; Wayman, C.M. Introduction. In Shape Memory Materials; Cambridge University Press: Cambridge, UK, 1998; pp. 1–26. [Google Scholar]
- Ren, X.; Otsuka, K. Origin of rubber-like behaviour in metal alloys. Nature 1997, 389, 579–582. [Google Scholar]
- Otsuka, K.; (NRIM, Tsukuba Japan). Personal communication, 2011.
- Saburi, T. Ti-Ni shape memory alloys. In Shape Memory Materials; Otsuka, K., Wayman, C.M., Eds.; Cambridge University Press: Cambridge, UK, 1998; pp. 49–96. [Google Scholar]
- Nishida, M.; Honma, T. All-round shape memory effect in Ni-rich TiNi alloys generated by constrained aging. Scr. Metall. 1984, 18, 1293. [Google Scholar] [CrossRef]
- Dunne, D.P. Transformation-induced anelasticity. J. Aust. Inst. Met. 1974, 19, 28–34. [Google Scholar]
- Lieberman, D.S. Phase Transformations; ASM: Geauga County, OH, USA, 1970; Volume 1. [Google Scholar]
- Lieberman, D.S.; Schmerling, M.A.; Karz, R.W. Ferroelastic ‘memory’ and mechanical properties of gold-cadmium. In Shape Memory Effects in Alloys; Perkins, J., Ed.; Plenum Press: New York, NY, USA, 1975; pp. 203–244. [Google Scholar]
- Stalmans, R.; Van Humbeeck, J.; Delaey, L. Training of copper based shape memory alloys. In Proceedings of the ICOMAT—92, Monterey, CA, USA, 20–24 July 1993; pp. 1065–1070. [Google Scholar]
- Otsuka, K.; Saxena, A.; Deng, J.; Ren, X. Mechanism of the shape memory effect in martensitic alloys: An assessment. Philos. Mag. 2011, 91, 4514–4535. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Conti, S.; Zanzotto, G.; Zimmer, J. Crystal symmetry and the reversibility of martensitic transformations. Nature 2004, 428, 55. [Google Scholar] [CrossRef] [PubMed]
- Maki, T. Microstructure and mechanical behaviour of ferrous martensite. In Proceedings of the International Conference on Martensitic Transformations (ICOMAT’89); Materials Science Forum; Muddle, B.C., Ed.; Trans Tech Publications Ltd: Stafa-Zurich, Switzerland, 1990; Volume 56–58, pp. 157–168. [Google Scholar]
- Ohmori, T.; Ando, K.; Okana, M.; Xu, X.; Tanaka, Y.; Ohnuma, I.; Kainuma, R.; Ishida, K. Superelastic Effect in Polycrystalline Ferrous Alloys. Science 2011, 333, 68. [Google Scholar] [CrossRef] [PubMed]
- Koval, Y.N.; Monastyrsky, G.E. Reversible martensite transformation and shape memory effect in Fe-Ni-Nb alloys. Scr. Metall. 1993, 28, 41–48. [Google Scholar] [CrossRef]
- Stockel, D. Status and Trends in Shape Memory Technology; Nitiol Development Corporation: Pittsburgh, PA, USA, 1992; pp. 79–84. [Google Scholar]

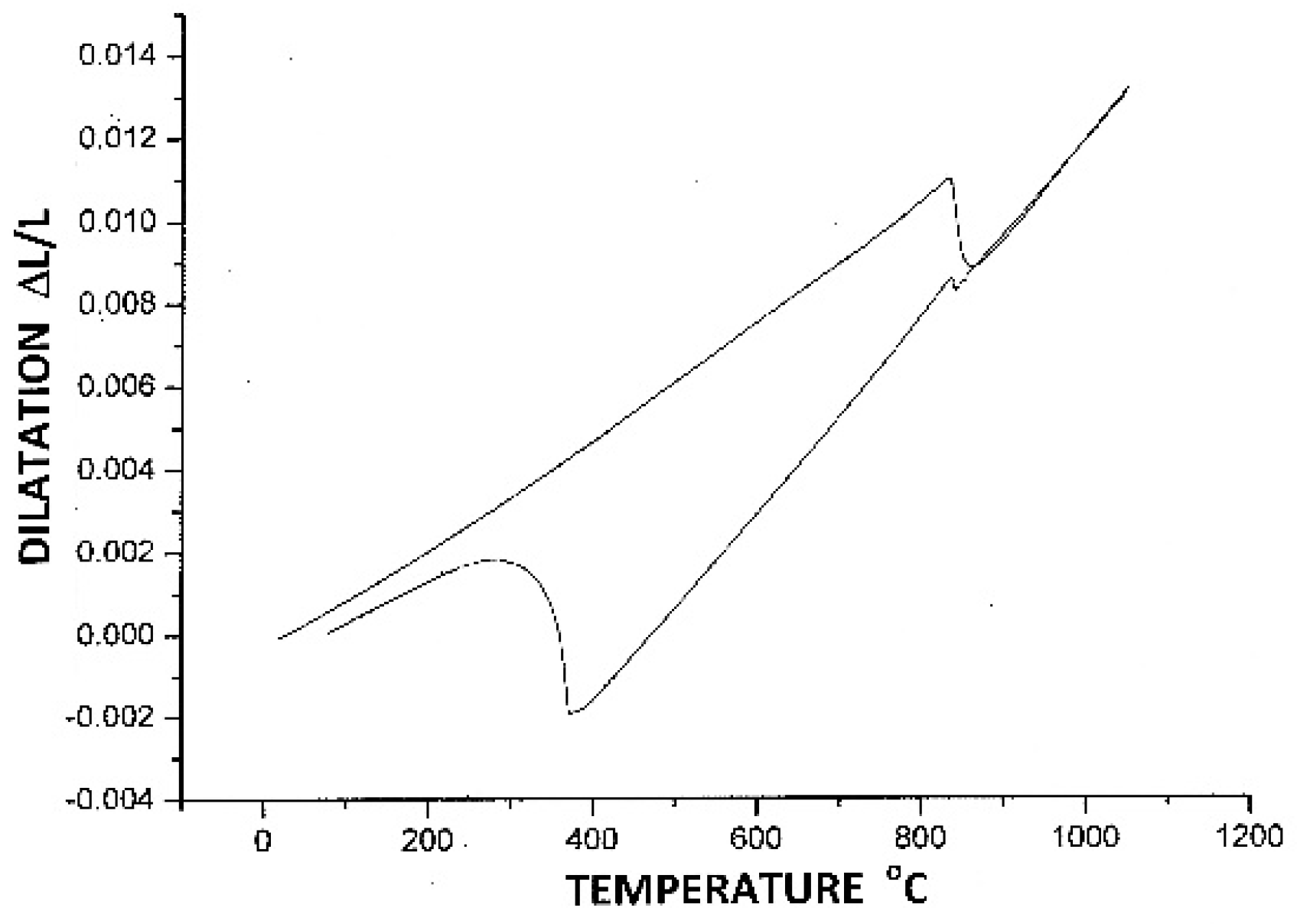

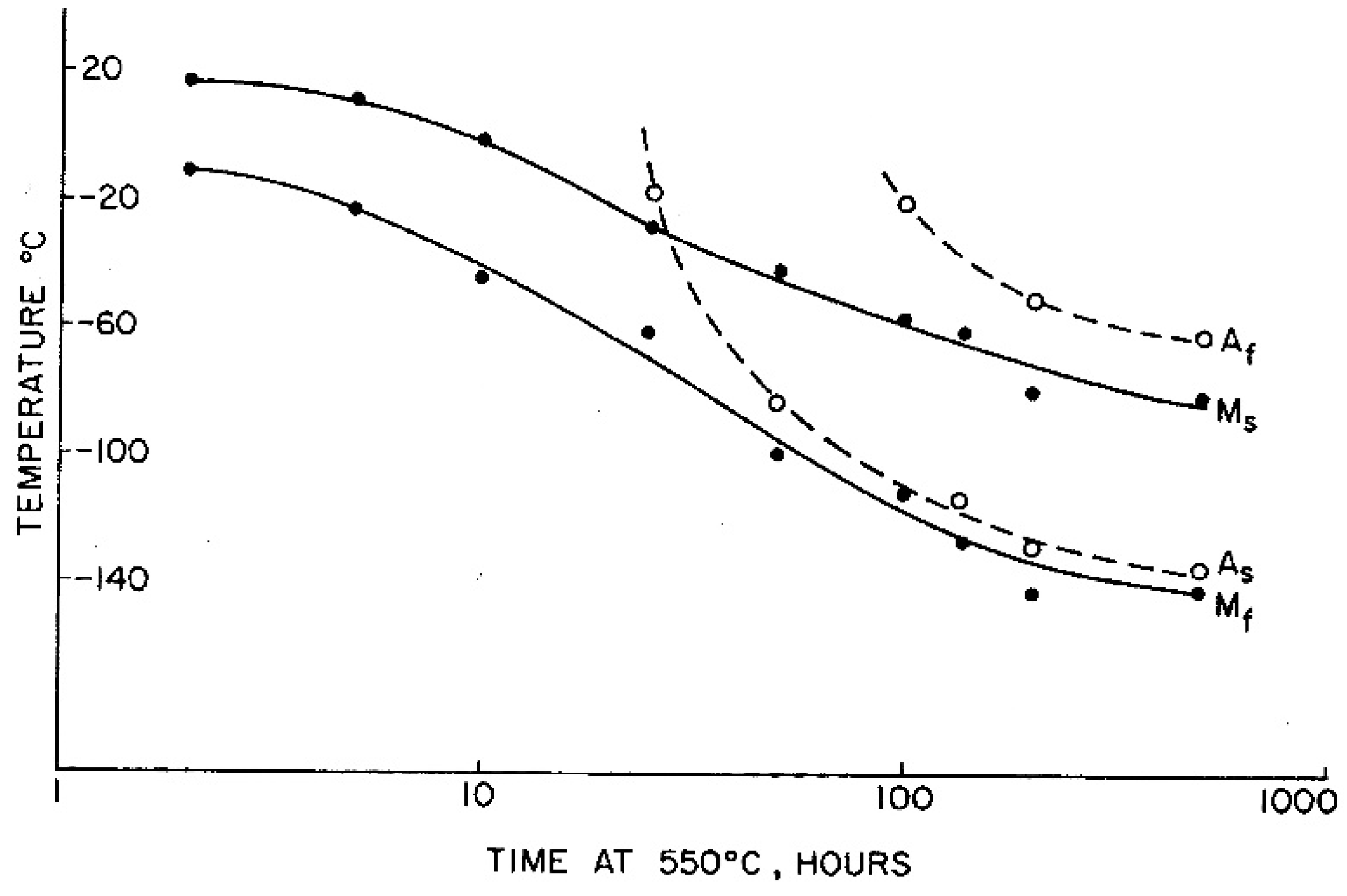
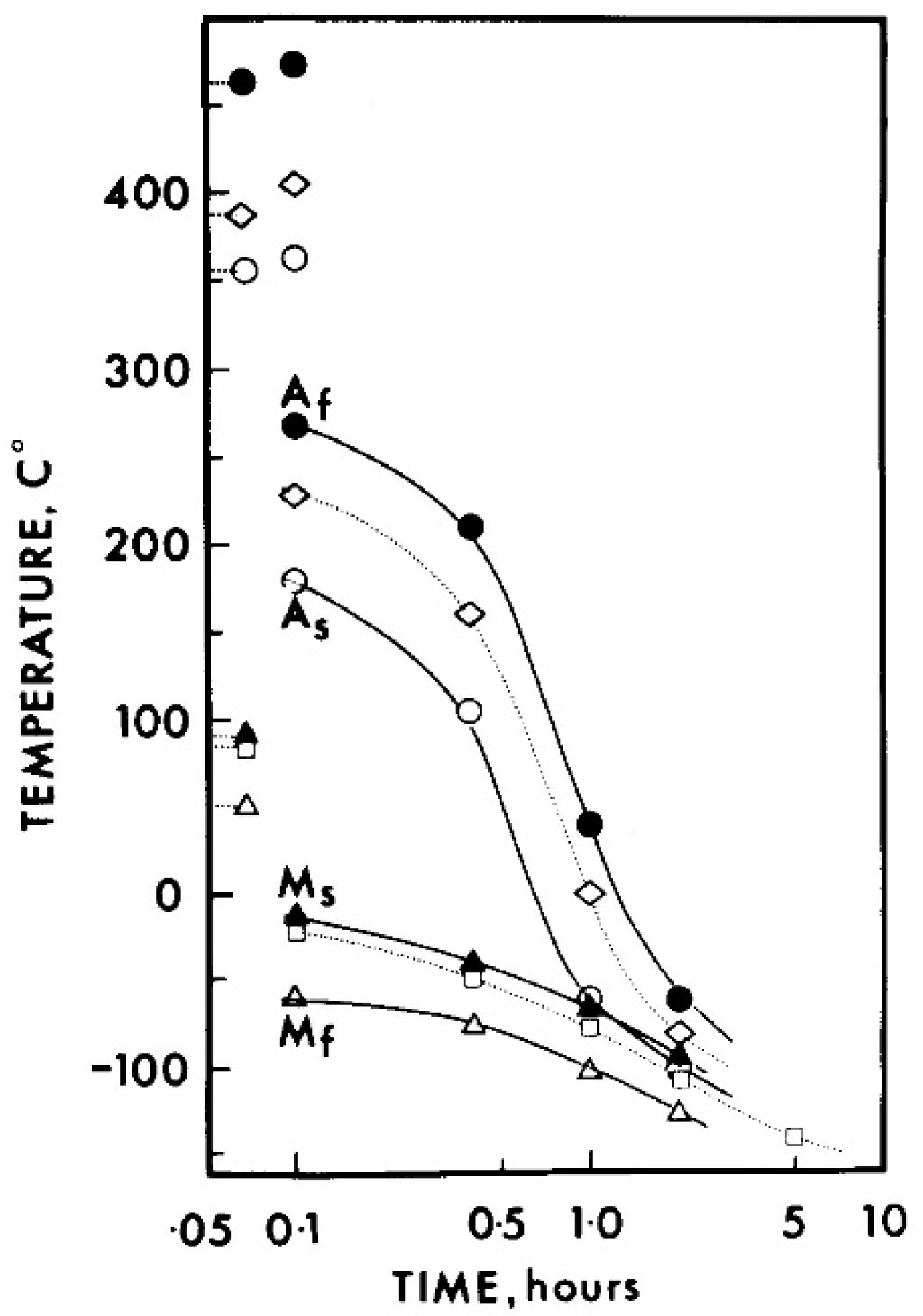
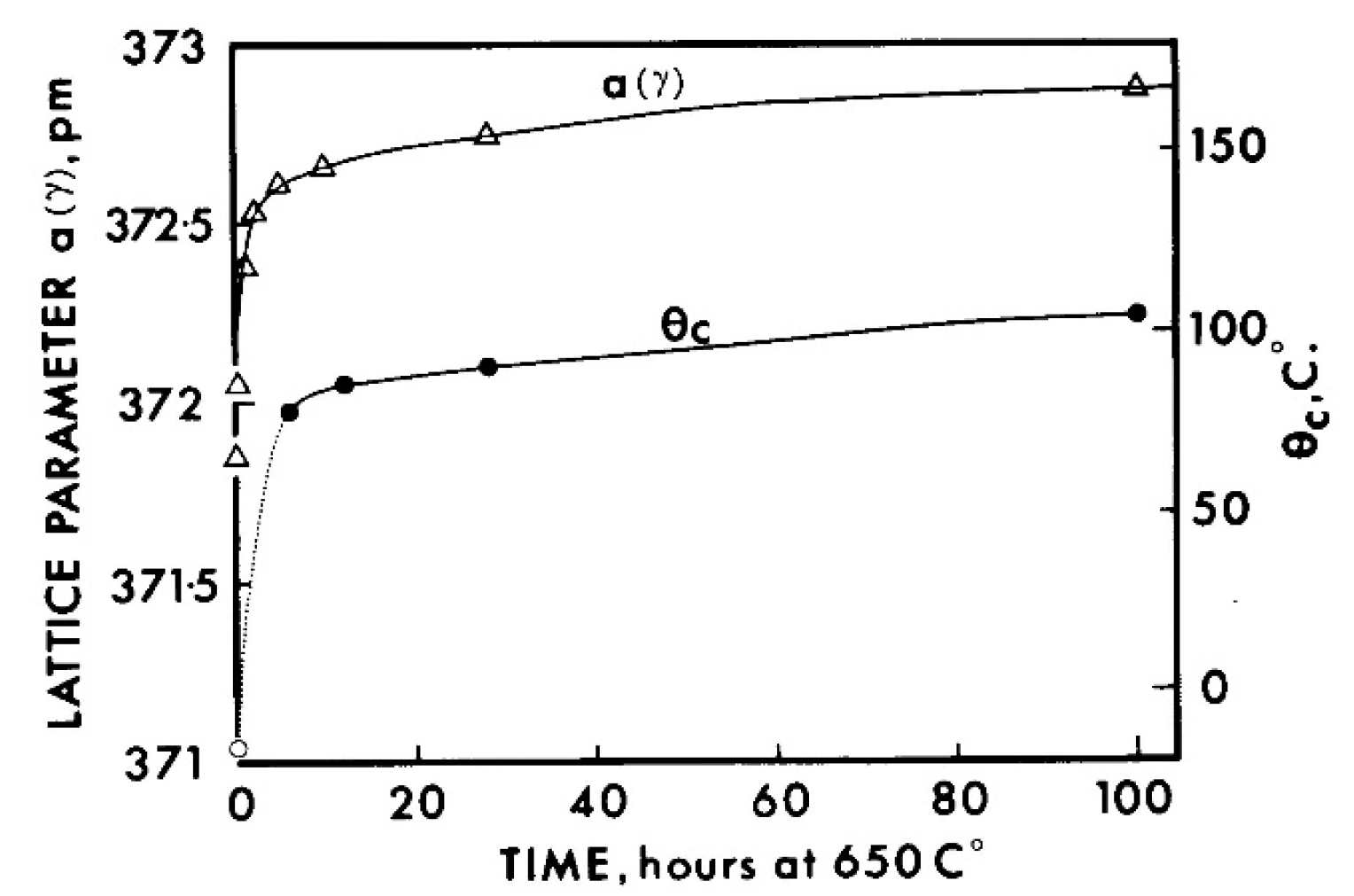
| Strain Limiting Factor | Effect of Increasing Order |
| Low volume change | Volume change decreases [36] |
| Low magnitude of shape strain | m1 decreases [51] |
| Low ∆Gc | Shear modulus decreases [53] |
| Strain Accommodating Factor | Effect of Increasing Order |
| Thin plate morphology | Coarse lenticular plates replaced by thin plates [11] |
| High elastic limit for austenite | Yield stress and hardness increase due to magnetostrictive and order strengthening [51] |
| Self-accommodating plate groups | p1′ approaches {hhk}F allowing the formation of self-accommodating four plate clusters [51] |
| Fine transformation twins | Twin boundary energy decreases with increase in martensite tetragonality [36,56,57] |
| Deformation Temperature TD | PSEUDO—PLASTICITY | PSEUDO—ELASTICITY | ||
|---|---|---|---|---|
| Description | Recovery Temperature TR | Description | Recovery Temperature TR | |
| TD < MF | PP(M) | TR > AF | PE(M) | TD |
| MS < TD < MD | PP(A) | TR > AF | PE(A) | TD |
| MF < TD < MS | PP(M+A) | TR > AF | PE(M+A) | TD |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunne, D. Martens-ite. Metals 2018, 8, 395. https://doi.org/10.3390/met8060395
Dunne D. Martens-ite. Metals. 2018; 8(6):395. https://doi.org/10.3390/met8060395
Chicago/Turabian StyleDunne, Druce. 2018. "Martens-ite" Metals 8, no. 6: 395. https://doi.org/10.3390/met8060395





