Mechanical Properties and Corrosion Behavior of WZ73 Mg Alloy/SiCp Composite Fabricated by Stir Casting Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
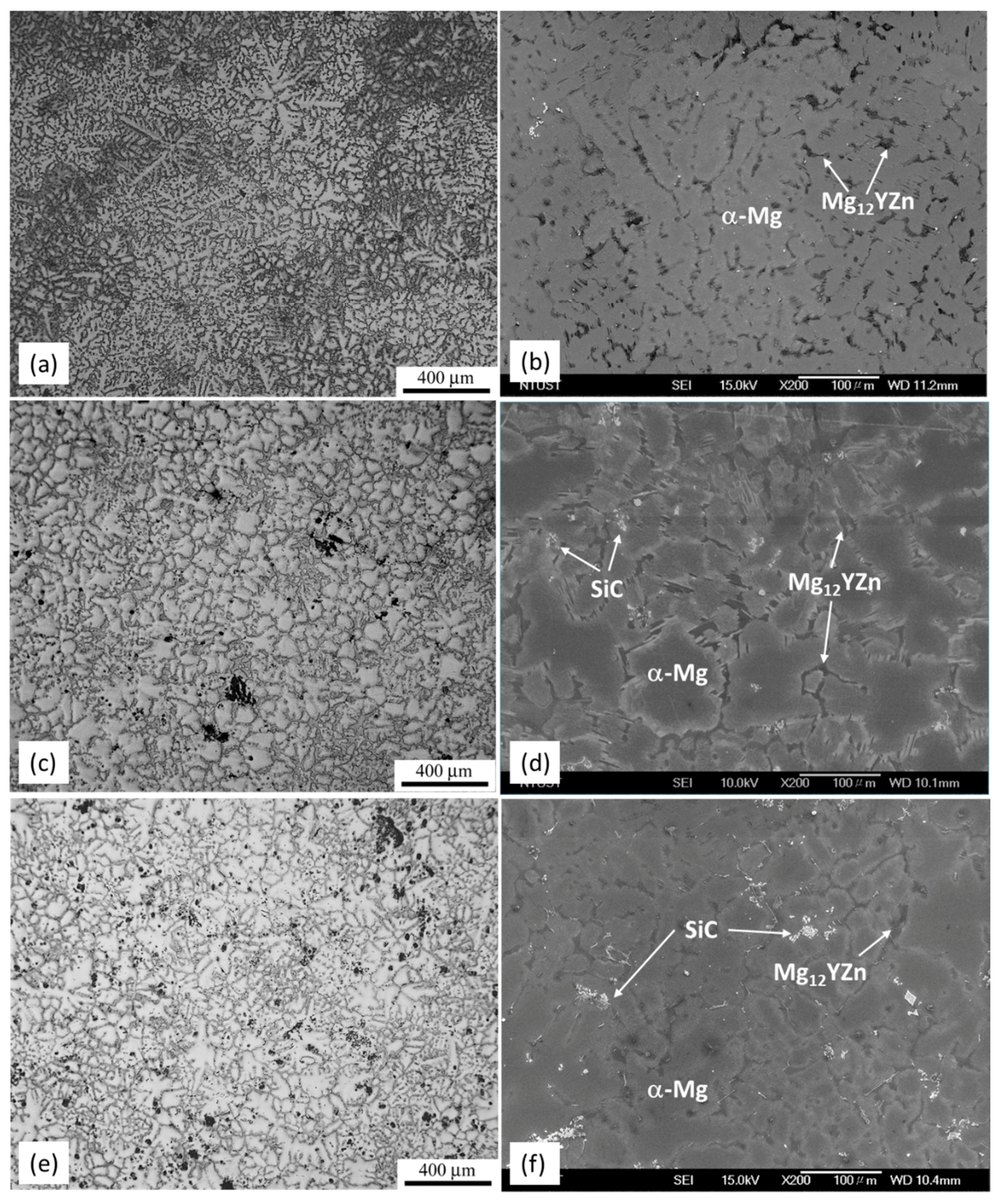
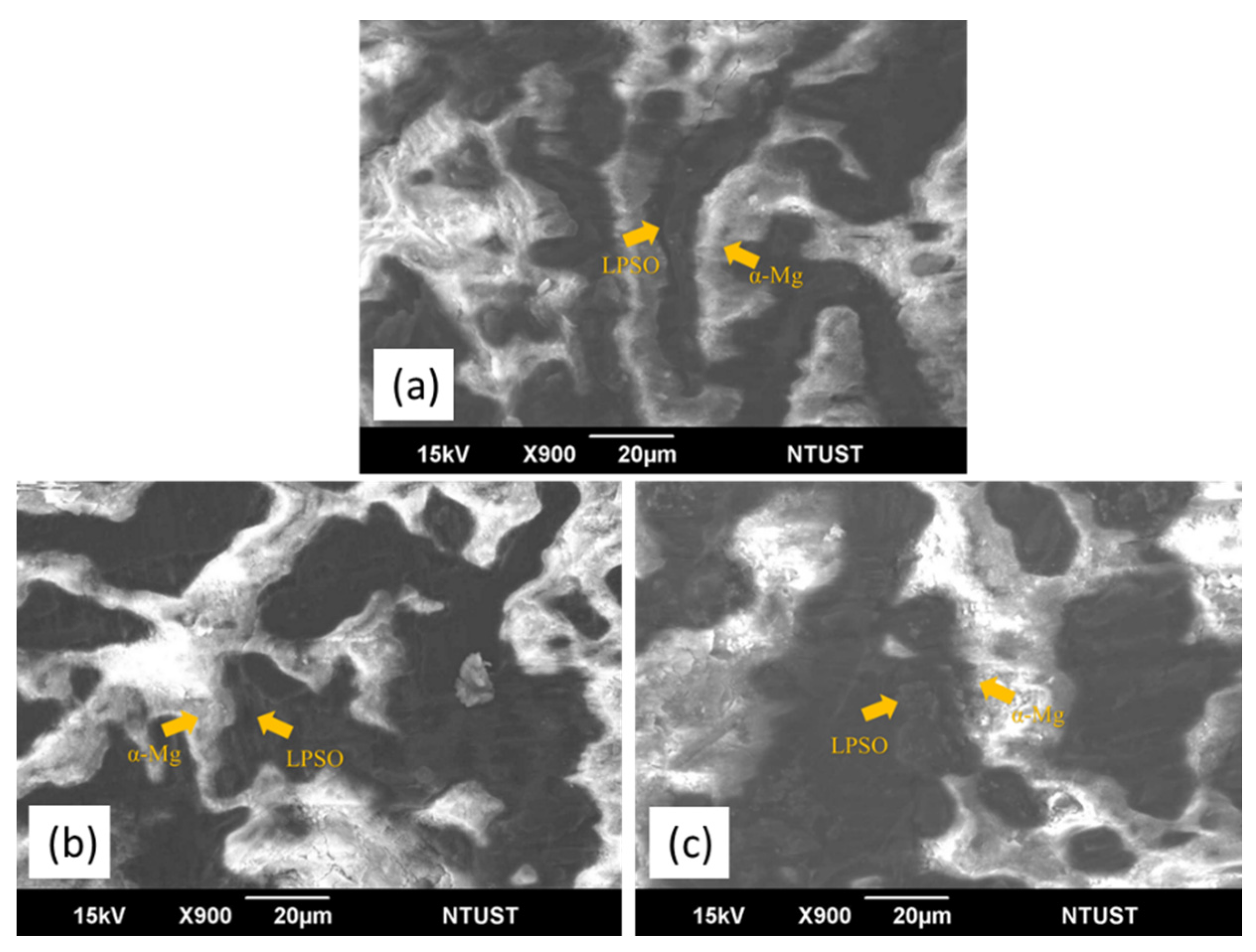
3.1. Microstructural Characterization
3.2. Mechanical Properties
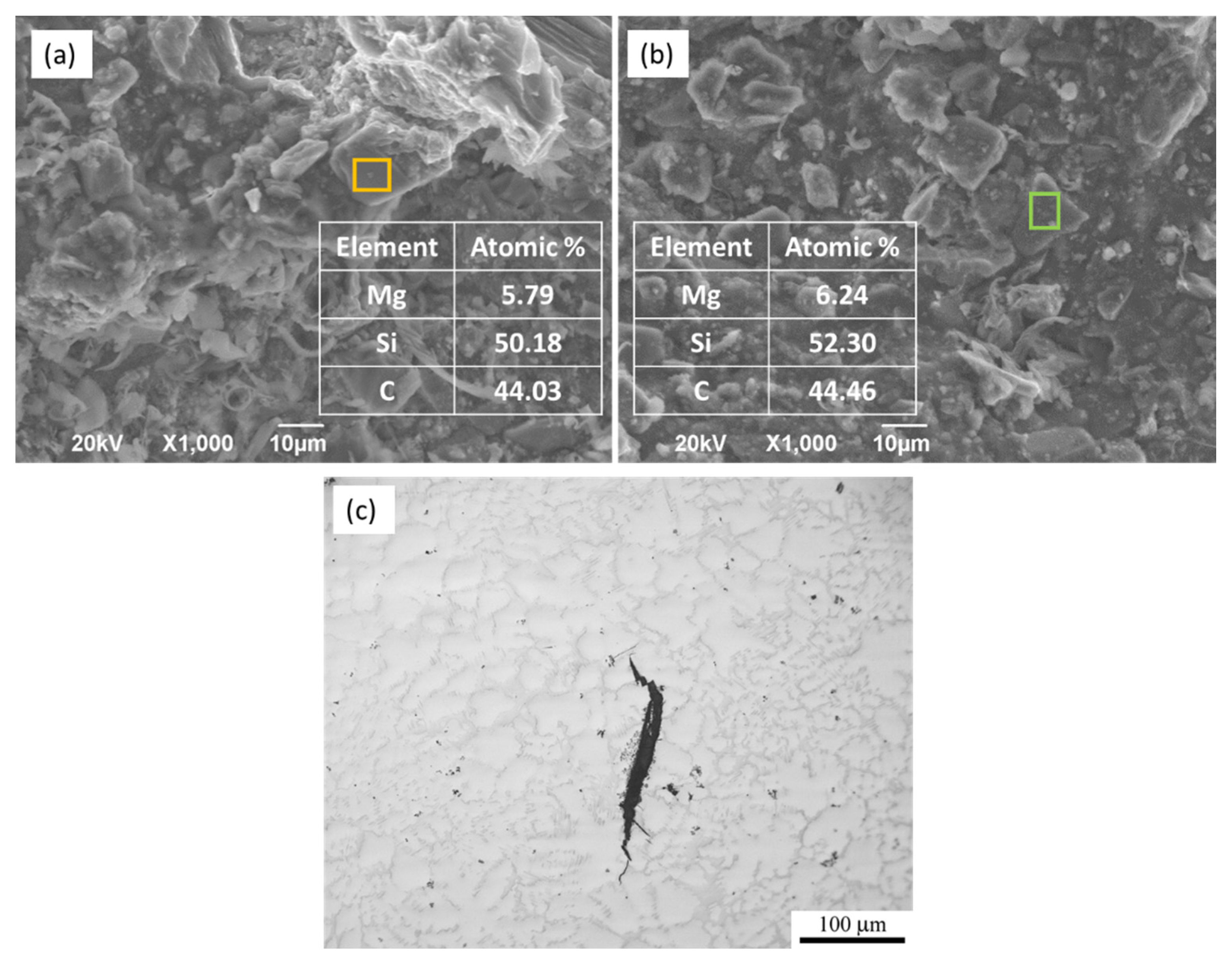
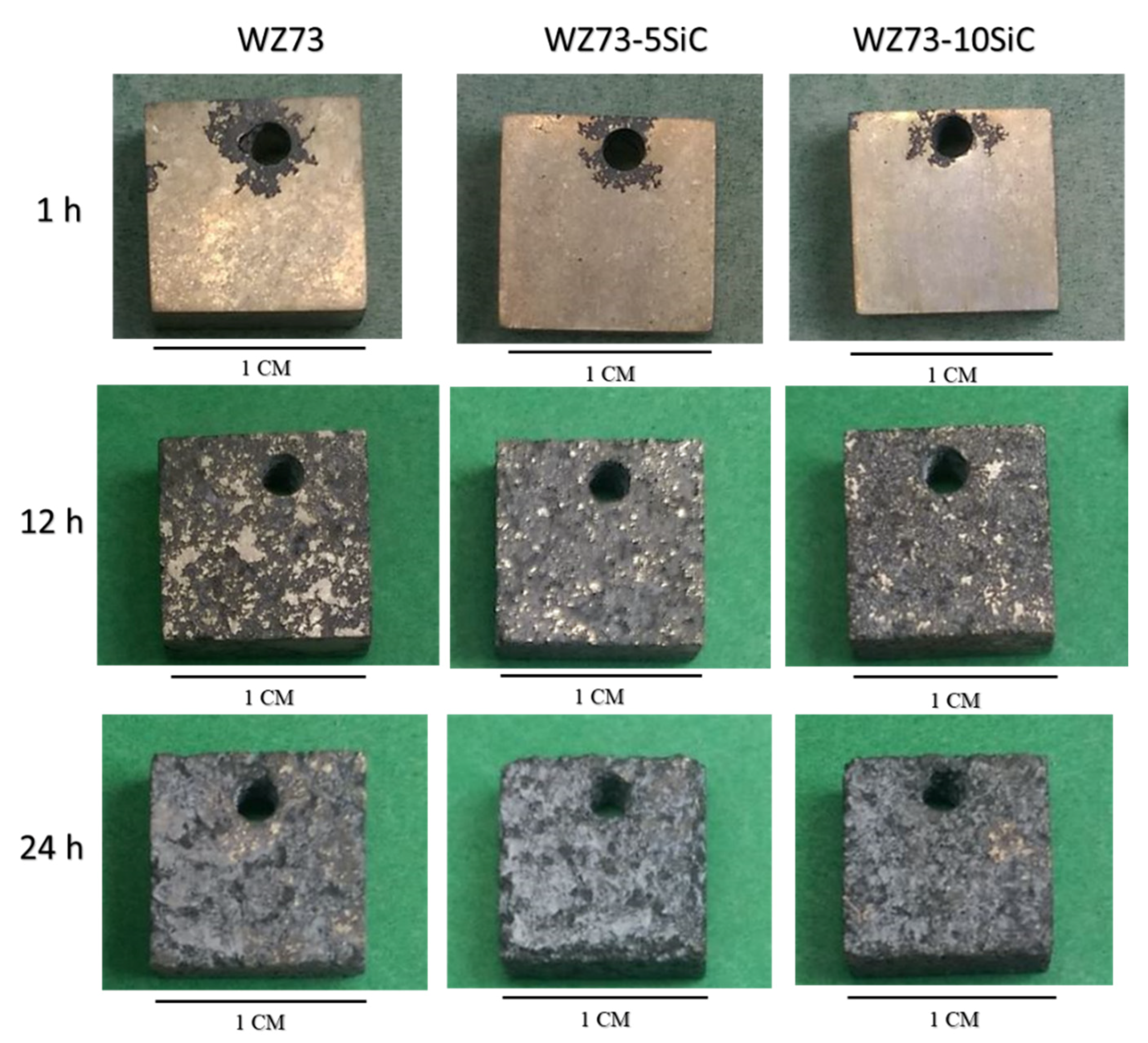
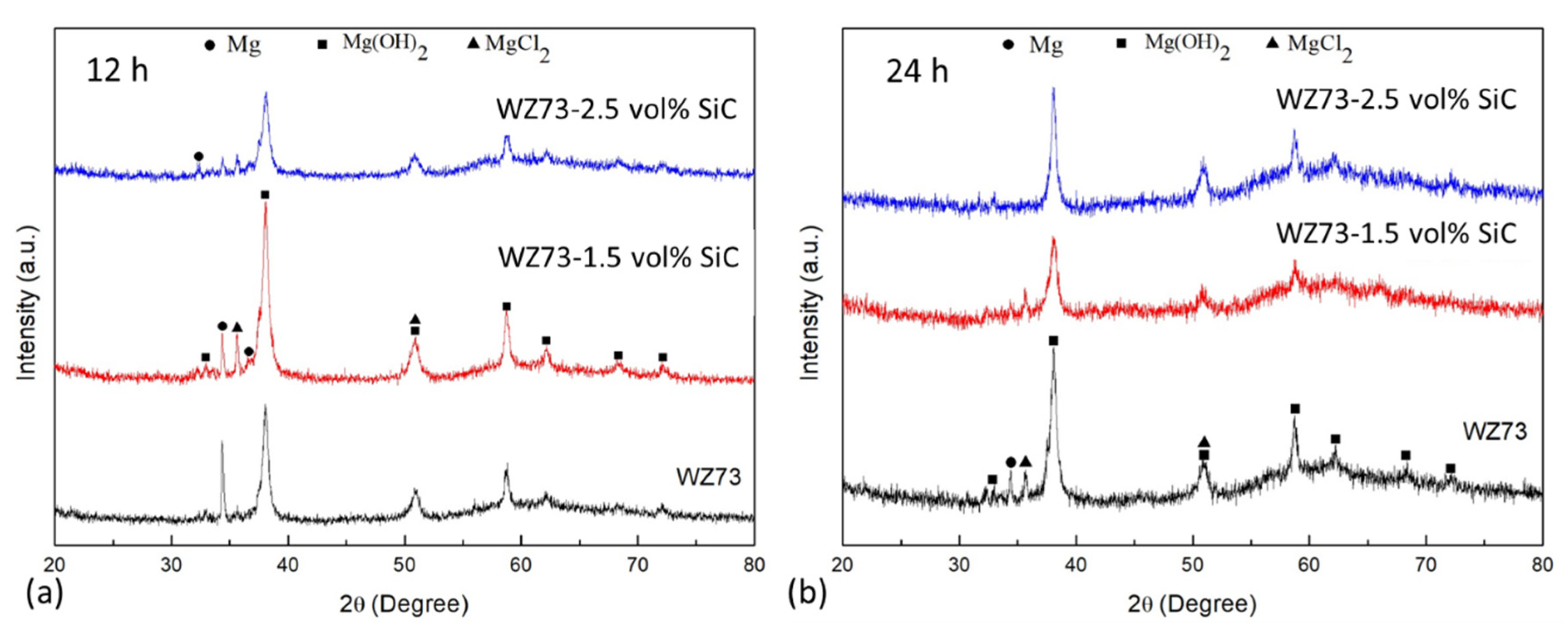
3.3. Corrosion Behavior
4. Conclusions
- The microstructure of the as-cast MMCs consist of α-Mg matrix and LPSO phase distributed discontinuously along the grain boundary. SiC particles are located in the vicinity of the LPSO phase.
- The LPSO phase is observed in the as-cast MMCs. The addition of SiC particles does not inhibit the formation of the LPSO phase in the MMCs during solidification.
- Grain refinement of Mg is observed in the MMCs, which is beneficial to mechanical properties. The addition of SiC has no influence on the morphology and distribution of the LPSO phase. The grain size strengthening and dispersion strengthening brought by SiC enhance the strength of MMC but reduce elongation. Increasing amounts of SiC do not significantly improve the mechanical properties, which is a result of the clustering of SiC particles.
- SiC has a deleterious effect on the corrosion resistance of WZ73 alloy. The results of the immersion test of SiC in 1 wt % NaCl solution indicate the MMCs has a higher corrosion rate. In the WZ73-SiC MMCs, SiC shows an indirect effect on the microgalvanic corrosion. The increased corrosion rate observed in MMCs is due to the presence of the Mg matrix/SiC interface in the vicinity of the LPSO phase, which breaks the continuity of the matrix, and accelerates the corrosion rate.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mordike, B.L.; Ebert, T. Magnesium: Properties-applications-potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Schumann, S. The paths and strategies for increased magnesium applications in vehicles. Mater. Sci. Forum 2005, 488–489, 1–8. [Google Scholar] [CrossRef]
- Cabibbo, M.; Spigarelli, S. A TEM quantitative evolution of strengthening in an Mg-RE alloy reinforced with SiC. Mater. Charact. 2011, 62, 959–969. [Google Scholar] [CrossRef]
- Pan, F.S.; Yang, M.B.; Chen, X.H. A review on casting magnesium alloys: Modification of commercial alloys and development of new alloys. J. Mater. Sci. Technol. 2016, 32, 1211–1221. [Google Scholar] [CrossRef]
- Liu, H.; Ju, J.; Bai, J.; Sun, J.; Song, D.; Yan, J.; Jiang, J.; Ma, A. Preparation, microstructure evolutions, and mechanical property of an ultra-fine grained Mg-10Gd-4Y-1.5Zn-0.5Zr alloy. Metals 2017, 7, 398. [Google Scholar] [CrossRef]
- Li, C.Q.; Xu, D.K.; Zeng, Z.R.; Wang, B.J.; Sheng, L.Y.; Chen, X.B.; Han, E.H. Effect of volume fraction of LPSO phases on corrosion and mechanical properties of Mg-Zn-Y alloys. Mater. Des. 2017, 121, 430–441. [Google Scholar] [CrossRef]
- Noda, M.; Mayama, T.; Kawamura, Y. Evolution of mechanical properties and microstructure in extruded Mg96Zn2Y2 alloys by annealing. Mater. Trans. 2009, 11, 2526–2531. [Google Scholar] [CrossRef]
- Shi, F.; Wang, C.Q.; Zhang, Z.M. Microstructures, corrosion and mechanical properties of as-cast Mg-Zn-Y-(Gd) alloys. Trans. Nonferrous Met. Soc. China 2015, 25, 2172–2180. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, L.L.; Xu, J. Biodegradable Mg-Zn-Y alloys with long-period stacking ordered structure: Optimization for mechanical properties. J. Mech. Behav. Biomed. Mater. 2013, 18, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, J.; Cheng, W.; Chen, C.; Kang, J. Corrosion behavior of Mg-Zn-Y alloy with long-period stacking ordered structures. J. Mater. Sci. Technol. 2012, 28, 1157–1162. [Google Scholar] [CrossRef]
- Kawamura, Y.; Hayashi, K.; Inoue, A.; Masumoto, T. Rapidly solidified powder metallurgy Mg97Zn1Y2 alloys with excellent tensile yield strength above 600 MPa. Mater. Trans. 2001, 42, 1172–1176. [Google Scholar] [CrossRef]
- Itoi, T.; Seimiya, T.; Kawamura, Y.; Hirohashi, M. Long period stacking structures observed in Mg97Zn1Y2 alloy. Scr. Mater. 2004, 51, 107–111. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, S.J.; Wu, Y.J.; Kang, C.W. Particle size and particle percentage effect of AZ61/SiCp magnesium matrix micro- and nano-composites on their mechanical properties due to extrusion and subsequent annealing. Metals 2017, 7, 293. [Google Scholar] [CrossRef]
- Rahmany-Gorji, R.; Alizadeh, A.; Jafari, H. Microstructure and mechanical properties of stir cast ZX51/Al2O3p magnesium matrix composites. Mater. Sci. Eng. A 2016, 674, 413–418. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, G.; Han, L.; Hu, N.; Han, F.; Zhao, Z.; Xia, X.; Wan, Y. Microstructure evolution of SiCp/ZM6 (Mg-Nd-Zn) magnesium matrix composite in the semi-solid state. J. Alloys Compd. 2016, 656, 67–76. [Google Scholar] [CrossRef]
- Tan, X.; Chee, W.K.H.; Chan, J.K.W.; Kwok, R.W.O.; Gupta, M. Stretching the engineering strain of high strength LPSO quaternary Mg-Y-Zn-Al alloy via integration of nano-Al2O3. J. Mater. Sci. 2016, 51, 4160–4168. [Google Scholar] [CrossRef]
- Matin, A.; Saniee, F.F.; Abedi, H.R. Microstructure and mechanical properties of Mg/SiC and AZ80/SiC nano-composites fabricated through stir casting method. Mater. Sci. Eng. A 2015, 625, 81–88. [Google Scholar] [CrossRef]
- Hashim, J.; Loony, L.; Hashmi, M. Metal matrix composites: Production by the stir casting method. J. Mater. Process. Technol. 1999, 92, 1–7. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, L.L.; Xu, J. Mg-Zn-Y Alloys with long-period stacking ordered structure: In vitro assessments of biodegradation behavior. Mater. Sci. Eng. C 2013, 33, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Balasubramaniam, R.; Gupta, M. Corrosion behavior of SiC reinforced magnesium composites. Corros. Sci. 2007, 49, 711–725. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, S.; Merino, M.C.; Barroso, I.; Mohedano, M.; Arrabal, R.; Viejo, F. Corrosion behavior of silicon-carbide-particle reinforced AZ92 magnesium alloy. Corros. Sci. 2009, 51, 841–849. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Pardo, A.; Matykina, E. Corrosion and wear of PEO coated AZ91/SiC composites. Surf. Coat. Technol. 2017, 309, 1023–1032. [Google Scholar] [CrossRef]
- Zucchi, F.; Trabanelli, G.; Grassi, V.; Frignani, A. Corrosion behavior in sodium sulfate and sodium chloride solutions of SiCp reinforced magnesium alloy metal matrix composites. Corrosion 2004, 60, 326–328. [Google Scholar] [CrossRef]
- Yamasaki, M.; Hashimoto, K.; Hagihara, K.; Kawamura, Y. Effect of multimodal microstructure evolution on mechanical properties of Mg-Zn-Y extruded alloy. Acta Mater. 2011, 59, 3646–3658. [Google Scholar] [CrossRef]
- Singh, I.B.; Singh, M.; Das, S. A comparative corrosion behavior of Mg, AZ31 and AZ91 alloys in 3.5% NaCl solution. J. Magnes. Alloys 2015, 3, 142–148. [Google Scholar] [CrossRef]
- Chen, B.; Lin, D.; Zeng, X.; Liu, C. Effects of yttrium and zinc addition on the microstructure and mechanical properties of Mg-Zn-Y alloys. J. Mater. Sci. 2010, 45, 2510–2517. [Google Scholar] [CrossRef]
- Su, Z.G.; Li, R.G.; An, J.; Lu, Y. Effect of rolling temperature on the microstructures and mechanical properties of Mg97Zn1Y2 magnesium alloy. J. Mater. Eng. Perform. 2010, 19, 70–76. [Google Scholar] [CrossRef]
- Oñorbe, E.; Garcés, G.; Pérez, P.; Adeva, P. Effect of the LPSO volume fraction on the microstructure and mechanical properties of Mg-Y2-x-Znx alloys. J. Mater. Sci. 2012, 47, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, C.; Que, Z.; Cheng, W.; Xu, J.; Kang, J. 18R and 14H long-period stacking ordered structures in the Mg93.96Zn2Y4Sr0.04 alloy and the modification effect of Sr on X-phase. Mater. Sci. Eng. A 2012, 552, 81–88. [Google Scholar] [CrossRef]
- Wang, B.S.; Xiong, S.M.; Liu, Y.B. Tensile fracture of as-cast and hot rolled Mg-Zn-Y alloy with long-period stacking phase. Trans. Nonferrous Met. Soc. China 2010, 20, s488–s492. [Google Scholar] [CrossRef]
- Park, S.H.; Jung, J.G.; Yoon, J. Influence of Sn addition on the microstructure and mechanical properties of extruded Mg-8Al-2Zn alloy. Mater. Sci. Eng. A 2015, 625, 128–135. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, K.K. Microstructures and mechanical properties of SiCp/Mg-xAl-2Ca composites collectively influenced by SiCp and Al content. Mater. Sci. Eng. A 2018, 725, 510–521. [Google Scholar] [CrossRef]
- Ramya, M.; Sarwat, S.G.; Udhayabanu, V.; Subramanian, S.; Raj, B.; Ravi, K.R. Role of partially amorphous structure and alloying elements on the corrosion behavior of Mg-Zn-Ca bulk metallic glass for biomedical applications. Mater. Des. 2015, 86, 829–835. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Fu, H.; Cai, X.; Wang, Y.; Liu, B.; Xu, Z. Degradation behavior of Mg-based biomaterials containing different long-period stacking ordered phases. Sci. Rep. 2014, 4, 3620. [Google Scholar] [CrossRef] [PubMed]
- Leng, Z.; Zhang, J.H.; Yin, T.T.; Zhang, L.; Guo, X.Y.; Peng, Q.M.; Zhang, M.L.; Wu, R.Z. Influence of biocorrosion on microstructure and mechanical properties of deformed Mg–Y–Er–Zn biomaterial containing 18R-LPSO phase. J. Mech. Behav. Biomed. Mater. 2013, 28, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Chen, F.; Wu, Y.; Wang, Z.; Wang, Q. Relation between LPSO structure and biocorrosion behavior of biodegradable GZ51K alloy. Mater. Lett. 2015, 138, 212–215. [Google Scholar] [CrossRef]
- Cao, F.; Song, G.-L.; Atrens, A. Corrosion and passivation of magnesium alloys. Corros. Sci. 2016, 111, 835–845. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, F.; Wu, Y.; Le, J.; He, M.; Zhu, J.; Hu, W. Protective diffusion coatings on magnesium alloys: A review of recent developments. J. Alloys Compd. 2012, 520, 11–21. [Google Scholar] [CrossRef]
- Sun, Y.-H.; Wang, R.-C.; Peng, C.-Q.; Feng, Y.; Yang, M. Corrosion behavior and surface treatment of superlight Mg-Li alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 1455–1475. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, X.J.; Gong, W.X.; Wu, K.; Wang, F.H. Effect of SiC particles on microarc oxidation process of magnesium matrix composites. Appl. Surf. Sci. 2013, 283, 906–913. [Google Scholar] [CrossRef]
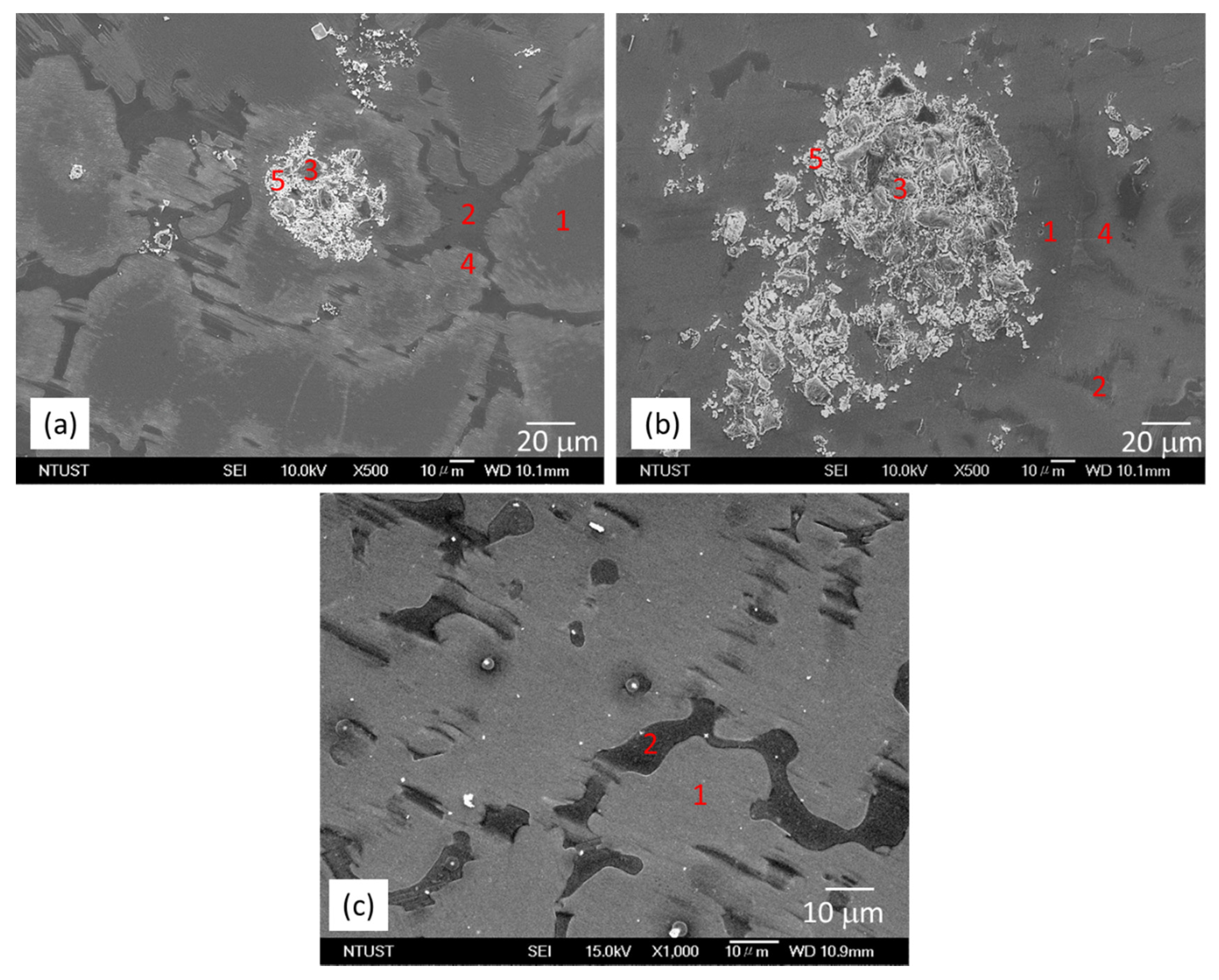


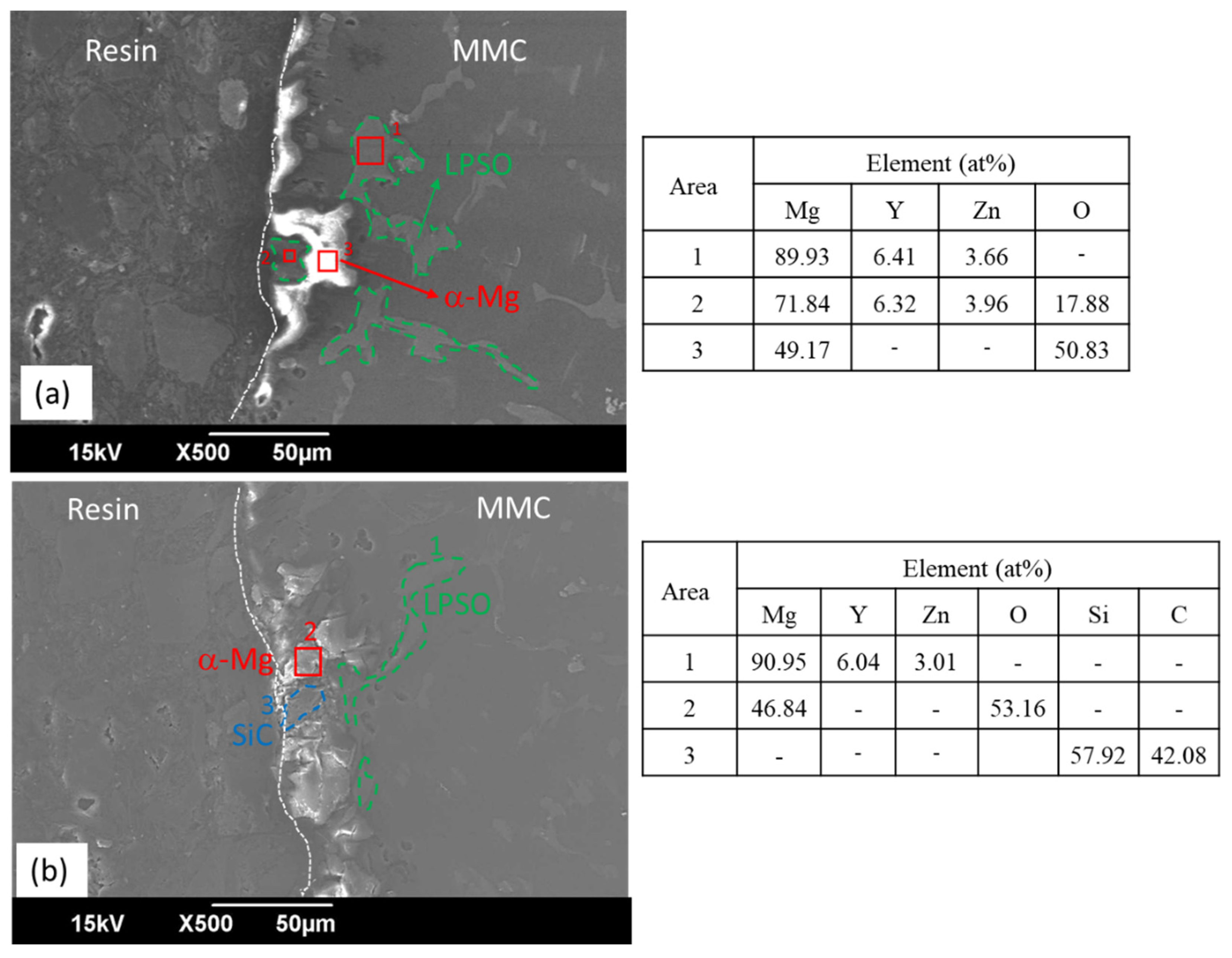

| Sample | Zone | Element | |||||
|---|---|---|---|---|---|---|---|
| Mg | Y | Zn | Si | C | O | ||
| WZ73 | 1 | 98.2 | 1.2 | 0.7 | - | - | - |
| 2 | 89.1 | 6.4 | 4.6 | - | - | - | |
| WZ73-1.5 vol % SiC | 1 | 98.8 | 0.9 | 0.3 | - | - | - |
| 2 | 90.0 | 5.8 | 4.2 | - | - | - | |
| 3 | 1.4 | - | - | 47.8 | 50.8 | - | |
| 4 | 98.0 | 1.4 | 0.6 | - | - | - | |
| 5 | 48.5 | 22.4 | - | 18.7 | - | 10.4 | |
| WZ73-2.5 vol % SiC | 1 | 99.3 | 0.3 | 0.4 | - | - | - |
| 2 | 90.1 | 5.3 | 4.5 | ||||
| 3 | 1.1 | - | - | 42.4 | 56.5 | - | |
| 4 | 98.2 | 0.9 | 0.9 | - | - | - | |
| 5 | 36.3 | 25.9 | - | 23.8 | - | 14.0 | |
| Sample | α-Mg (%) | LPSO (%) | Grain Size of α-Mg(µm) | YS (MPa) | UTS (MPa) | Elongation (%) |
|---|---|---|---|---|---|---|
| WZ73 | 77.2 | 22.8 | 143 | 126 ± 13 | 172 ± 9 | 9 ± 1 |
| WZ73-1.5 vol % SiC | 80.3 | 19.7 | 118 | 160± 1 | 223 ± 8 | 6 ± 2 |
| WZ73-2.5 vol % SiC | 84.1 | 15.9 | 114 | 154 ± 9 | 238 ± 9 | 7± 1 |
| Sample | α-Mg | α-Mg (Near Particles) | LPSO |
|---|---|---|---|
| WZ73 | 77 ± 3 | - | 115 ± 6 |
| WZ73-1.5 vol % SiC | 81 ± 9 | 86 ± 13 | 121 ± 5 |
| WZ73-2.5 vol % SiC | 79 ± 5 | 89 ± 8 | 119 ± 5 |
| Sample | Mass Loss (mg/cm2) | Corrosion Rate (mm/year) |
|---|---|---|
| WZ73 | 6.3 | 16 |
| WZ73-1.5 vol % SiC | 13.8 | 27 |
| WZ73-2.5 vol % SiC | 12.8 | 25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.; Liu, H.-C. Mechanical Properties and Corrosion Behavior of WZ73 Mg Alloy/SiCp Composite Fabricated by Stir Casting Method. Metals 2018, 8, 424. https://doi.org/10.3390/met8060424
Chiu C, Liu H-C. Mechanical Properties and Corrosion Behavior of WZ73 Mg Alloy/SiCp Composite Fabricated by Stir Casting Method. Metals. 2018; 8(6):424. https://doi.org/10.3390/met8060424
Chicago/Turabian StyleChiu, Chun, and Hsu-Chieh Liu. 2018. "Mechanical Properties and Corrosion Behavior of WZ73 Mg Alloy/SiCp Composite Fabricated by Stir Casting Method" Metals 8, no. 6: 424. https://doi.org/10.3390/met8060424




