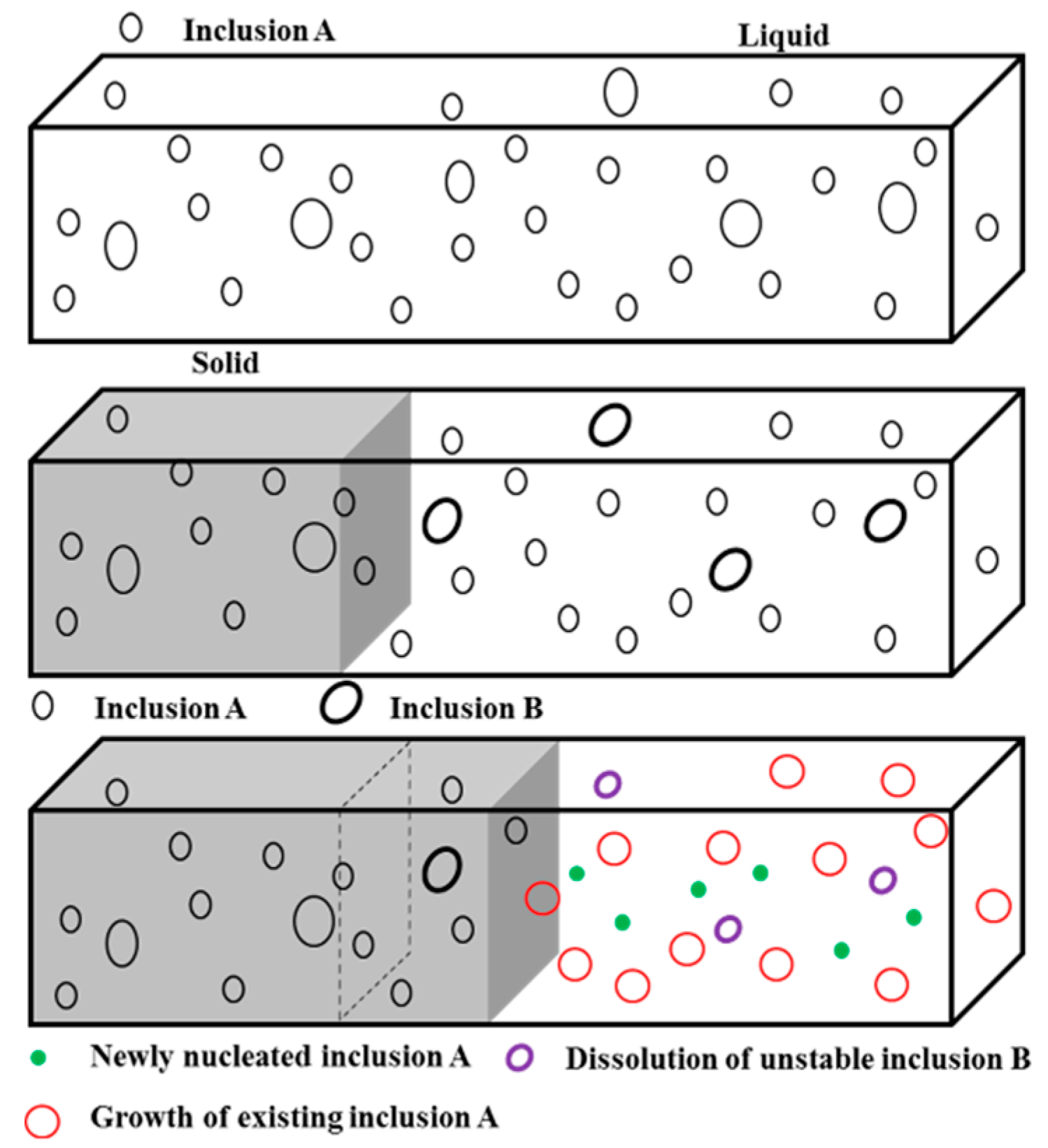
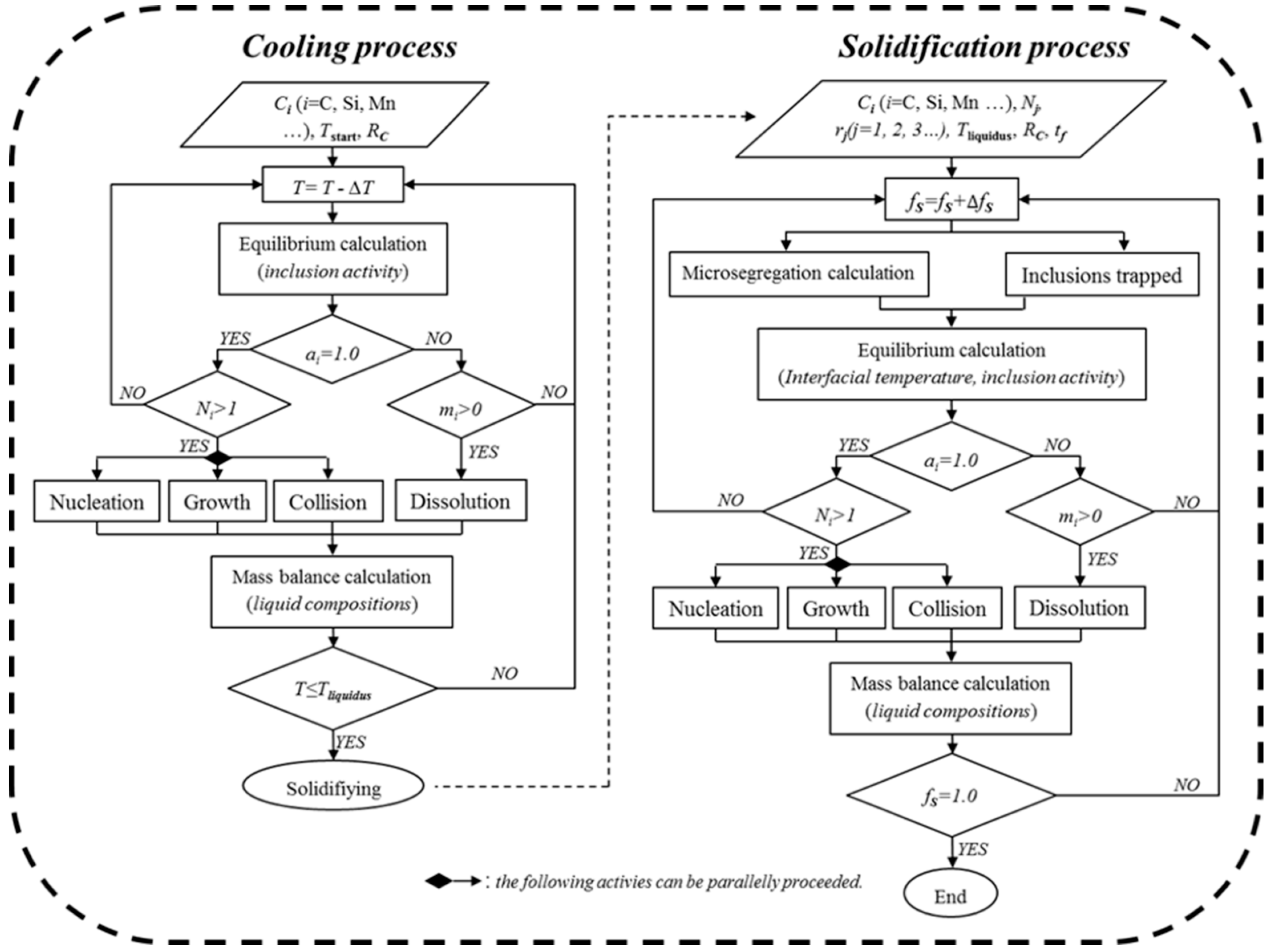
3.1. Influence of the Alloying Temperature
The alloying temperature here means the temperature of the addition of Ti and Al. The alloys were assumed to be instantly well-mixed with the melt after the addition Ti and A1. Without considering the holding process, the alloying temperature turned out to be the starting cooling temperature in the simulations. In laboratory experiments, the melt would be cooled down after a short period of time, following the addition of alloy, to prevent the floating of inclusions. Hence, the importance of the alloying temperature during the cooling and solidification process was studied using the model. The formation of inclusions under alloying temperatures of 1650 and 1600 °C were simulated.
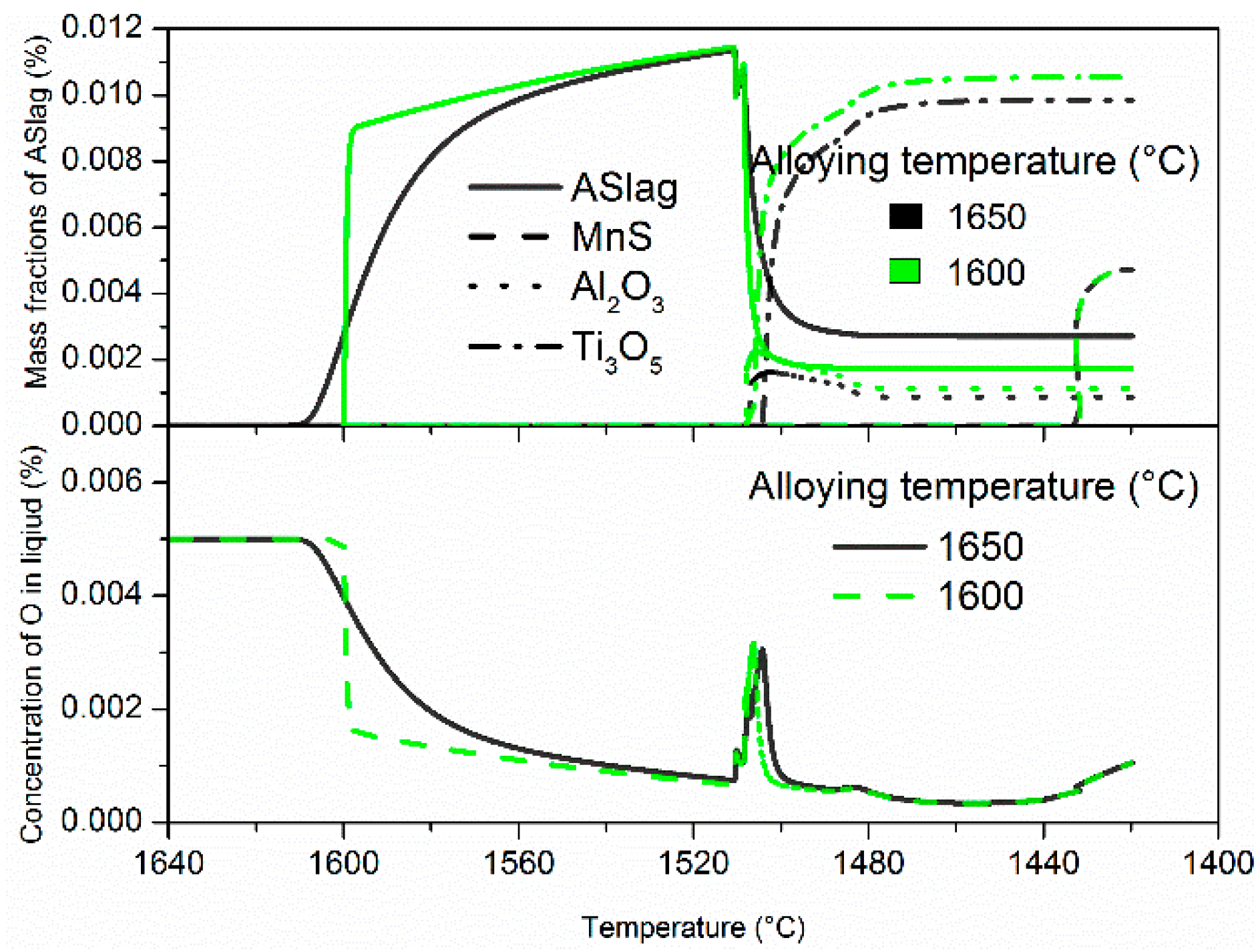
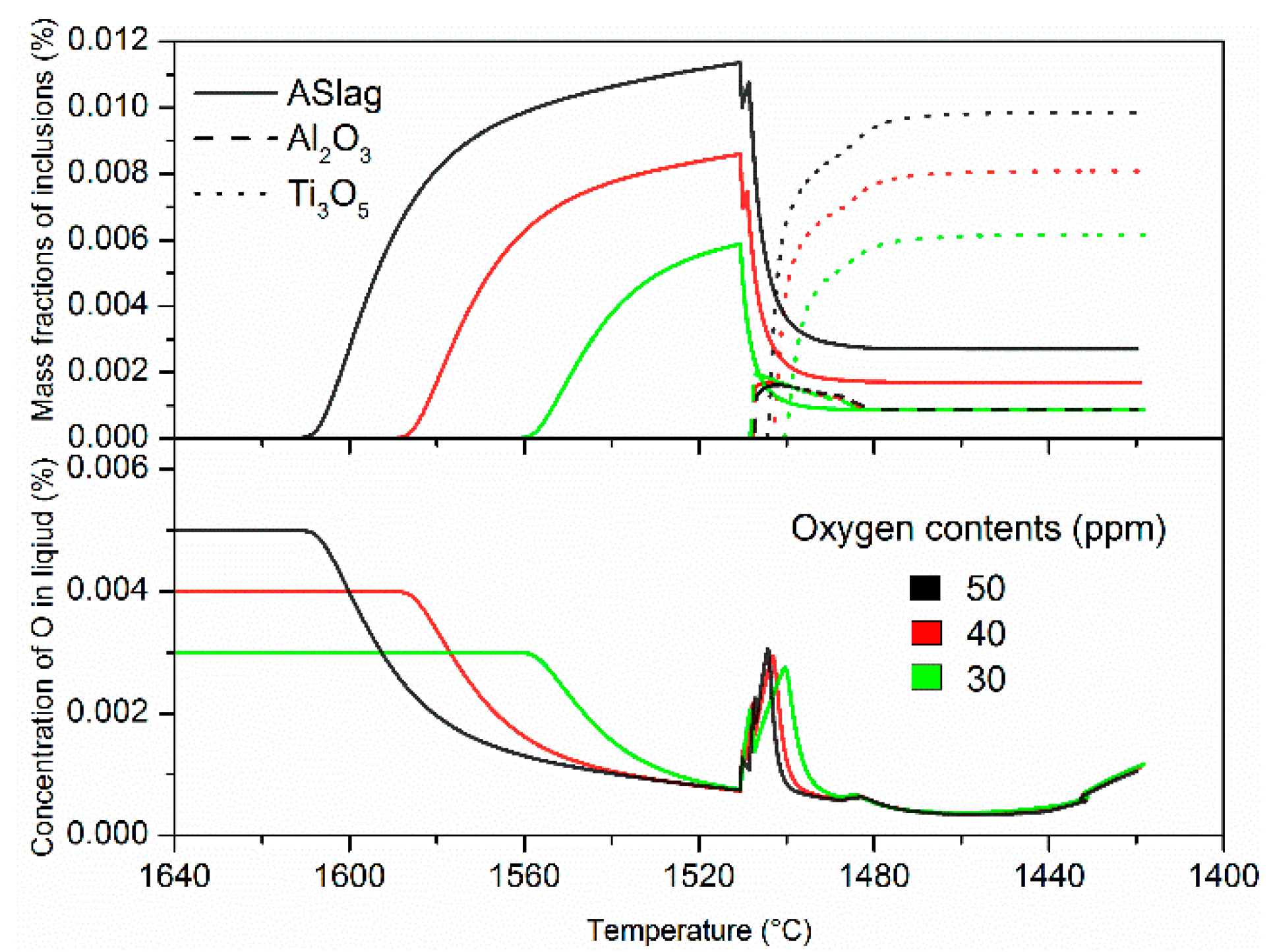
The evolution of mass fractions of various inclusions (top) and the corresponding oxygen concentration changes (bottom) are displayed in
Figure 3. The calculations under the different alloying temperatures show the same inclusions types: ASlag, Ti
3O
5, Al
2O
3 and MnS. ASlag is a solution phase that mainly consists of Al
2O
3, MnO, Ti
2O
3 and TiO
2. ASlag is the only inclusion formed in the selected steel in the cooling process, as shown in
Figure 3. After solidification starts, ASlag is transformed into Al
2O
3 and Ti
3O
5. MnS precipitates at the late stage of solidification due to its strong enrichment of Mn and S.
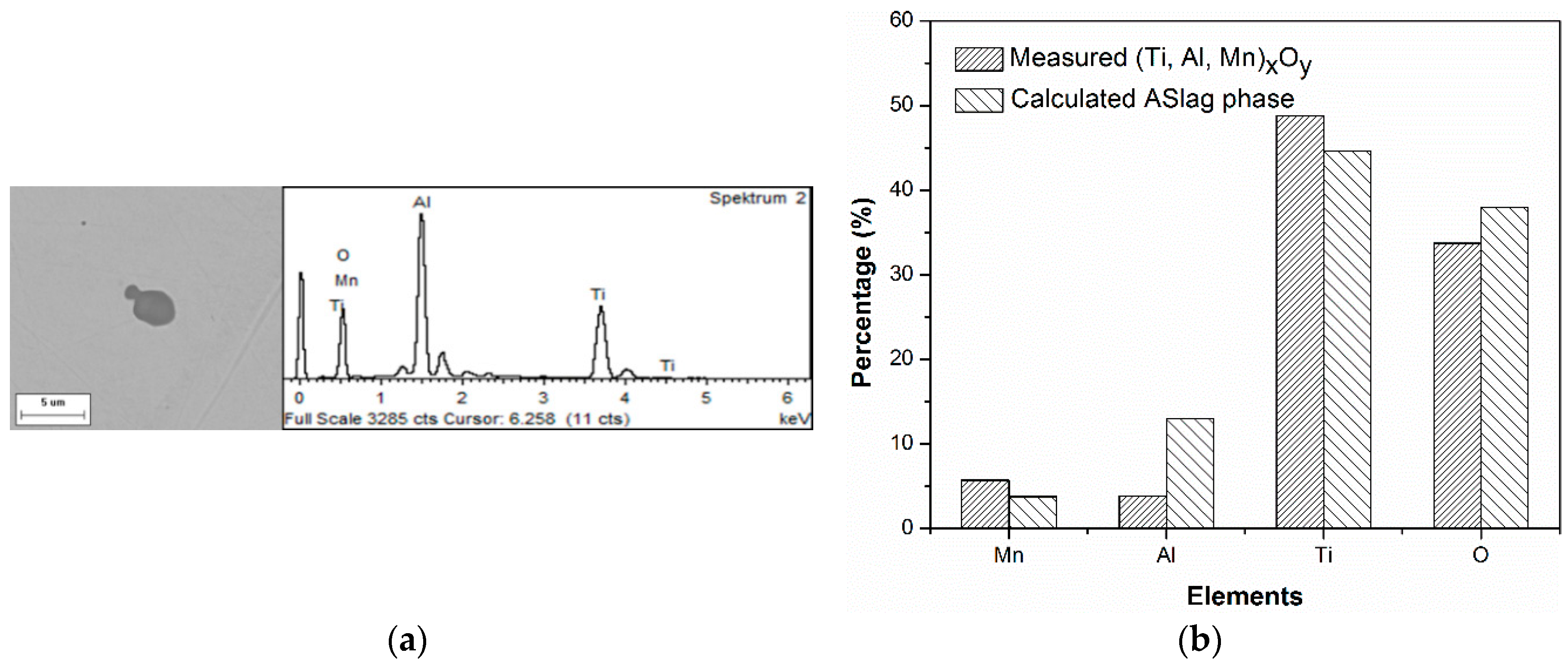
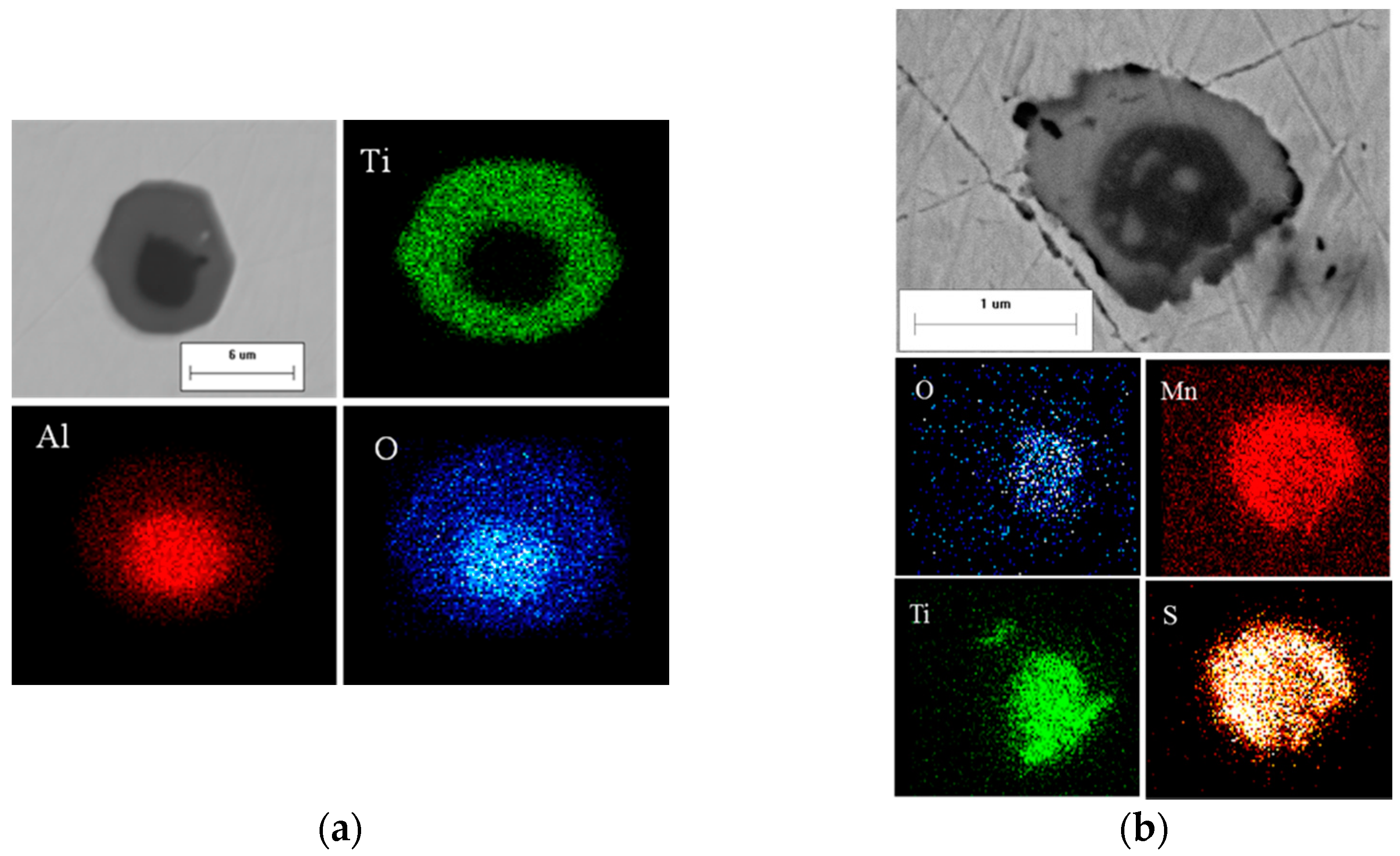
To evaluate the predicted types and composition of the inclusions, selected steel with 50 ppm oxygen was also melted in the laboratory. The prepared sample was observed using both manual and automated scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS) measurement. The details of the experiment and measurements can be found elsewhere [
15]. The predicted and measured inclusion types are compared in
Table 3. All the homogeneous inclusions, including (Ti, Al, Mn)
xO
y, TiO
x and MnS from calculation and experiment were in good accordance (Types 1, 2, and 3). The (Ti, Al, Mn)
xO
y particle was named ASlag in the prediction, which was a liquid solution phase. The detected (Ti, Al, Mn)
xO
y particle and a comparison of the compositions are shown in
Figure 4. The shape and nature of this inclusion type indicates its liquid occurrence at the processing temperature, as shown in
Figure 4a. There was reasonable agreement between the average composition of the measured and calculated oxides, as illustrated in
Figure 4b. Both the aforementioned aspects indicate that this complex oxide was well-predicted. The heterogeneous inclusions, Al
2O
3-TiO
x and TiO
x-MnS (Types 4 and 5 in
Figure 5), could not be predicted by the model. However, the formation order of the inclusions could be indirectly applied to illustrate the stability of the inclusions. As shown in
Figure 5a,b, Al
2O
3 is likely to act as a heterogeneous nucleus for TiO
x, and TiO
x can also act as a nucleus for the precipitation of MnS. This indicates the following formation order of the inclusions: Al
2O
3, Ti
3O
5, and MnS. The simulations displayed the same order of formation of the inclusions, as shown in
Figure 3. The agreement between the predicted and experimental inclusion types, and the composition and formation order indicated to some extent the reasonability of the modeling thermodynamics. Based on reliable thermodynamics, the competitive formation kinetics of the inclusions was studied. Note that only homogeneous nucleation was considered in the presented model, while the heterogeneous nucleation of different types of inclusions (as shown in
Figure 5) is beyond the capacity of the model.
In addition, there were considerable differences in the mass fraction profiles at different alloying temperatures, while the mass evolutions of MnS remained the same; these are displayed in
Figure 3. At an alloying temperature of 1650 °C, ASlag precipitated until the temperature cooled down to 1610 °C and the mass fraction gradually increased. When the alloying temperature was lowered to 1600 °C, the mass fraction of ASlag initially soared to approximately 85% (95 ppm) of its total amount and then increased at a much lower speed due to the limited oxygen content. At the liquidus temperature (about 1510 °C), the mass fractions in both cases reached similar peak values. After solidification started, ASlag became unstable and steeply dissolved, resulting in minor stability problems and mass fraction fluctuations. ASlag dissolved much more quickly and its mass fraction became less with an alloying temperature of 1600 °C, as compared to an alloying temperature of 1650 °C. This led to earlier formations and higher mass fractions in the later-transformed Al
2O
3 and Ti
3O
5 in the sample. The phenomenon can be explained by the fact that the smaller particles are more easily dissolved (Equation (7) and
Figure 4) and promote the transformations. At both alloying temperatures, below 1480 °C, the mass fraction of Ti
3O
5, ASlag and Al
2O
3 remained constant even though ASlag and Al
2O
3 are thermodynamically unstable and completely dissolved in the residual liquid steel. Their existence in the solid steel was attributed only to the entrapment by the solidification interface. MnS precipitates at the end of solidification, which is little influenced by the alloying temperature. Throughout the whole process, the oxygen concentrations are in agreement with the mass fraction of oxides. While the oxygen concentration increased slightly at the latest stage of solidification due to microsegregation, the limited content in the residual liquid did not result in the significant growth of the oxides.
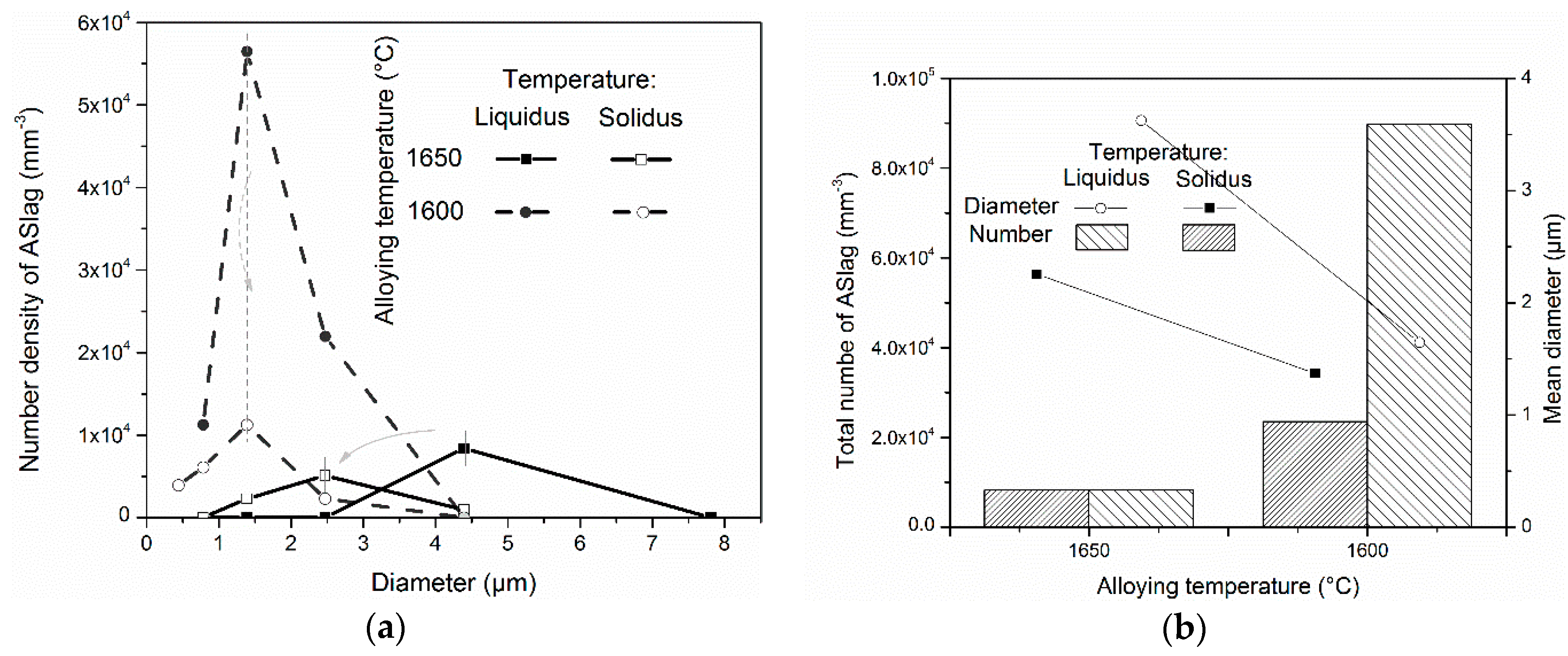
The influence of the alloying temperature on the size distribution, mean diameter and total amount of ASlag is displayed in
Figure 6. The particle size distributions are described by the histograms with the lognormal group sizes. It was found that at the liquidus temperature (before dissolution) the size distribution of ASlag in the simulation at the alloying temperature of 1650 °C was significantly flatter and larger in size than the one with an alloying temperature of 1600 °C, as shown in
Figure 6a. Specifically, the ASlag diameter (4.5 µm) with a peak number density in the simulation with an alloying temperature of 1650 °C was approximately three times higher than that in the simulation with an alloying temperature of 1600 °C (1.5 µm), and the peak number density of the latter was just one-seventh of the former (0.8 × 10
4 mm
−3). At the solidus temperature (after dissolution), the size distribution of ASlag in the two cases had a similar shape as that which had not undergone dissolution, although the size and number densities were considerably reduced. The remarkable differences in mean diameter and total number at the different alloying temperatures are shown in
Figure 6b. At both the liquidus and solidus temperatures, the ASlag in the simulation at an alloying temperature of 1600 °C showed a significantly smaller mean diameter, but much higher number density. Specifically at the liquidus temperature, the total amount of ASlag in the simulation at an alloying temperature of 1600 °C (9 × 10
4 mm
−3) was more than ten times that of the simulation with an alloying temperature of 1650 °C (0.8 × 10
4 mm
−3), and remained approximately three times higher after dissolution. The mean diameters at the liquidus and solidus temperatures in the simulation with a lower alloying temperature (1.6 and 1.3 µm) were approximately half those with higher alloying temperatures (3.8 and 2.4 µm). In total, the size of ASlag was reduced and the number was increased at the lower alloying temperature, before and after experiencing dissolution. The explanations will be further discussed below.
On the other hand, from
Figure 6 it can be seen that dissolution influences the various size distributions in different ways. For the pointed shape distribution, which has a larger number density and smaller size (ASlag in the simulation with a lower alloying temperature), the dissolution of the particles mainly reduced their number, while their size was decreased in a limited manner, from 1.6 to 1.3 µm in the present case. In contrast, for the relatively flat size distribution, which offered a smaller number and a larger diameter, the dissolution of the particles preferably consumed the size, and the number had few changes (
Figure 6b). In addition, it was illustrated that the alloying temperature influences the ASlag at liquidus and solidus (before and after disillusion) in the same way. This means that the dissolution does not affect the size distribution trend.
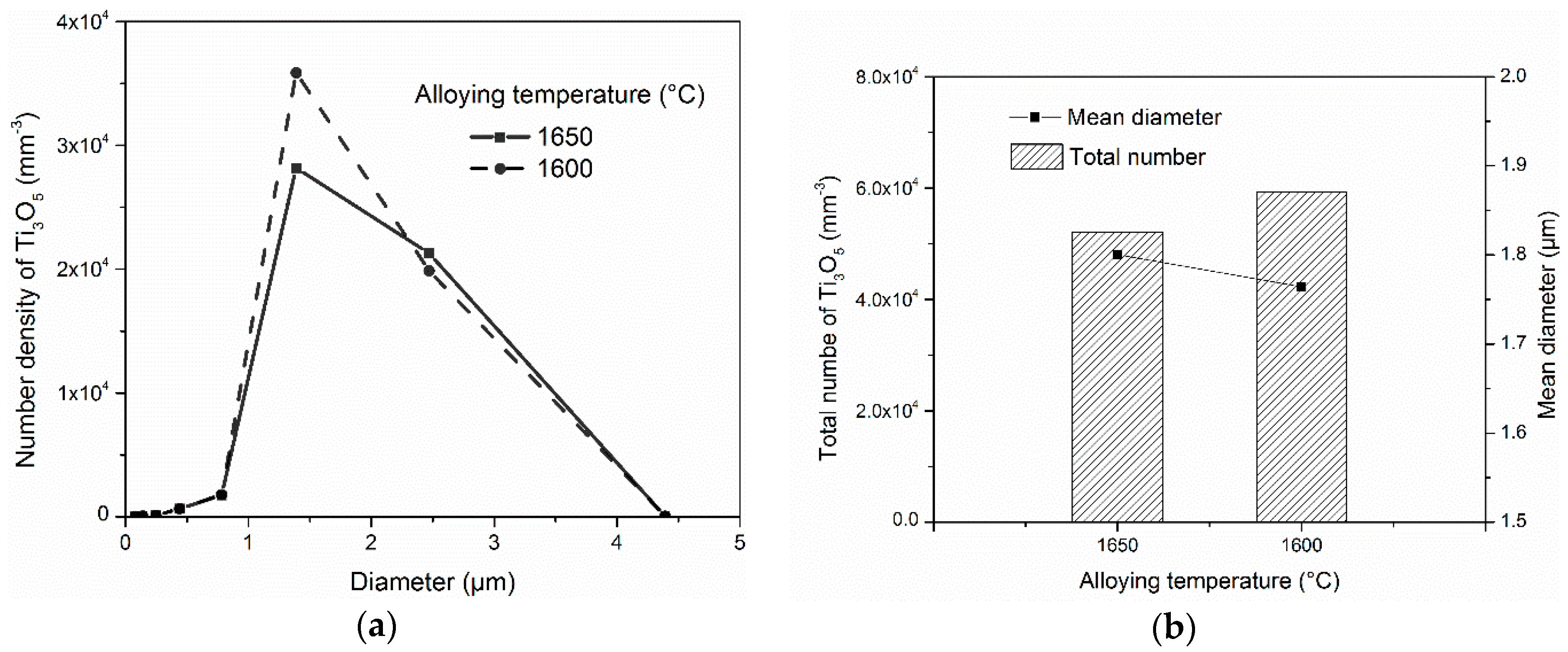
In the investigated steel, ASlag was the first precipitated inclusion and affiliated almost all the oxygen before dissolution. As discussed above, the alloying temperature obviously affected the size distribution of ASlag, while the influence on the later-transformed oxides (Al
2O
3 and Ti
3O
5) was limited. The effect of the alloying temperature on the final size distribution, mean diameter and total amount of Ti
3O
5, as an example, is shown in
Figure 7. In
Figure 7a, it can be seen that the size distribution of Ti
3O
5 in simulations with different alloying temperatures is similar. They have the same size (1.5 µm) as the peak number density and size range, but a difference in the peak number density can be observed. From
Figure 7b, it was found that the total number of Ti
3O
5 in the simulations with different alloying temperatures were quite close, while their mean diameters had a difference within 0.1 µm. The alloying temperature had a minor indirect influence on the later-transformed inclusions. The smaller size and higher number density of ASlag in the simulation with the lower alloying temperature (1600 °C) resulted in an increased dissolution of the particles and a higher oxygen content. This led to a high number density of Ti
3O
5, but a similar size.
3.2. Influence of Oxygen Content
To understand and control the inclusion formation, the influence of oxygen content needs to be studied. The alloying temperature was set at 1650 °C in the calculations of this section. The calculated results including the evolution of the mass fraction, size distribution, mean size and total number are shown in
Figure 8,
Figure 9 and
Figure 10.
The changes in the mass fraction of the inclusions and the corresponding oxygen concentrations during cooling and solidification are compared in
Figure 8. With a lower oxygen content, ASlag inclusions precipitated later and, as expected, their mass fraction was lower, the oxygen content in the liquid steel decreased accordingly. At the beginning of solidification, ASlag steeply dissolved, with a decrease of just ten degrees in temperature due to the thermodynamic instability. The entrapment of particles at the liquid/solid interface was again responsible for the remaining ASlag phase results. Throughout the whole process, the mass fraction profiles of ASlag in the simulations with different oxygen contents evolved in parallel. Note that the minor fluctuation was caused by the instability problem due to the fast dissolution of ASlag. The simulation containing only 30 ppm of oxygen finally achieved a lower ASlag mass fraction than the others. The change in the amount of Al
2O
3 in all the simulations was similar; the mass fraction rapidly reached the maximum and then reduced gradually due to dissolution, while the final mass fractions of Al
2O
3 in the different simulations were nearly the same. The mass fraction profiles of Ti
3O
5 were similar but distinct. The amount of Ti
3O
5 increased as the oxygen content increased. The fluctuations in the oxygen concentration curves resulted from the changes in the amount of ASlag, Al
2O
3 and Ti
3O
5. The dissolution of ASlag led to the increase in the oxygen concentration of the residual liquid at the beginning of the solidification. The Al
2O
3 formation caused a sharp decrease of dissolved oxygen, and after the Al
2O
3 became unstable, the oxygen concentration increased again until the formation of Ti
3O
5 started. Ti
3O
5 consumed almost all the oxygen content in the residual liquid. Though the oxygen concentration increased slightly at the end of solidification due to microsegregation, its content was quite limited. While MnS formed at the end of solidification and is also displayed in
Figure 3, oxygen had little influence on it in the current calculations.
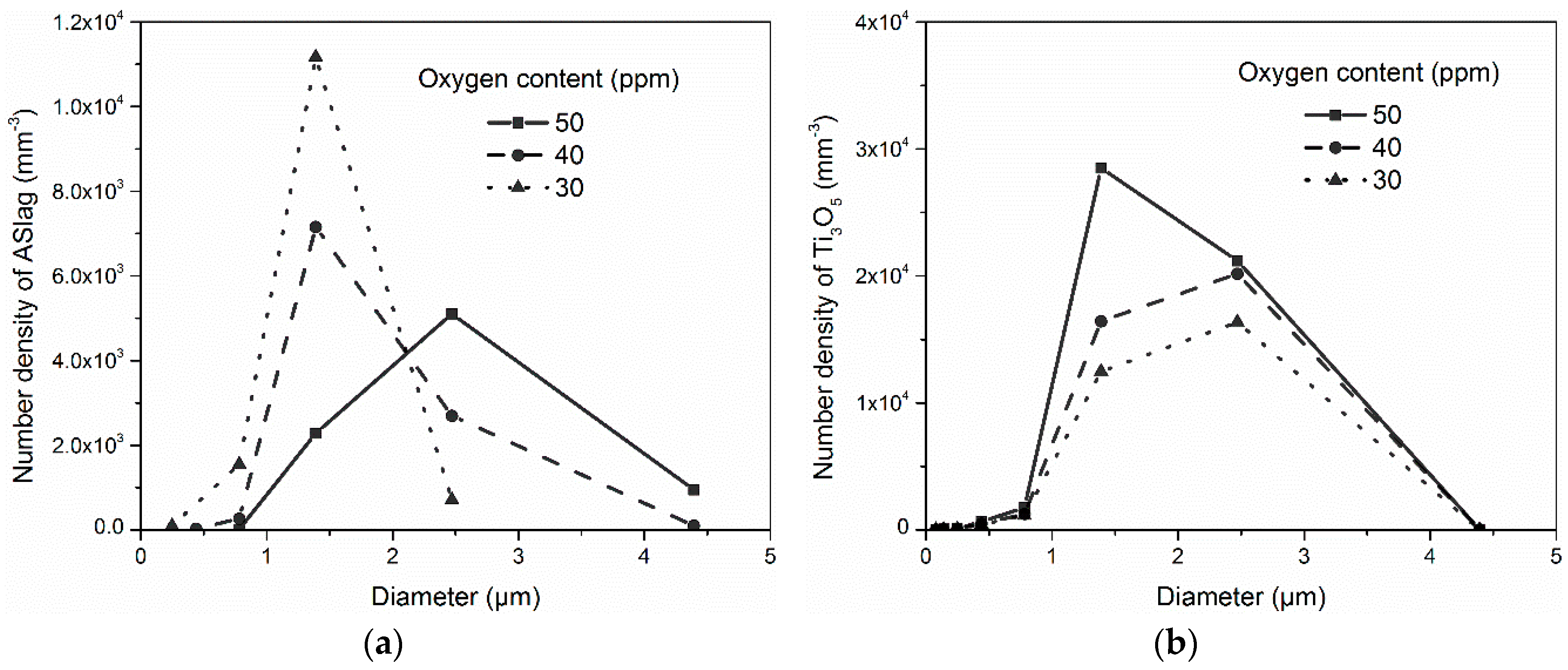
In
Figure 9, the influence of oxygen content on the size distribution of the oxides after solidification is presented. The maximum number density of ASlag decreased as the oxygen content increased, while their corresponding size enlarged from 1.5 µm to 2.5 µm, as shown in
Figure 9a. The maximum size of ASlag in the simulation containing 30 ppm oxygen was 2.5 µm, while the largest particles in the simulations with a higher oxygen content were in a diameter range of 4.4 µm. The number density of Ti
3O
5 decreased as the oxygen content decreased, while the size with the largest number increased from 1.5 µm to 2.5 µm, as displayed in
Figure 9b. The maximum sizes in the different simulations were the same, with a diameter of 4.5 µm. The peak number density of Al
2O
3 decreased with the lower oxygen content, as shown in
Figure 9c. The corresponding particle sizes ranged within the same diameter of approximately 0.45 µm. The size ranges of the Al
2O
3 particles in the three cases were also similar (from 0.08 to 1.35 µm).
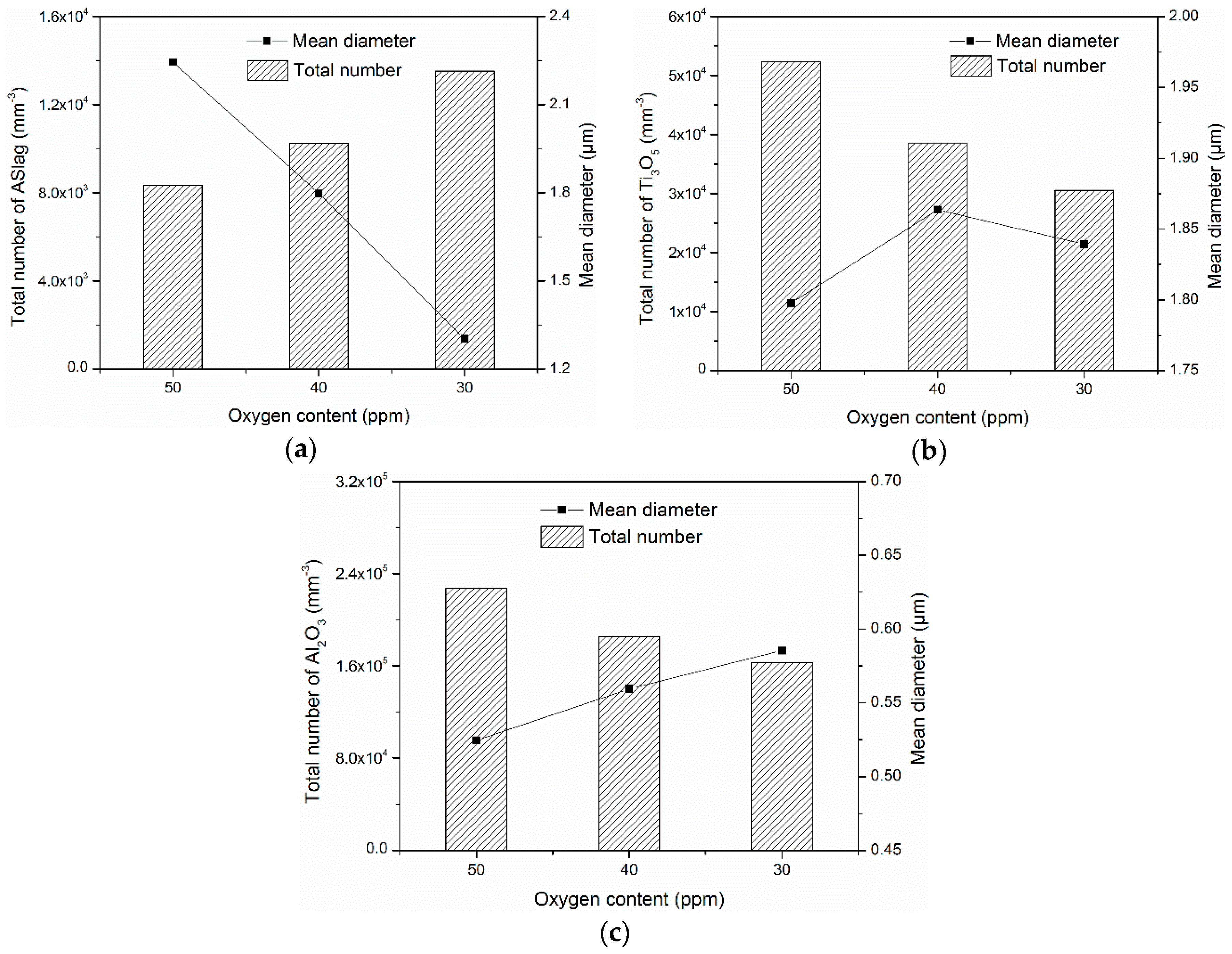
The influence of the oxygen content on the total number and mean diameter of the oxides is displayed in
Figure 10. Due to the different formation conditions, the oxygen content affected the total number and mean diameter of the various oxides in different ways. For ASlag (
Figure 10a), increasing the oxygen content led to a large mean diameter and a lower number density. In contrast to ASlag, the total number of Ti
3O
5 increased with the higher oxygen content and the mean diameters were similar (from 1.8 µm to 1.9 µm) (
Figure 10b). As shown in
Figure 10c, Al
2O
3 had a higher number density and a smaller but similar size (from 0.53 µm to 0.58 µm) when the oxygen content was higher. The mechanisms related to the influence of the oxygen content on the formation of oxides are discussed in the following.
3.3. Discussion
As described in the former sections, both alloying temperature and oxygen content have a significant influence on the formation of various oxides. In contrast to the effects resulting from metallurgical parameters, they could be explained by the mechanisms of competitive nucleation and growth.
As shown in
Figure 6, lowering the alloying temperature reduces the energy obstacle and enhances the chemical driving force of the nucleation (Equations (4) and (5)) of ASlag in the selected steel. Thus the nucleation rate and number density are greatly increased. The high number density further limits the growth of the particles with the given oxygen content. This results in a relatively pointed shape size distribution and a smaller size (
Figure 6a). Comparatively, the ASlag in the simulation with a higher alloying temperature had a larger size but were fewer in number. As the first-precipitated oxide, the size distribution of ASlag further influenced the formation of later-transformed oxides. From Equation (7), it was found that smaller particles show a faster dissolution rate and disappear more easily. This explains the lower mass fraction of ASlag and higher mass fraction of Ti
3O
5 and Al
2O
3 in the simulation with a lower alloying temperature (
Figure 3). While Ti
3O
5 and Al
2O
3 formed at comparable temperatures in different cases, the higher dissolution rate of ASlag released more oxygen and contributed to higher nucleation rates and number densities of Ti
3O
5 and Al
2O
3.
In
Figure 9 and
Figure 10, it can be seen that the initial oxygen content affects the first precipitated ASlag phase and the later-transformed Ti
3O
5 and Al
2O
3 in different manners. As the oxygen content in the simulations increased, ASlag starts to form at significantly higher temperatures, over 30 °C (
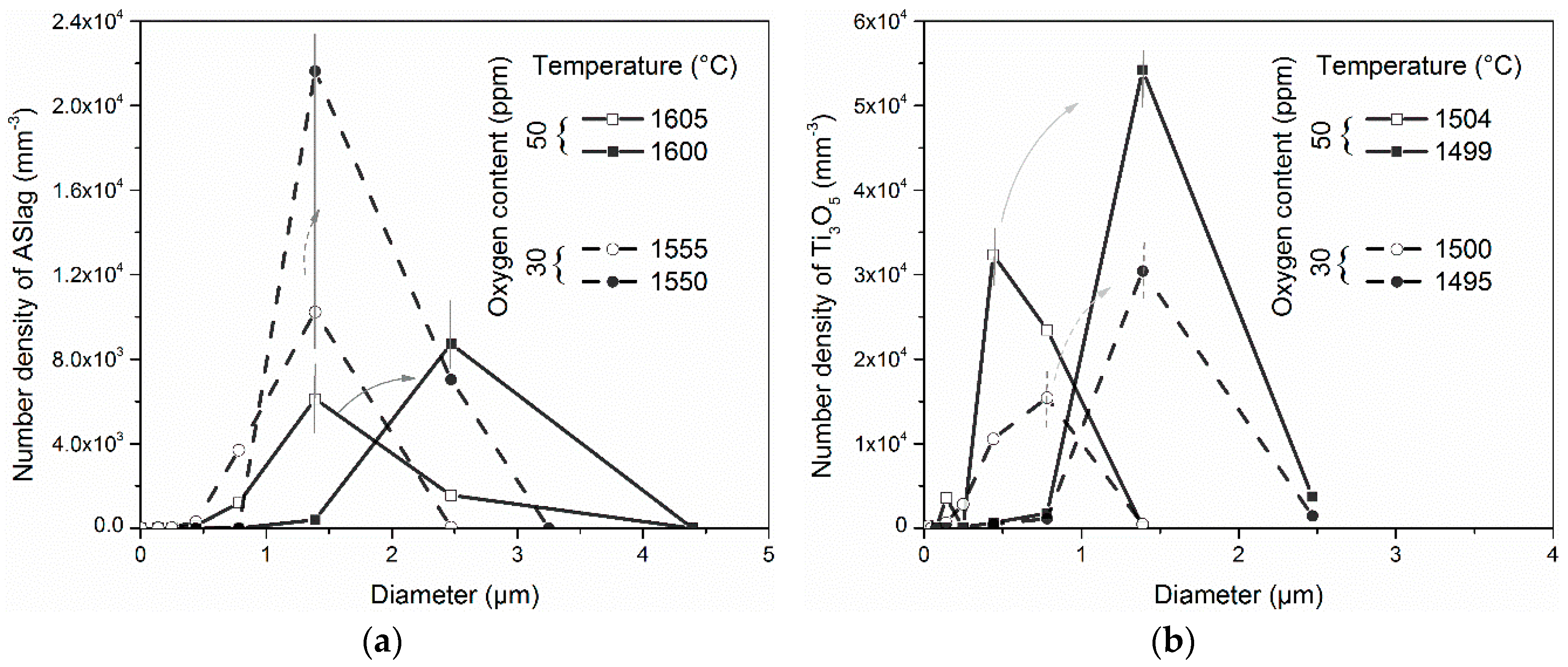
Figure 8). From Equation (14), we can see that oxygen diffuses faster at higher temperatures. This led to the faster growth of ASlag particles in the simulation containing more oxygen. In the meantime, the growth of ASlag was more favorable than its nucleation in the simulation with a higher oxygen content, as illustrated in
Figure 11a. In the figure, the size and number evolution of ASlag in the simulations with 50 and 30 ppm oxygen are compared. In both cases, ASlag can nucleate and increase in size during the temperature periods; the temperatures decreased by 5 °C under the same cooling rate, which ensures that the evolution time of ASlag is the same. It showed that the size distribution of ASlag in the simulation with 50 ppm oxygen at 1605 °C was similar to that in the simulation containing 30 ppm oxygen at 1555 °C. When the temperature decreased from 1605 to 1600 °C, the ASlag in the simulation with 50 ppm oxygen grew significantly, with a limited increase in number, the size with the largest number density almost doubled and the number density increased by just 30%. In contrast, in the simulation with 30 ppm oxygen cooled down by 5 °C, the peak number density of the particles became 2.2 times higher and the corresponding diameter remained unchanged. By comparing these two cases at a higher formation temperature, ASlag was more likely to increase in size than its nucleate. In addition, ASlag precipitated earlier in the simulation with more oxygen and had a longer time to grow, which also contributed to its larger size. Based on the above discussion, it was reasonable to find that the total number of ASlag decreased and the mean diameter increased as the oxygen content increased (
Figure 10a). Further, the size distribution of the particles trapped by the solid steel can reflect their character in the formation process due to the assumed homogeneous distribution in the residual liquid, even though the particles undergo dissolution.
In the cases of Al
2O
3 and Ti
3O
5, which formed during the solidification process, the oxides in the simulations containing different oxygen contents started to precipitate at quite similar temperatures. In the simulation with a higher oxygen content before nucleation (
Figure 8), the supersaturation and driving force of the oxide formation were higher. This led to a smaller nucleation barrier, as referred to in Equation (5). From Equation (5), at the given temperature, the less-critical nucleation energy change enlarged the nucleation rate. Thus, the number densities of Al
2O
3 and Ti
3O
5 increased as the oxygen content increased. At a similar temperature, the diffusivity of the reactants was regarded as the same in the different simulations and the growth rates of the particles were similar. In
Figure 11b, the evolution of the Ti
3O
5 in the simulation with 50 and 30 ppm oxygen were taken as an example to instruct this. Similar to the case of ASlag (
Figure 11a), the size distribution of Ti
3O
5 that experienced the same evolution period (5 °C decrease) were compared. This shows that the number densities were larger in the simulation with a higher oxygen content, before and after evolution. The size distribution shapes of the two cases were quite similar, and changes in them after nucleation and growth were in parallel. The mean size growth rates of Ti
3O
5 in the simulations with 50 and 30 ppm oxygen were almost the same at 1.89 and 1.82 (µm/s), respectively. A large number of particles limits the growth driving force of each single particle and further reduces the growth rate. This explains why the final mean diameters of the Al
2O
3 and Ti
3O
5 particles increased insignificantly in the simulations with less oxygen (
Figure 10b,c). The case of Ti
3O
5 in the simulation containing 30 ppm oxygen was an exception, with both a smaller number and mean diameter compared to that in the simulation containing 40 ppm oxygen (
Figure 10b). This is attributed to the limited amount of oxygen in the residual liquid.
Based on the calculation results and the mechanisms of the competitive formation of the multi-type inclusions, decreasing the alloy temperature effectively avoided the formation of large oxides and improved the steel properties. Additionally, it generated a high number of particles with a small size, which were beneficial for promoting acicular ferrite formation and grain refinement. On the other hand, the available oxygen content, which was nothing other than the total oxygen content, neither stands automatically for the total amount of various oxides nor for their number or size. According to the different objectives, a specific focus on the inclusions in different steels and the metallurgical conditions is needed. In the selected steel, the large ASlag particles in the simulation with a higher oxygen content that formed at a high temperature should be avoided; considering both the cleanness of the steel and the creation of desirable oxides, 30 ppm of oxygen is the preferable choice. The comprehensive adjustment of the alloying temperature and steel composition is a promising approach to obtaining preferable inclusions. In sum, to combine a preferably high steel cleanness with the creation of specific inclusion types and sizes for microstructure evolution, comprehensive knowledge of the inclusion formation is needed.