Thermal Stability of Ru–Al Multilayered Thin Films on Inconel 617
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
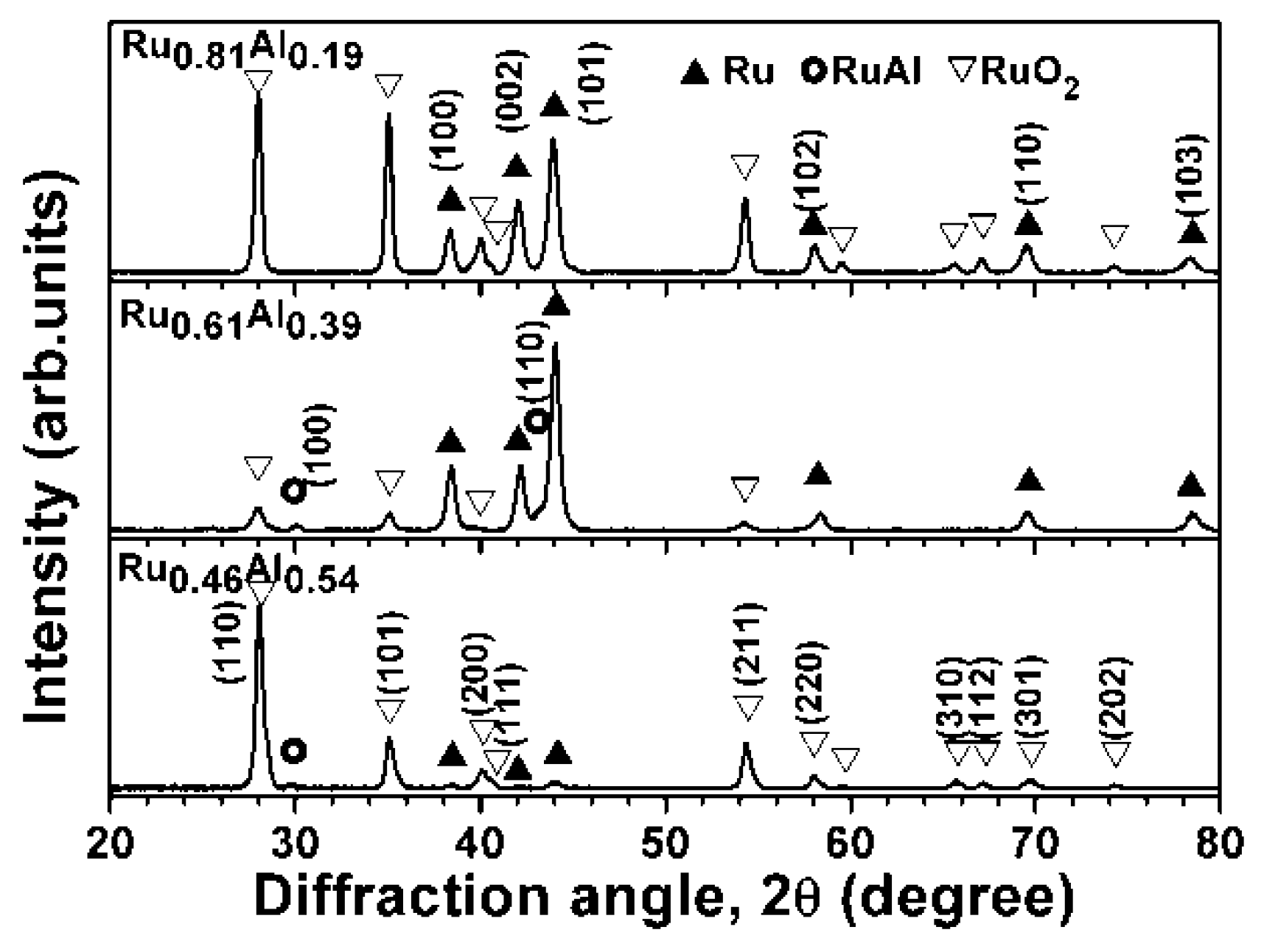
3.1. As-Deposited Ru–Al Thin Films
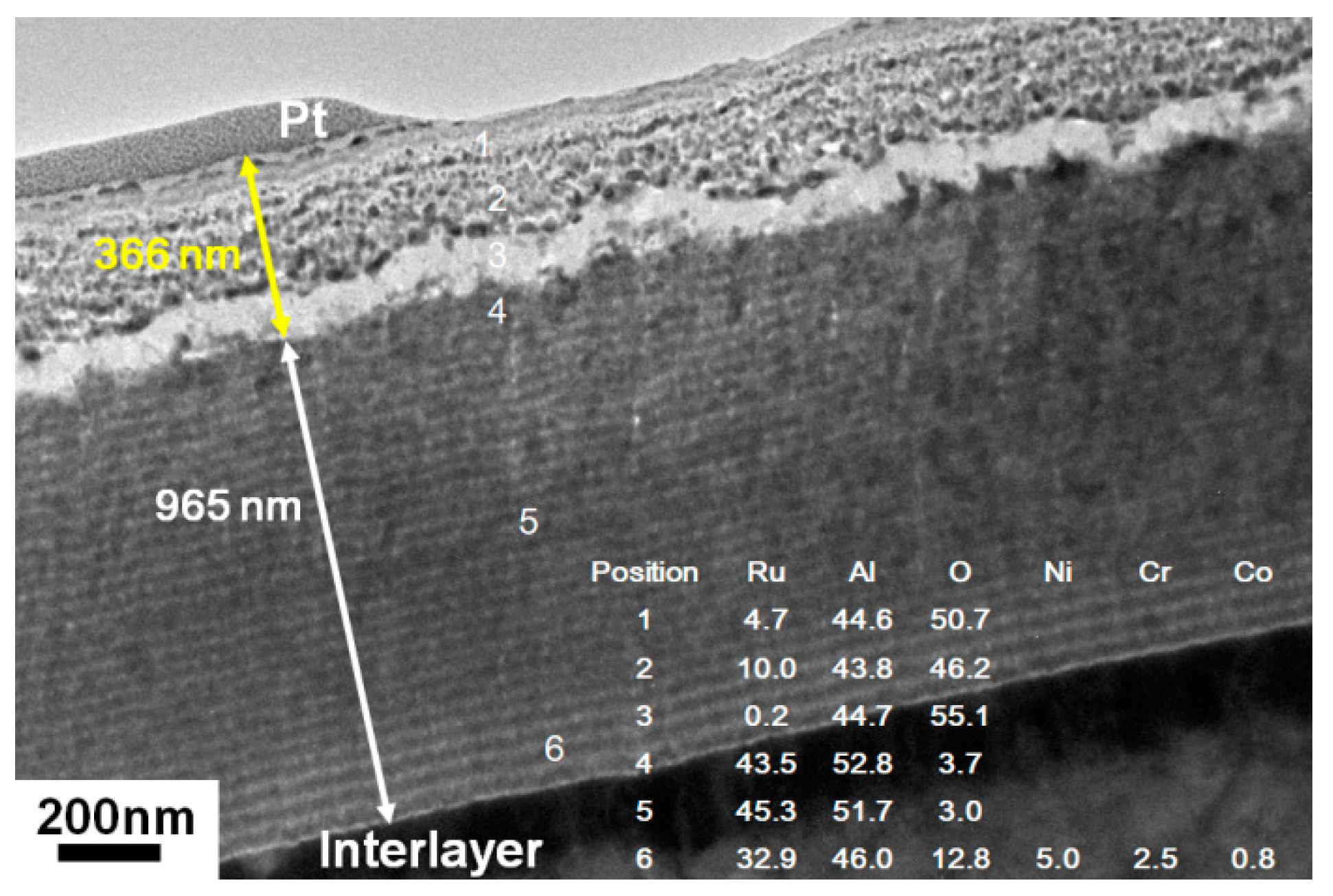
3.2. Ru0.48Al0.52 Thin Films Annealed in 1% O2–99% Ar at 600 °C
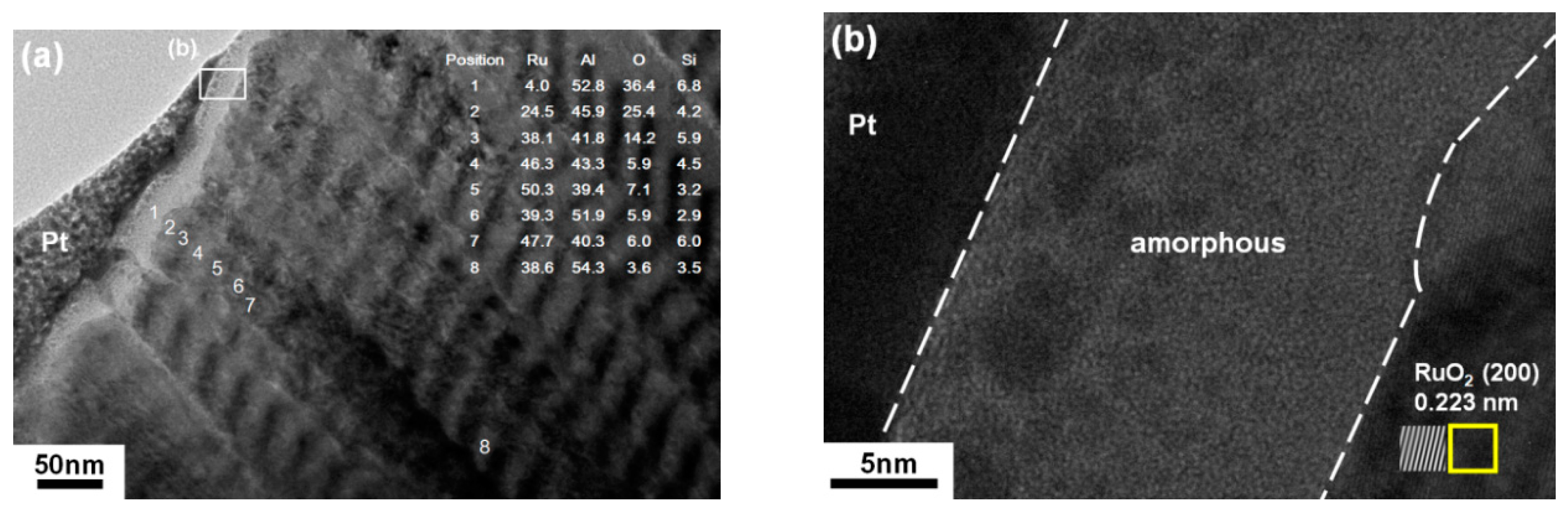
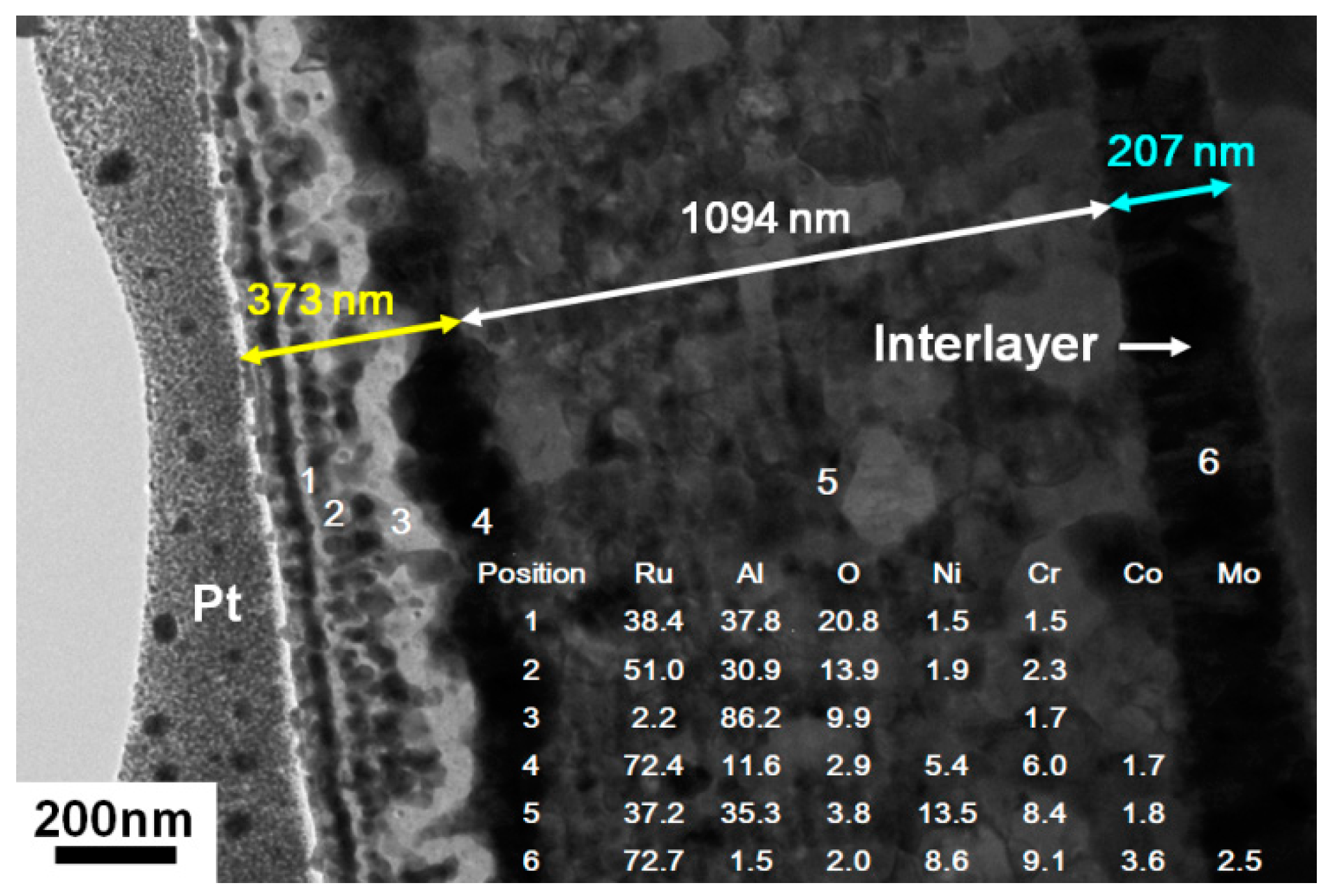
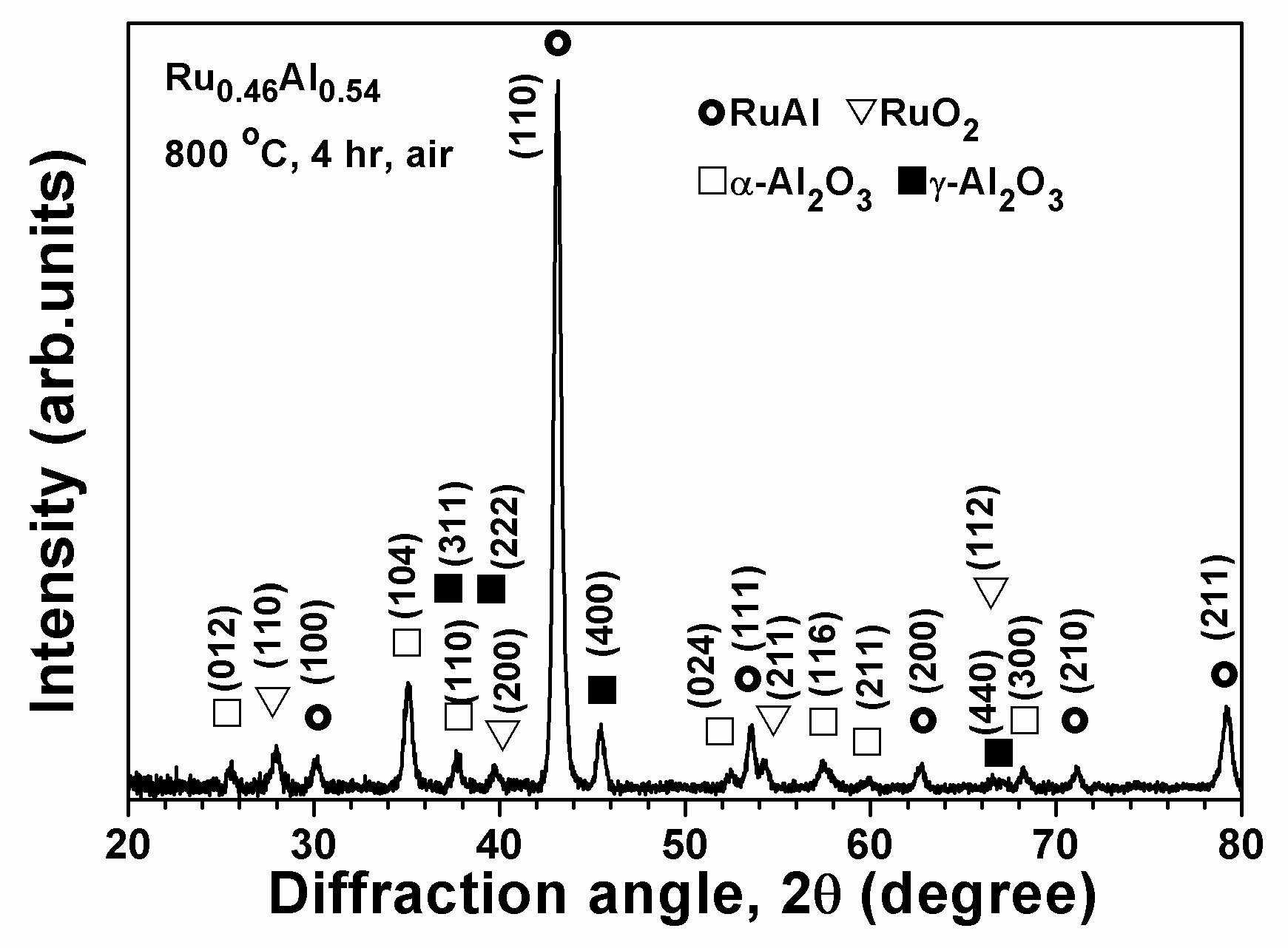
3.3. Ru–Al Thin Films Annealed in Air at 800 °C
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahman, M.d.S.; Priyadarshan, G.; Raja, K.S.; Nesbitt, C.; Misra, M. Characterization of high temperature deformation behavior of INCONEL 617. Mech. Mater. 2009, 41, 261–270. [Google Scholar] [CrossRef]
- Gariboldi, E.; Cabibbo, M.; Spigarelli, S.; Ripamonti, D. Investigation on precipitation phenomena of Ni–22Cr–12Co–9Mo alloy aged and crept at high temperature. Int. J. Press. Vessel. Pip. 2008, 85, 63–71. [Google Scholar] [CrossRef]
- DeMasi-Marcin, J.T.; Gupta, D.K. Protective coatings in the gas turbine engine. Surf. Coat. Technol. 1994, 68–69, 1–9. [Google Scholar] [CrossRef]
- Vaßen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Miller, R.A. Current status of thermal barrier coatings—An overview. Surf. Coat. Technol. 1987, 30, 1–11. [Google Scholar] [CrossRef]
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Aygun, A.; Vasiliev, A.L.; Padture, N.P.; Ma, X. Novel thermal barrier coatings that are resistant to high-temperature attack by glassy deposits. Acta Mater. 2007, 55, 6734–6745. [Google Scholar] [CrossRef]
- Huang, J.; Wang, W.; Lu, X.; Hu, D.; Feng, Z.; Guo, T. Effect of particle size on the thermal shock resistance of plasma-sprayed YSZ coatings. Coatings 2017, 7, 150. [Google Scholar] [CrossRef]
- Hu, N.; Khan, M.; Wang, Y.; Song, X.; Lin, C.; Chang, C.; Zeng, Y. Effect of Microstructure on the Thermal Conductivity of Plasma Sprayed Y2O3 Stabilized Zirconia (8% YSZ). Coatings 2017, 7, 198. [Google Scholar] [CrossRef]
- Mücklich, F.; Ilić, N. RuAl and its alloys. Part I. Structure physical properties, microstructure and processing. Intermetallics 2005, 13, 5–21. [Google Scholar] [CrossRef]
- Mücklich, F.; Ilić, N.; Wol, K. RuAl and its alloys, part II: Mechanical properties, environmental resistance and applications. Intermetallics 2008, 16, 593–608. [Google Scholar] [CrossRef]
- Tryon, B.; Pollock, T.M.; Gigliotti, M.F.X.; Hemker, K. Thermal expansion behavior of ruthenium aluminides. Scr. Mater. 2014, 50, 845–848. [Google Scholar] [CrossRef]
- Tryon, B.; Feng, Q.; Wellman, R.G.; Murphy, K.S.; Yang, J.; Levi, C.G.; Nicholls, J.R.; Pollock, T.M. Multilayered ruthenium-modified bond coats for thermal barrier coatings. Metall. Mater. Trans. A 2006, 37A, 3347–3358. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.B.; Peng, H.; Peng, L.Q.; Gong, S.K. Diffusion barrier behaviors of (Ru,Ni)Al/NiAl coatings on Ni-based superalloy substrate. Intermetallics 2011, 19, 191–195. [Google Scholar] [CrossRef]
- Guitar, M.A.; Woll, K.; Ramos-Moore, E.; Mücklich, F. Study of grain growth and thermal stability of nanocrystalline RuAl thin films deposited by magnetron sputtering. Thin Solid Films 2013, 527, 1–8. [Google Scholar] [CrossRef]
- Guitar, M.A.; Ramos-Moore, E.; Mücklich, F. The influence of impurities on the formation of protective aluminum oxides on RuAl thin films. J. Alloys Compd. 2014, 594, 165–170. [Google Scholar] [CrossRef]
- Seifert, M.; Menzel, S.B.; Rane, G.K.; Hoffmann, M.; Gemming, T. RuAl thin films on high–temperature piezoelectric substrates. Mater. Res. Express 2015, 2, 085001. [Google Scholar] [CrossRef]
- Seifert, M.; Rane, G.K.; Menzel, S.B.; Gemming, T. TEM studies on the changes of the composition in LGS and CTGS substrates covered with a RuAl metallization and on the phase formation within the RuAl film after heat treatment at 600 and 800 °C. J. Alloys Compd. 2016, 664, 510–517. [Google Scholar] [CrossRef]
- Seifert, M.; Rane, G.K.; Menzel, S.B.; Gemming, T. The influence of barrier layers (SiO2, Al2O3, W) on the phase formation and stability of RuAl thin films on LGS and CTGS substrates for surface acoustic wave technology. J. Alloys Compd. 2016, 688, 228–240. [Google Scholar] [CrossRef]
- Seifert, M.; Rane, G.K.; Oswald, S.; Menzel, S.B.; Gemming, T. The influence of the composition of Ru100–xAlx (x = 50, 55, 60, 67) thin films on their thermal stability. Materials 2017, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.I.; Zheng, Z.T.; Kai, W.; Huang, Y.R. Oxidation behavior of Ru–Al multilayer coatings. Appl. Surf. Sci. 2017, 406, 1–7. [Google Scholar] [CrossRef]
- Liu, S.C.; Chen, Y.I.; Tsai, H.Y.; Lin, K.C.; Chen, Y.H. Thermal stability of Ir–Re coatings annealed in oxygen-containing atmospheres. Surf. Coat. Technol. 2013, 237, 105–111. [Google Scholar] [CrossRef]
- Chen, Y.I. Laminated structure in internally oxidized Ru–Ta coatings. Thin Solid Films 2012, 524, 205–210. [Google Scholar] [CrossRef]
- Chen, Y.I.; Lu, T.S.; Zheng, Z.T. Internally oxidized Ru–Zr multilayer coatings. Coatings 2017, 7, 46. [Google Scholar] [CrossRef]
- Janssen, G.C.A.M.; Abdalla, M.M.; van Keulen, F.; Pujada, B.R.; van Venrooy, B. Celebrating the 100th anniversary of the Stoney equation for film stress: Developments from polycrystalline steel strips to single crystal silicon wafers. Thin Solid Films 2009, 517, 1858–1867. [Google Scholar] [CrossRef]
- Deng, Y.L.; Lee, J.W.; Lou, B.S.; Duh, J.G.; Chu, J.P.; Jang, J.S.C. The fabrication and property evaluation of Zr–Ti–B–Si thin film metallic glass materials. Surf. Coat. Technol. 2014, 259, 115–122. [Google Scholar] [CrossRef]
- Bell, W.E.; Tagami, M. High-temperature chemistry of the ruthenium–oxygen system. J. Phys. Chem. 1963, 67, 2432–2436. [Google Scholar] [CrossRef]
- Huang, J.H.; Chen, J.S. Material characteristics and electrical property of reactively sputtered RuO thin films. Thin Solid Films 2001, 382, 139–145. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; VCH: New York, NY, USA, 1995. [Google Scholar]
- Chen, Y.I.; Chu, H.N.; Kai, W. Internal oxidation of laminated Nb–Ru coatings. Appl. Surf. Sci. 2016, 389, 477–483. [Google Scholar] [CrossRef]
- Bellina, P.J.; Catanoiu, A.; Morales, F.M.; Rühle, M. Formation of discontinuous Al2O3 layers during high-temperature oxidation of RuAl alloys. J. Mater. Res. 2006, 21, 276–286. [Google Scholar] [CrossRef]
- Soldera, F.; Ilić, N.; Brännström, S.; Barrientos, I.; Gobran, H.; Mücklich, F. Formation of Al2O3 scales on single-phase RuAl produced by reactive sintering. Oxid. Met. 2003, 59, 529–542. [Google Scholar] [CrossRef]
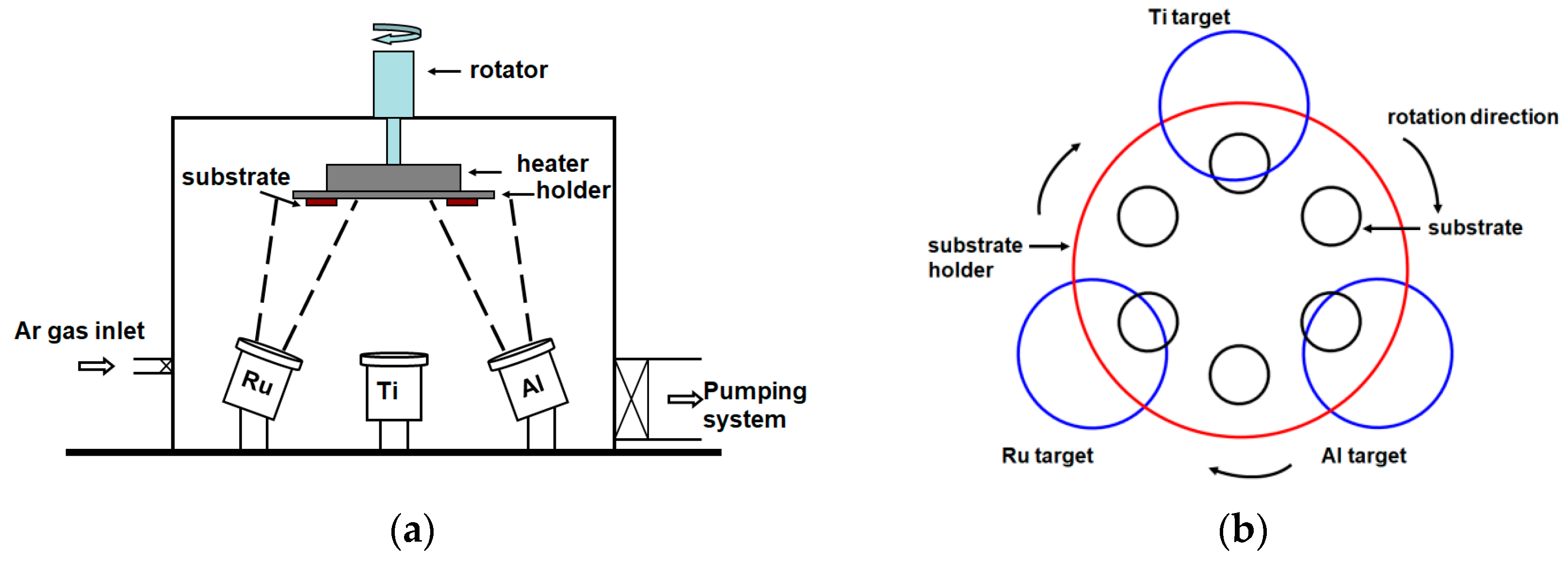
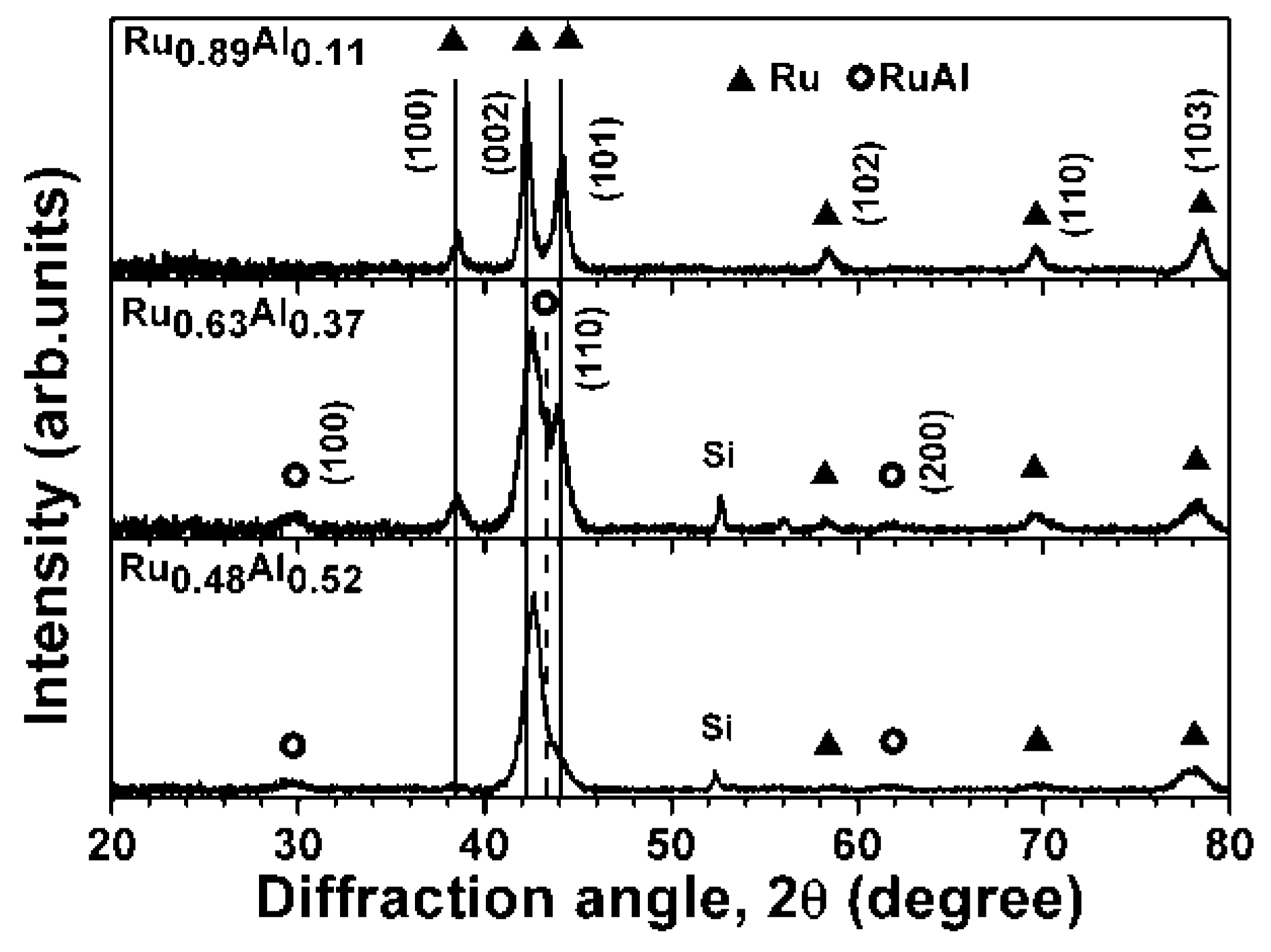
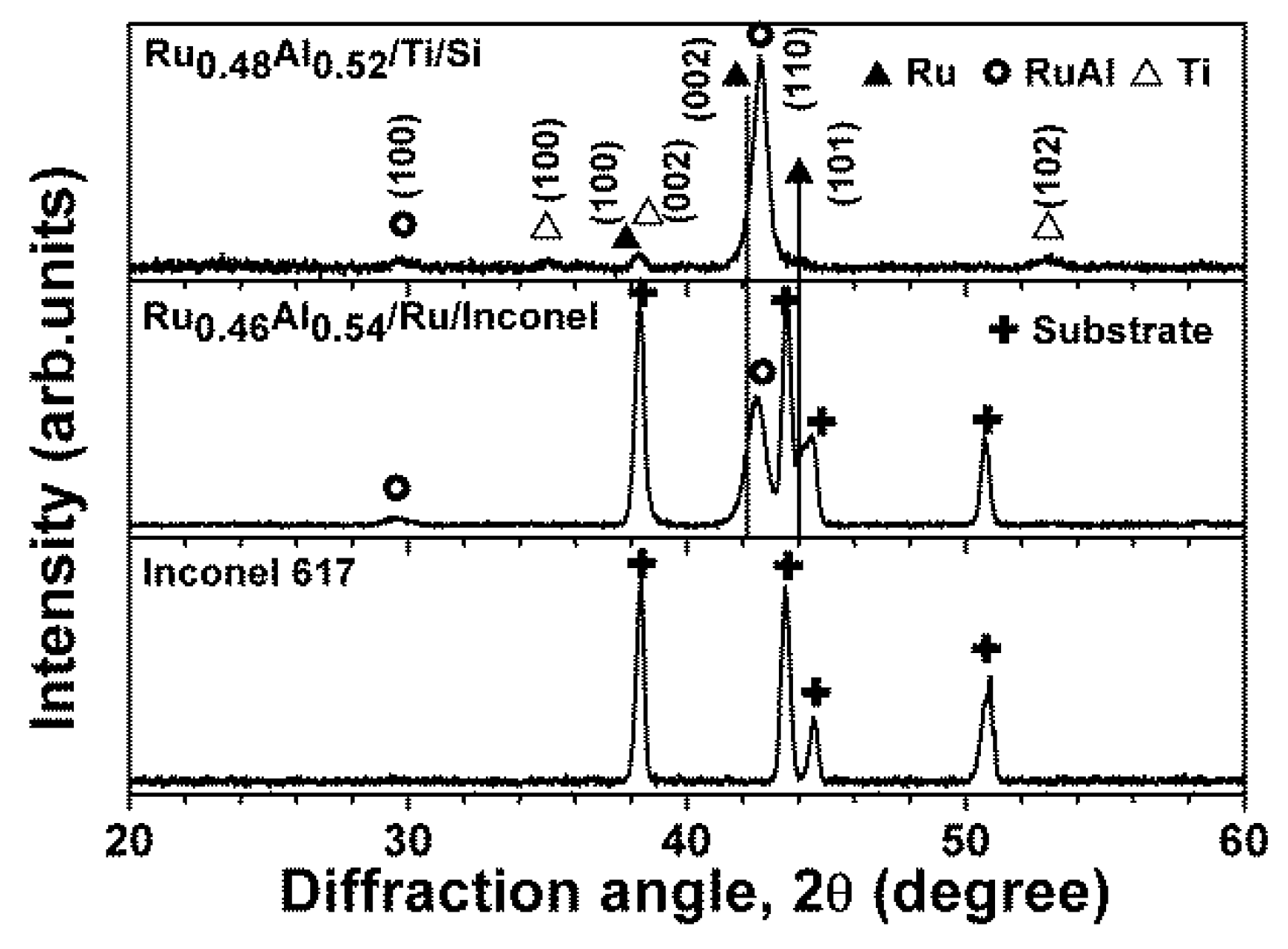

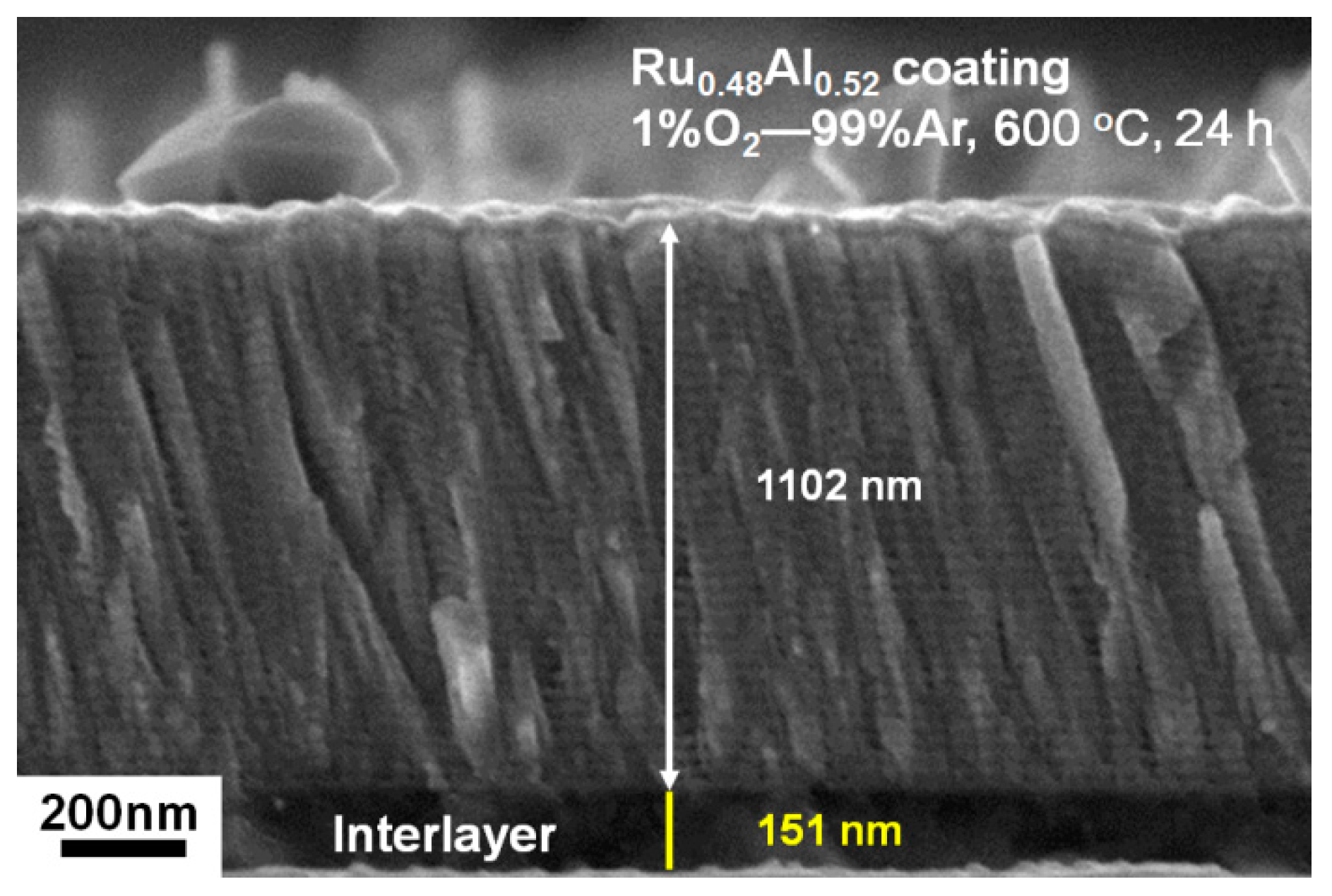




| Sample | Sputter Power (W) | Chemical Composition (at.%) | Thickness (nm) | Period | ||||
|---|---|---|---|---|---|---|---|---|
| WRu | WAl | Ru | Al | O | Film | Interlayer | (nm) | |
| Ru0.89Al0.11 | 200 | 100 | 86.69 ± 0.39 | 10.89 ± 0.02 | 2.42 ± 0.37 | 1305 | 50 | 37 |
| Ru0.63Al0.37 | 150 | 150 | 59.33 ± 0.32 | 34.75 ± 0.32 | 5.92 ± 0.03 | 1312 | 50 | 37 |
| Ru0.48Al0.52 | 100 | 200 | 47.35 ± 0.39 | 52.06 ± 0.34 | 0.59 ± 0.14 | 1083 | 50 | 31 |
| Sample | Sputter Power (W) | Chemical Composition (at.%) | |||
|---|---|---|---|---|---|
| WRu | WAl | Ru | Al | O | |
| as-deposited | |||||
| Ru0.81Al0.19 | 200 | 100 | 80.02 ± 0.58 | 19.16 ± 0.24 | 0.82 ± 0.78 |
| Ru0.61Al0.39 | 150 | 150 | 57.16 ± 0.20 | 37.16 ± 0.06 | 5.68 ± 0.15 |
| Ru0.46Al0.54 | 100 | 200 | 44.80 ± 0.03 | 52.54 ± 0.15 | 2.66 ± 0.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-I.; Zheng, Z.-T.; Jhang, J.-W. Thermal Stability of Ru–Al Multilayered Thin Films on Inconel 617. Metals 2018, 8, 514. https://doi.org/10.3390/met8070514
Chen Y-I, Zheng Z-T, Jhang J-W. Thermal Stability of Ru–Al Multilayered Thin Films on Inconel 617. Metals. 2018; 8(7):514. https://doi.org/10.3390/met8070514
Chicago/Turabian StyleChen, Yung-I, Zhi-Ting Zheng, and Jia-Wei Jhang. 2018. "Thermal Stability of Ru–Al Multilayered Thin Films on Inconel 617" Metals 8, no. 7: 514. https://doi.org/10.3390/met8070514




