Dissolution and Precipitation Behavior for Hot Forming of 7021 and 7075 Aluminum Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results & Discussion
3.1. Heating Experiments
3.2. Experiments on Isothermal Soaking
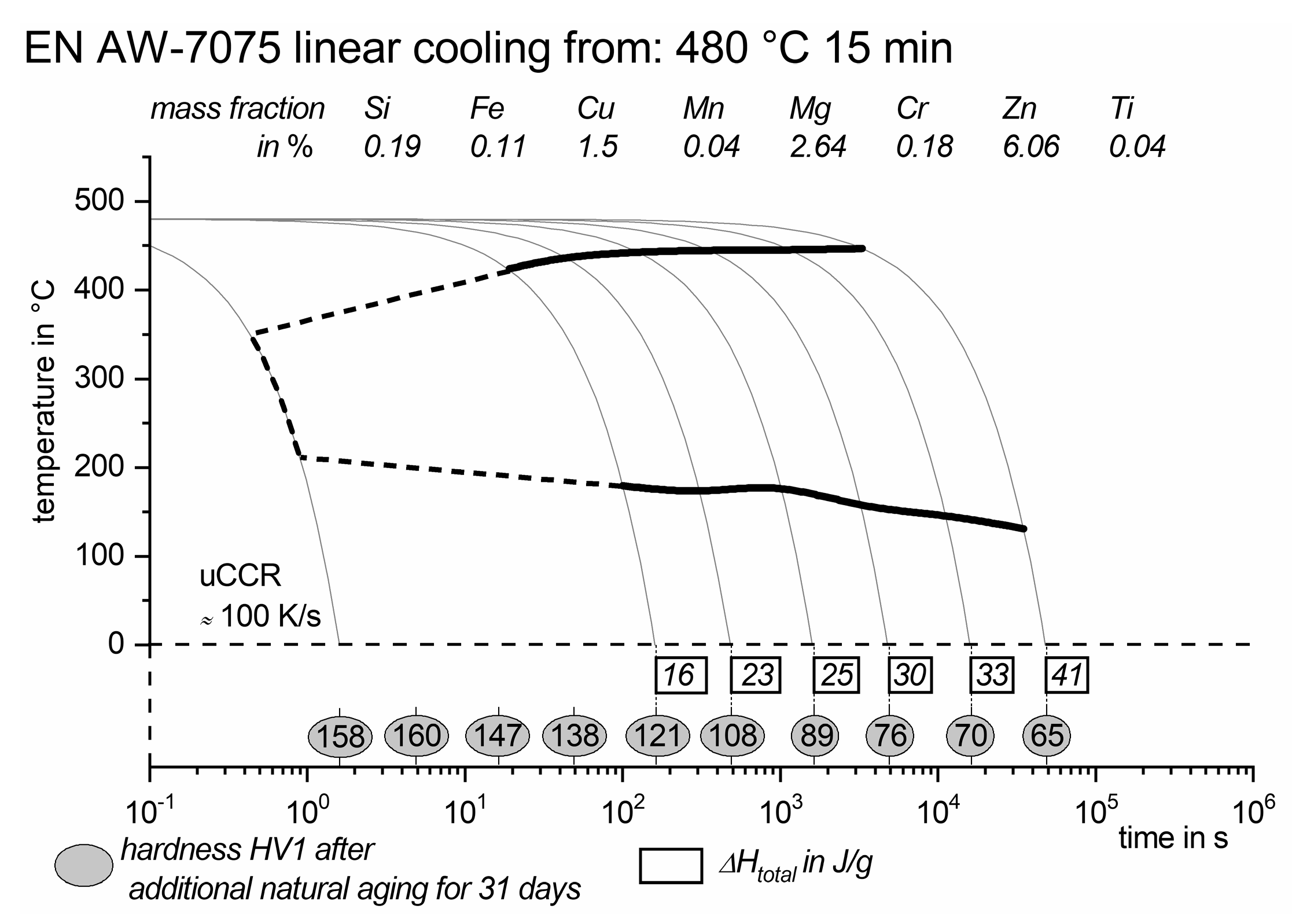
3.3. Cooling Experiments
3.4. Comparison with Real Hot Forming Processes
4. Conclusions
- ▪
- Applying a heating rate of 1 K/s results in the following solution annealing parameters to be appropriate for:
- ○
- EN AW-7021 T4: 515 °C for 5 min
- ○
- EN AW-7075 T6: 480 °C for 15 min
- ▪
- Cooling from solution treatment might lead to substantial quench induced precipitation resulting in a severe loss of age hardening potential. If using the above-mentioned solution temperatures, the transfer of parts from the solution treatment furnace to the quenching operation must not exceed a loss down to 380 °C for 7021 and down to 425 °C for 7075 (for the given cooling rate of calm air cooling).
- ▪
- The critical cooling rate to avoid any quench induced precipitation or loss of age hardening potential is about 10 K/s for EN AW-7021 and about 100 K/s for EN AW-7075.
- ▪
- Those relatively high cooling rates should be retained down to below 200 °C to avoid quench induced precipitation or loss of age hardening potential.
- ▪
- Real hot forming of 2 mm thick sheet is suitable to fulfill the above mentioned critical process parameters, for 7021 with a large safety margin, for 7075 near the borderline.
Author Contributions
Funding
Conflicts of Interest
References
- Jaburek, N.; Merklein, M. Influence of a retrogression and reaging (RRA)-treatment on the mechanical and microstructural characteristics of the aluminium alloy AlZn4, 5Mg1. Prod. Eng. Res. Dev. 2015, 9, 161–166. [Google Scholar] [CrossRef]
- Bardelcik, A.; Bouhier, A.; Worswick, M.J. Three Point Bend Performance of Solutionized, Die Quenched and Heat Treated AA7075 Beam Members. MSF 2014, 794–796, 431–436. [Google Scholar] [CrossRef]
- Kumar, M.; Sotirov, N.; Chimani, C.M. Investigations on warm forming of AW-7020-T6 alloy sheet. J. Mater. Process. Technol. 2014, 214, 1769–1776. [Google Scholar] [CrossRef]
- Keci, A.; Harrison, N.R.; Luckey, S.G. Experimental Evaluation of the Quench Rate of AA7075. In Proceedings of the SAE 2014 World Congress & Exhibition, Detroit, MI, USA, 8–10 April 2014; SAE International400 Commonwealth Drive: Warrendale, PA, USA, 2014. [Google Scholar]
- Polmear, I.J. Light Alloys. From Traditional Alloys to Nanocrystals, 4th ed.; Elsevier Butterworth-Heinemann: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Starink, M.J.; Milkereit, B.; Zhang, Y.; Rometsch, P.A. Predicting the quench sensitivity of Al-Zn-Mg-Cu alloys: A model for linear cooling and strengthening. Mater. Des. 2015, 88, 958–971. [Google Scholar] [CrossRef]
- Adler, P.N.; DeIasi, R. Calorimetric studies of 7000 series aluminum alloys: II. Comparison of 7075, 7050 and RX720 alloys. Metall. Trans. A 1977, 8, 1185–1190. [Google Scholar] [CrossRef]
- DeIasi, R.; Adler, P.N. Calorimetric studies of 7000 series aluminum alloys: I. Matrix precipitate characterization of 7075. Metall. Mater. Trans. A 1977, 8, 1177–1183. [Google Scholar]
- Lloyd, D.J.; Chaturvedi, M.C. A calorimetric study of aluminium alloy AA-7075. J. Mater. Sci. 1982, 17, 1819–1824. [Google Scholar] [CrossRef]
- Papazian, J.M. Calorimetric studies of precipitation and dissolution kinetics in aluminum alloys 2219 and 7075. Metall. Mater. Trans. A 1982, 13, 761–769. [Google Scholar] [CrossRef]
- Park, J.K.; Ardell, A.J. Correlation between microstructure and calorimetric behavior of aluminum alloy 7075 and Al-Zn-Mg alloys in various tempers. Mater. Sci. Eng. A 1989, 114, 197–203. [Google Scholar] [CrossRef]
- Paulisch, M.C.; Wanderka, N.; Haupt, M.; Selve, S.; Driehorst, I.; Reimers, W. The influence of heat treatments on the microstructure and the mechanical properties in commercial 7020 alloys. Mater. Sci. Eng. A 2015, 626, 254–262. [Google Scholar] [CrossRef]
- Milkereit, B.; Kessler, O.; Schick, C. Recording of continuous cooling precipitation diagrams of aluminium alloys. Thermochim. Acta 2009, 492, 73–78. [Google Scholar] [CrossRef]
- Milkereit, B.; Wanderka, N.; Schick, C.; Kessler, O. Continuous cooling precipitation diagrams of Al-Mg-Si alloys. Mater. Sci. Eng. A 2012, 550, 87–96. [Google Scholar] [CrossRef]
- Milkereit, B.; Giersberg, L.; Kessler, O.; Schick, C. Isothermal time-temperature-precipitation diagram for an aluminum alloy 6005A by in situ DSC experiments. Materials 2014, 7, 2631–2649. [Google Scholar] [CrossRef] [PubMed]
- Zohrabyan, D.; Milkereit, B.; Schick, C.; Kessler, O. Continuous cooling precipitation diagram of high alloyed Al-Zn-Mg-Cu 7049A alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 2018–2024. [Google Scholar] [CrossRef]
- Zhang, Y.; Milkereit, B.; Kessler, O.; Schick, C.; Rometsch, P.A. Development of continuous cooling precipitation diagrams for aluminium alloys AA7150 and AA7020. J. Alloy. Compd. 2014, 584, 581–589. [Google Scholar] [CrossRef]
- Yang, B.; Milkereit, B.; Zhang, Y.; Rometsch, P.A.; Kessler, O.; Schick, C. Continuous cooling precipitation diagram of aluminium alloy AA7150 based on a new fast scanning calorimetry and interrupted quenching method. Mater. Charact. 2016, 120, 30–37. [Google Scholar] [CrossRef]
- Osten, J.; Milkereit, B.; Schick, C.; Kessler, O. Dissolution and precipitation behaviour during continuous heating of Al-Mg-Si alloys in a wide range of heating rates. Materials 2015, 8, 2830–2848. [Google Scholar] [CrossRef]
- Milkereit, B.; Beck, M.; Reich, M.; Kessler, O.; Schick, C. Precipitation kinetics of an aluminium alloy during Newtonian cooling simulated in a differential scanning calorimeter. Thermochim. Acta 2011, 522, 86–95. [Google Scholar] [CrossRef]
- Milkereit, B.; Kessler, O. Continuous-Cooling Precipitation Diagrams. In ASM Handbook Volume 4E: Heat Treating of Nonferrous Alloys; Totten, G.E., Ed.; ASM International: Materials Park, OH, USA, 2016; pp. 191–197. [Google Scholar]
- Fröck, H.; Milkereit, B.; Wiechmann, P.; Springer, A.; Sander, M.; Kessler, O.; Reich, M. Influence of Solution-Annealing Parameters on the Continuous Cooling Precipitation of Aluminum Alloy 6082. Metals 2018, 8, 265. [Google Scholar] [CrossRef]
- Poznak, A.; Thole, V.; Sanders, P. The Natural Aging Effect on Hardenability in Al-Mg-Si. A Complex Interaction between Composition and Heat Treatment Parameters. Metals 2018, 8, 309. [Google Scholar] [CrossRef]
- Banhart, J.; Chang, C.S.T.; Liang, Z.; Wanderka, N.; Lay, M.D.H.; Hill, A.J. Natural Aging in Al-Mg-Si Alloys—A Process of Unexpected Complexity. Adv. Eng. Mater. 2010, 12, 559–571. [Google Scholar] [CrossRef]
- Chang, C.S.T.; Wieler, I.; Wanderka, N.; Banhart, J. Positive effect of natural pre-ageing on precipitation hardening in Al–0.44 at% Mg–0.38 at% Si alloy. Ultramicroscopy 2009, 109, 585–592. [Google Scholar] [CrossRef]
- Pogatscher, S.; Antrekowitsch, H.; Leitner, H.; Ebner, T.; Uggowitzer, P.J. Mechanisms controlling the artificial aging of Al-Mg-Si Alloys. Acta Mater. 2011, 59, 3352–3363. [Google Scholar] [CrossRef]
- Serizawa, A.; Hirosawa, S.; Sato, T. Three-Dimensional Atom Probe Characterization of Nanoclusters Responsible for Multistep Aging Behavior of an Al-Mg-Si Alloy. Metall. Mater. Trans. A 2008, 39, 243–251. [Google Scholar] [CrossRef]
- Österreicher, J.A.; Kirov, G.; Gerstl, S.S.A.; Mukeli, E.; Grabner, F.; Kumar, M. Stabilization of 7xxx aluminium alloys. J. Alloy. Compd. 2018, 740, 167–173. [Google Scholar] [CrossRef]









| Alloys | Si | Fe | Cu | Mn | Mg | Zn | Cr | Ti | Zr |
|---|---|---|---|---|---|---|---|---|---|
| EN AW-7075-T6 | 0.19 | 0.11 | 1.50 | 0.04 | 2.64 | 6.06 | 0.18 | 0.04 | 0.02 |
| EN AW-7021-T4 | max. 0.25 | max. 0.40 | max. 0.16 | max. 0.10 | 1.60–2.10 | 6.00–6.80 | max. 0.05 | max. 0.1 | max. 0.18 |
| Setaram Sensys Evo | Perkin Elmer Pyris 1 DSC | |
|---|---|---|
| type | heat flow | power compensating |
| heating rate | max. 0.1 K/s | max. 5 K/s |
| cooling rate | 0.01–0.1 K/s | 0.3–5 K/s |
| sample geometry | Ø 6.0 mm × 9.0 mm (6 × 1.5 mm stacked) [19] | Ø 6.4 mm × 1.0 mm |
| Heat Treating Device | Setaram Sensys Evo | Perkin Elmer Pyris 1 DSC |
|---|---|---|
| heating rate in K/s | 0.1 | 1.0 |
| cooling rates in K/s | 0.01, 0.03, 0.1 | 0.3, 1.0, 3.0 |
| number of measurements per cooling rate | at least two | at least six |
| Calorimeter Perkin Elmer Pyris 1 | Dilatometer Bähr DIL 805 | |
|---|---|---|
| sample geometry | Ø 6.4 mm × 1.0 mm | Ø 6.4 mm × 1.0 mm |
| heating rate in K/s | 1.0 | |
| solution annealing | 480 °C/15 min (EN AW-7075) 515 °C/5 min (EN AW-7021) | |
| cooling rates in K/s | 0.01/0.03/1.0/3.0 | 10/ 30/100/300 1 |
| natural aging duration | 31 d | 10 min/30 min/2 h/6 h/1 d/3 d/10 d/30 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milkereit, B.; Österreich, M.; Schuster, P.; Kirov, G.; Mukeli, E.; Kessler, O. Dissolution and Precipitation Behavior for Hot Forming of 7021 and 7075 Aluminum Alloys. Metals 2018, 8, 531. https://doi.org/10.3390/met8070531
Milkereit B, Österreich M, Schuster P, Kirov G, Mukeli E, Kessler O. Dissolution and Precipitation Behavior for Hot Forming of 7021 and 7075 Aluminum Alloys. Metals. 2018; 8(7):531. https://doi.org/10.3390/met8070531
Chicago/Turabian StyleMilkereit, Benjamin, Martin Österreich, Philipp Schuster, Georg Kirov, Ermal Mukeli, and Olaf Kessler. 2018. "Dissolution and Precipitation Behavior for Hot Forming of 7021 and 7075 Aluminum Alloys" Metals 8, no. 7: 531. https://doi.org/10.3390/met8070531





