Recovery of Gallium from Corundum Flue Dust by Two-Stage Alkali Leaching, Carbonation, Acid Leaching and Solvent Extraction Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
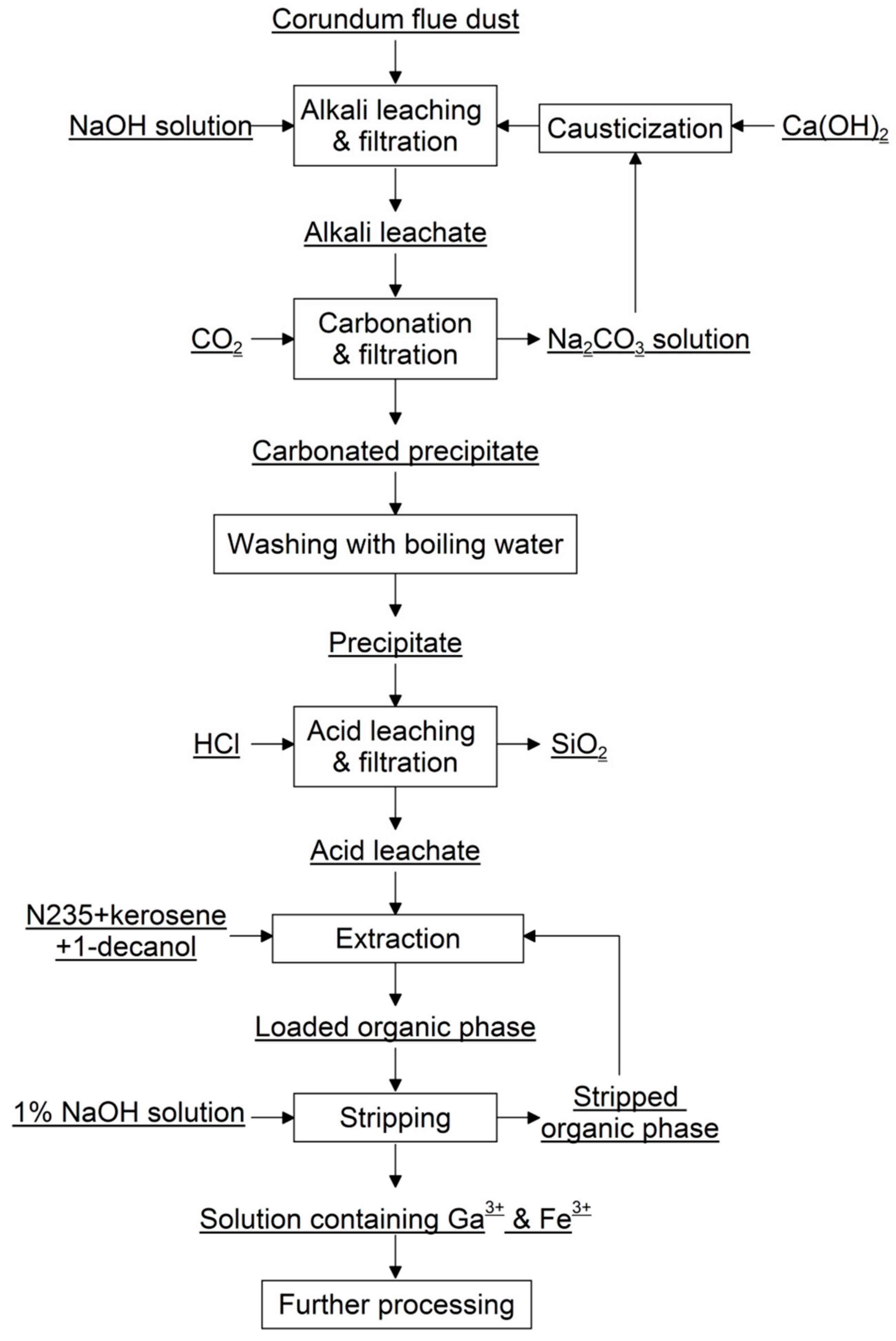
2.2. Experimental Procedure
2.2.1. Leaching
2.2.2. Solvent Extraction
2.3. Analysis Method
3. Results and Discussion
3.1. Characterization of Corundum Flue Dust
3.2. Alkali Leaching
3.2.1. Effect of Alkali-to-Ore Mass Ratio
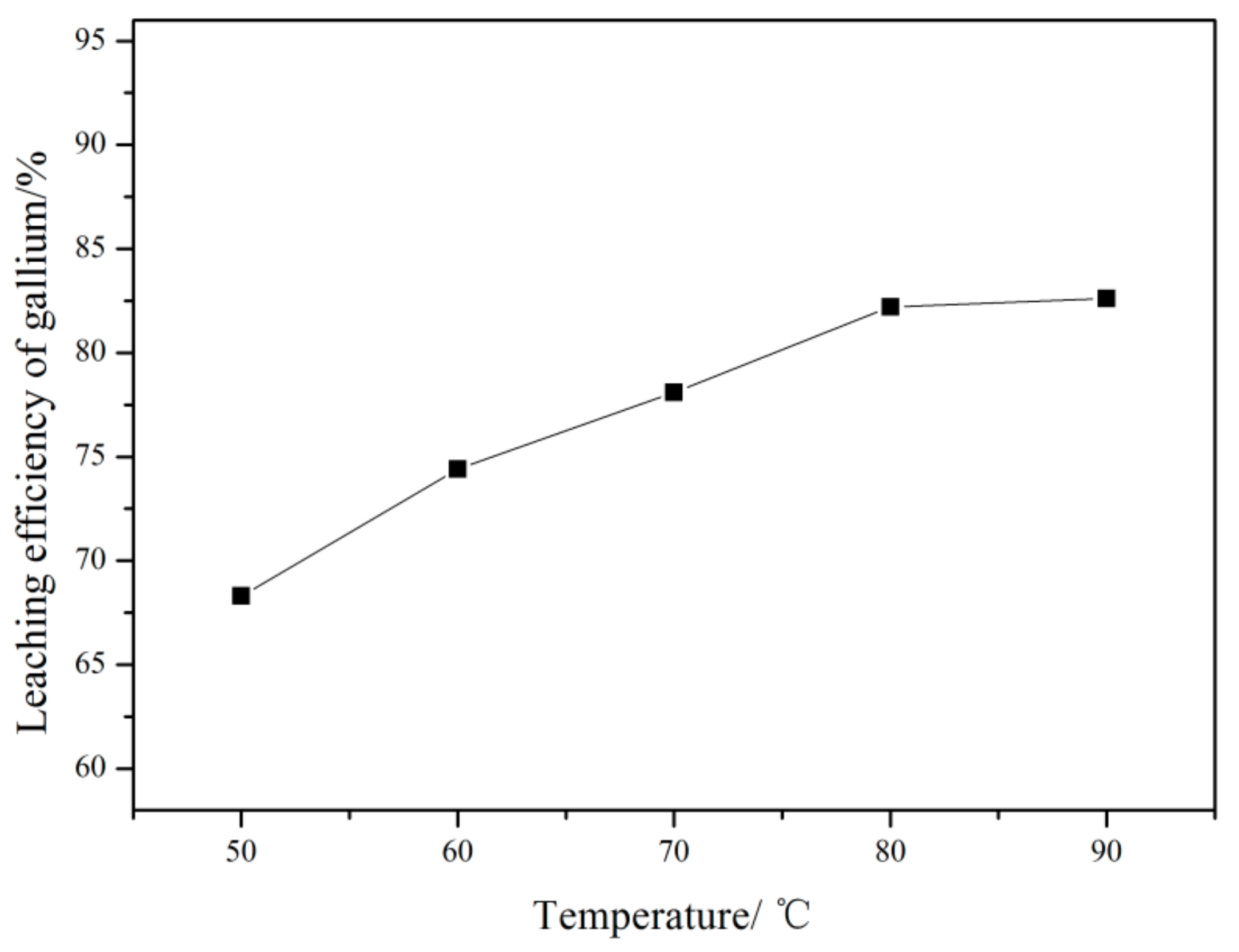
3.2.2. Effect of Leaching Temperature
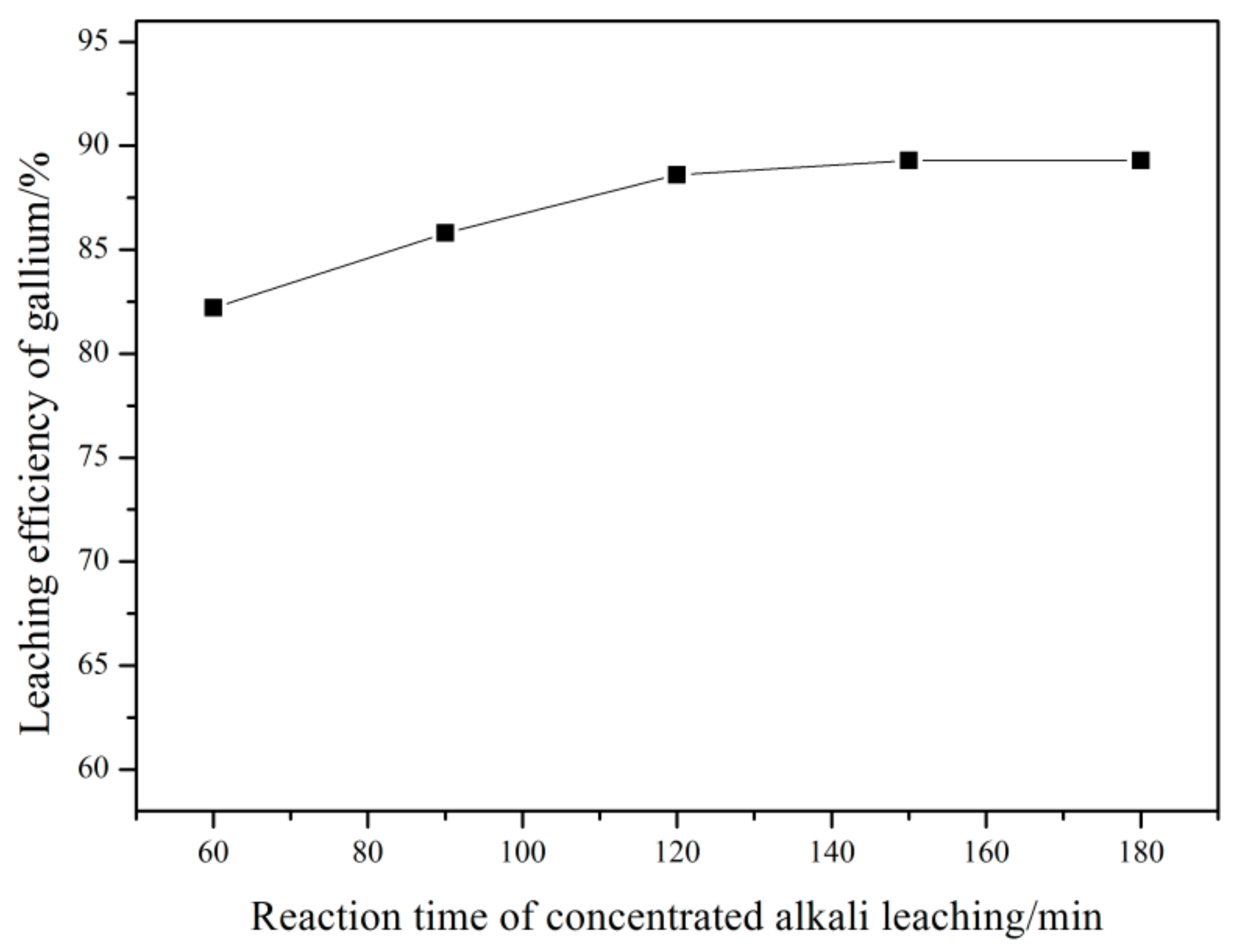
3.2.3. Effect of Reaction Time of the First Alkali Leaching Stage (Concentrated Alkali Leaching Stage)
3.2.4. Effect of (L/S)1 (L/S for Concentrated Alkali Leaching)
3.2.5. Effect of (L/S)2 (L/S for Dilute Alkali Leaching)
3.3. Carbonation and Acid Leaching
3.4. Solvent Extraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallium-A Smart Metal. Available online: https://pubs.usgs.gov/fs/2013/3006/ (accessed on 13 July 2018).
- Gray, F.; Kramer, D.; Bliss, J. Gallium and Gallium Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Kirk-Othmer, R., Ed.; VCH: New York, NY, USA, 2005; Volume 12, pp. 337–364. [Google Scholar]
- Naumov, A. Status and prospects of world gallium production and the gallium market. Metallurgist 2013, 57, 367–371. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Wang, X.; Niu, X. Research progress in gallium recovery technology. Nonferrous Met. 2008, 60, 105–108. [Google Scholar]
- Zhao, Z.; Yang, Y.; Xiao, Y.; Fan, Y. Recovery of gallium from Bayer liquor: A review. Hydrometallurgy 2012, 125, 115–124. [Google Scholar] [CrossRef]
- Vind, J.; Alexandri, A.; Vassiliadou, V.; Panias, D. Distribution of selected trace elements in the bayer process. Metals 2018, 8, 327. [Google Scholar] [CrossRef]
- Carvalho, M.; Neto, K.; Nobrega, A.; Medeiros, J. Recovery of gallium from aluminum industry residues. Sep. Sci. Technol. 2000, 35, 57–67. [Google Scholar] [CrossRef]
- Gutierrez, B.; Pazos, C.; Coca, J. Recovery of gallium from coal fly ash by a dual reactive extraction process. Waste Manag. Res. 1997, 15, 371–382. [Google Scholar] [CrossRef]
- Zou, J.; Tian, H.; Wang, Z. Leaching process of rare earth elements, gallium and niobium in a coal-bearing strata-hosted rare metal deposit—A study from the late Permian tuff in the Zhongliangshan Mine, Chongqing. Metals 2017, 7, 174. [Google Scholar] [CrossRef]
- Wu, X.; Wu, S.; Qin, W.; Ma, X.; Niu, Y.; Lai, S.; Yang, C.; Jiao, F.; Ren, L. Reductive leaching of gallium from zinc residue. Hydrometallurgy 2012, 113, 195–199. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Z.; Li, Y.; Liu, Z.; Li, Q.; Zeng, L. Extraction of gallium and germanium from zinc refinery residues by pressure acid leaching. Hydrometallurgy 2016, 164, 313–320. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Z.; Li, Y.; Wilson, B.; Lundström, M. Extraction of Ga and Ge from zinc refinery residues in H2C2O4 solutions containing H2O2. Int. J. Miner. Process. 2017, 163, 14–23. [Google Scholar] [CrossRef]
- Xu, K.; Deng, T.; Liu, J.; Peng, W. Study on the recovery of gallium from phosphorus flue dust by leaching with spent sulfuric acid solution and precipitation. Hydrometallurgy 2007, 86, 172–177. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, S.; Yang, H.; Zhang, Y.; Zhang, S.; Li, Q. Experimental research on recovering gallium, iron and aluminium from corundum slags. Multipurp. Util. Miner. Resour. 1997, 3, 14–17. [Google Scholar]
- Zhou, L. Rare Metals Metallurgy, 1st ed.; Metallurgical Industry Press: Beijing, China, 1988. [Google Scholar]
- Tian, Y.; Li, W.; Zeng, T.; Deng, H. Method for Recovering Gallium from Electric Arc Furnace Flue Dust of Corundum Production. CN Patent 1375564, 23 October 2002. [Google Scholar]
- Li, W.; Lu, P.; Yin, Z.; Li, X.; Han, D.; Yang, Q. Method for Recovering Gallium from Corundum Flue Dust. CN 102676829A, 19 September 2012. [Google Scholar]
- Fan, S.; Jia, Q.; Song, N.; Su, R.; Liao, W. Synergistic extraction study of indium from chloride medium by mixtures of sec-nonylphenoxy acetic acid and trialkyl amine. Sep. Purif. Technol. 2010, 75, 76–80. [Google Scholar] [CrossRef]
- Yu, X.; Xie, G.; Wang, J.; Li, Y. Study on Indium Extraction in Acidic Medium. Yunnan Met. 2006, 35, 28–32. [Google Scholar]
- Li, H.; Wang, N.; Chen, Y.; Tian, Y. The Environmental Significance of the Removal of Corundum Dusts and the Mineralogical Study of Such Dusts. Acta Pet. Miner. 1999, 18, 348–356. [Google Scholar]
- Xu, F.; Xu, N. Gallium production of three stage carbonization process. Henan Chem. Ind. 2002, 10, 21–24. [Google Scholar]
- Sullivan, R.; Stern, W.; Vance, B. Process for Recovering Gallium. U.S. Patent 4193968, 18 March 1980. [Google Scholar]
- Good, M.; Holland, F. Extraction of In(III) and Ga(III) from aqueous chloride media by long chain alkyl amines and quaternary salts. J. Inorg. Nucl. Chem. 1964, 26, 321–327. [Google Scholar] [CrossRef]
- Good, M.; Srivastava, S. The nature of the halide complexes of Fe(III), Co(III), Ga(III) and In(III) extracted from aqueous chloride media by high molecular weight substituted alkyl ammonium compounds. J. Inorg. Nucl. Chem. 1965, 27, 2429–2436. [Google Scholar] [CrossRef]
- Sato, T.; Nakamura, T.; Ishikawa, S. Liquid-liquid extraction of gallium (III) from hydrochloric acid solutions by organophosphorus compounds and high-molecular weight amines. Solv. Extr. Ion Exch. 1984, 2, 201–212. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Ning, Z.; Long, Q.; Xiao, L.; Huang, F.; Wang, W.; Xiao, Q.; Lan, X.; et al. Resources and extraction of gallium: A review. Hydrometallurgy 2017, 174, 105–115. [Google Scholar] [CrossRef]
- Mihaylov, I.; Distin, P. Gallium solvent extraction in hydrometallurgy: An overview. Hydrometallurgy 1992, 28, 13–27. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Zhang, D.; Deng, Y.; Cui, H.; Chen, J.; Li, D. Recovery of trace rare earths from high-level Fe3+ and Al3+ waste of oil shale ash (Fe-Al-OSA). Ind. Eng. Chem. Res. 2010, 49, 11645–11651. [Google Scholar] [CrossRef]






| Compounds and Elements | Content/wt. % |
|---|---|
| MgO | 0.26 |
| CaO | 0.13 |
| Fe2O3 | 3.52 |
| Al2O3 | 18.97 |
| SiO2 | 56.65 |
| Ga | 0.15 |
| K2O | 4.39 |
| Na2O | 1.36 |
| Loss on ignition | 14.22 |
| Test Parameters and Result | Value |
|---|---|
| Alkali-to-ore mass ratio | 1.2:1 |
| Temperature | 80 °C |
| Reaction Time (1st + 2nd stage) | 120 min + 30 min |
| L/S for the 1st alkali leaching stage | 1.5:1 |
| L/S for the 2nd alkali leaching stage | 8:1 |
| Leaching efficiency | 93.6% |
| Element | Concentration/wt. % |
|---|---|
| Ga | 0.468 |
| Si | 0.110 |
| Al | 1.823 |
| Fe | 0.244 |
| Test Parameters and Results | Value |
|---|---|
| Extraction | - |
| Solvent | 25% N235 + 5% 1-Decanol + 70% kerosene |
| A/O | 4.5:1 |
| Temperature | Ambient temperature |
| Extraction efficiency (Ga) | 99.8% |
| Extraction efficiency (Fe) | 99.1% |
| Stripping | - |
| Stripping agent | 1% NaOH solution |
| A/O | 1:1 |
| Temperature | Ambient temperature |
| Stripping efficiency (Ga) | 97.4% |
| Stripping efficiency (Fe) | 96.5% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, K.; Jiang, F.; Zhou, X.; Sun, Z. Recovery of Gallium from Corundum Flue Dust by Two-Stage Alkali Leaching, Carbonation, Acid Leaching and Solvent Extraction Process. Metals 2018, 8, 545. https://doi.org/10.3390/met8070545
Wen K, Jiang F, Zhou X, Sun Z. Recovery of Gallium from Corundum Flue Dust by Two-Stage Alkali Leaching, Carbonation, Acid Leaching and Solvent Extraction Process. Metals. 2018; 8(7):545. https://doi.org/10.3390/met8070545
Chicago/Turabian StyleWen, Kang, Feng Jiang, Xiangyang Zhou, and Zhaoming Sun. 2018. "Recovery of Gallium from Corundum Flue Dust by Two-Stage Alkali Leaching, Carbonation, Acid Leaching and Solvent Extraction Process" Metals 8, no. 7: 545. https://doi.org/10.3390/met8070545




