Laser Cladding In-Situ Ti(C,N) Particles Reinforced Ni-Based Composite Coatings Modified with CeO2 Nanoparticles
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials Used
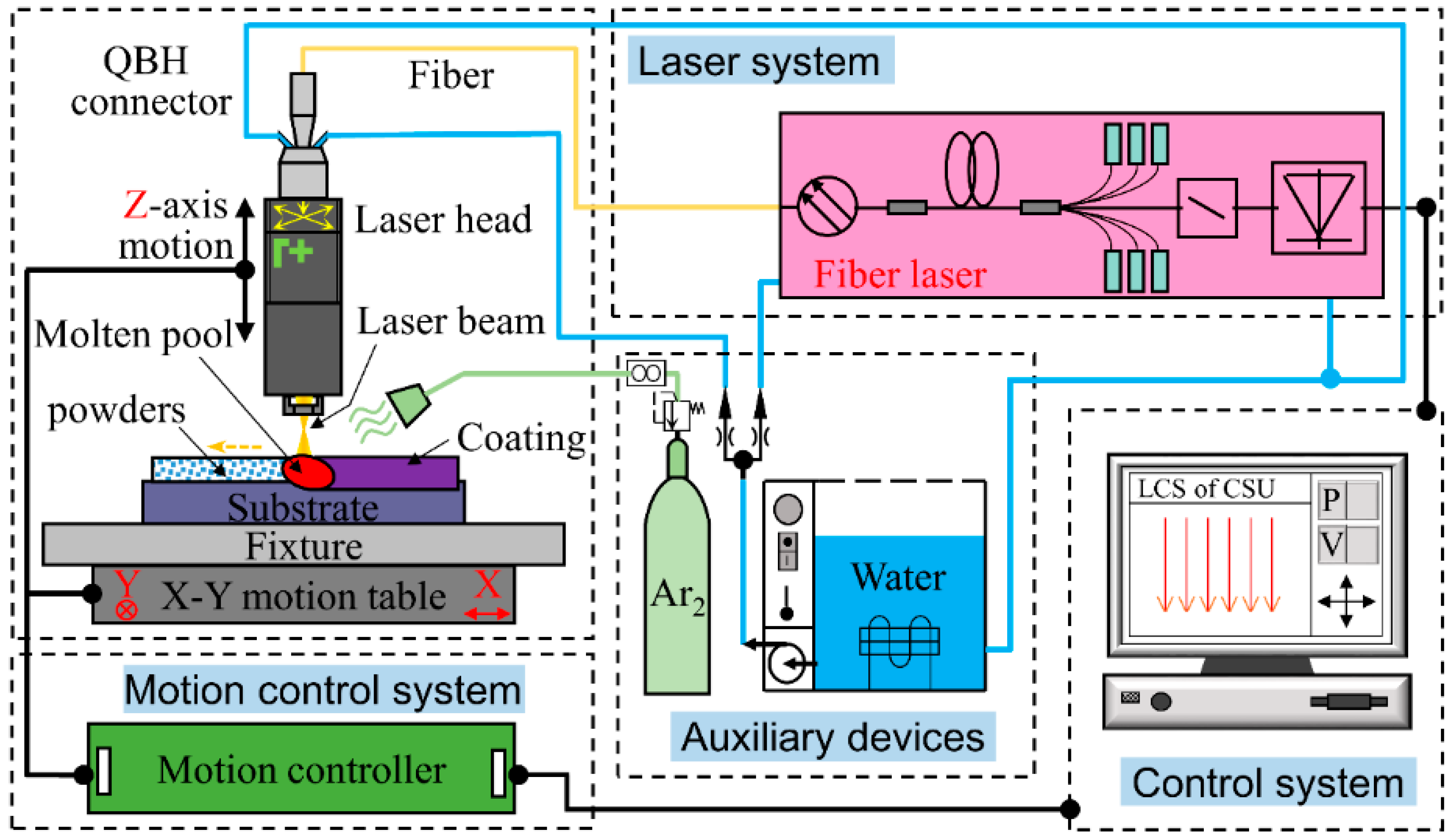
2.2. Laser Cladding Setup and Process
2.3. Characterization Test Methods of the Coatings
3. Results and Discussion
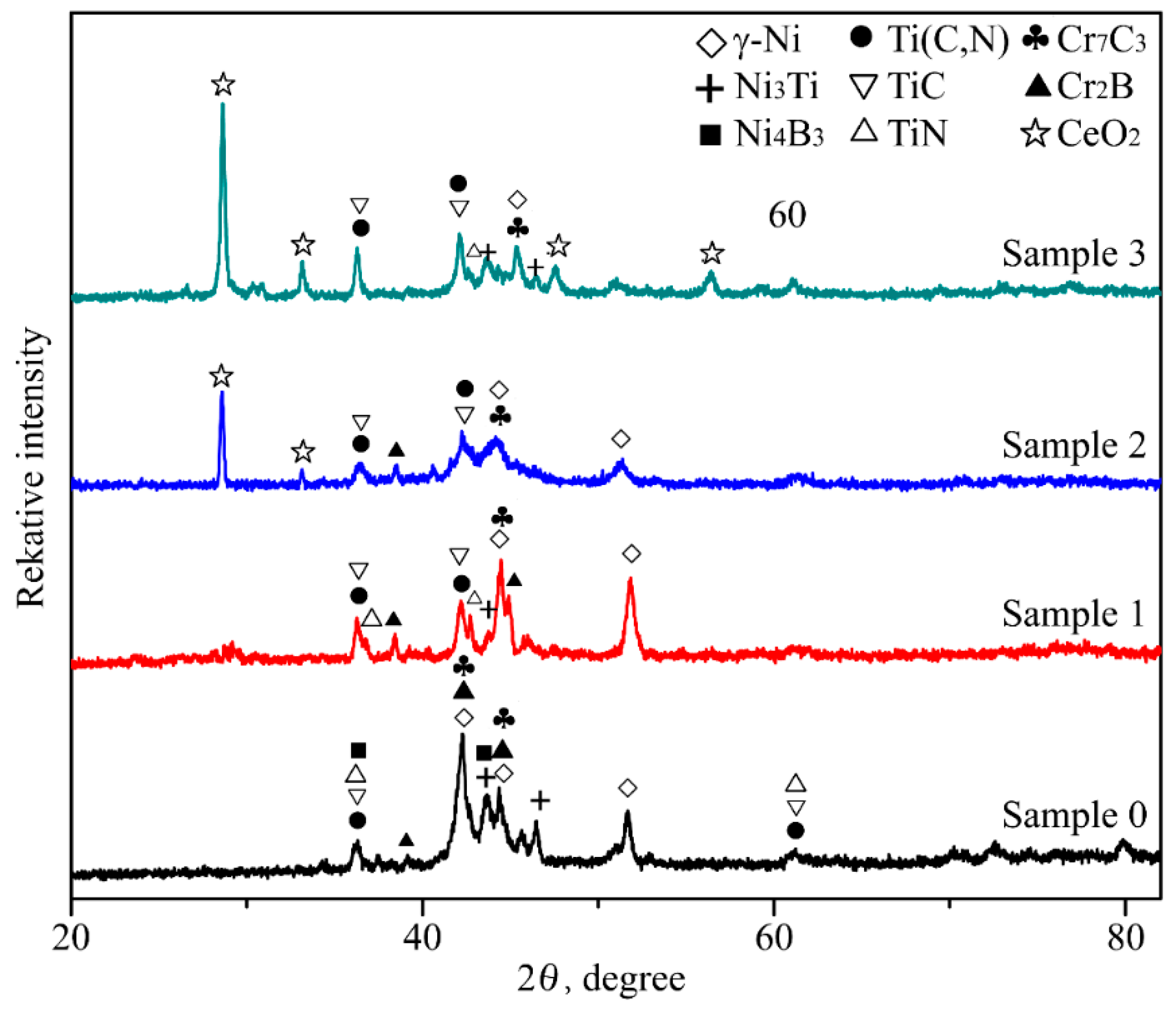
3.1. Phase Constituents of the Coatings
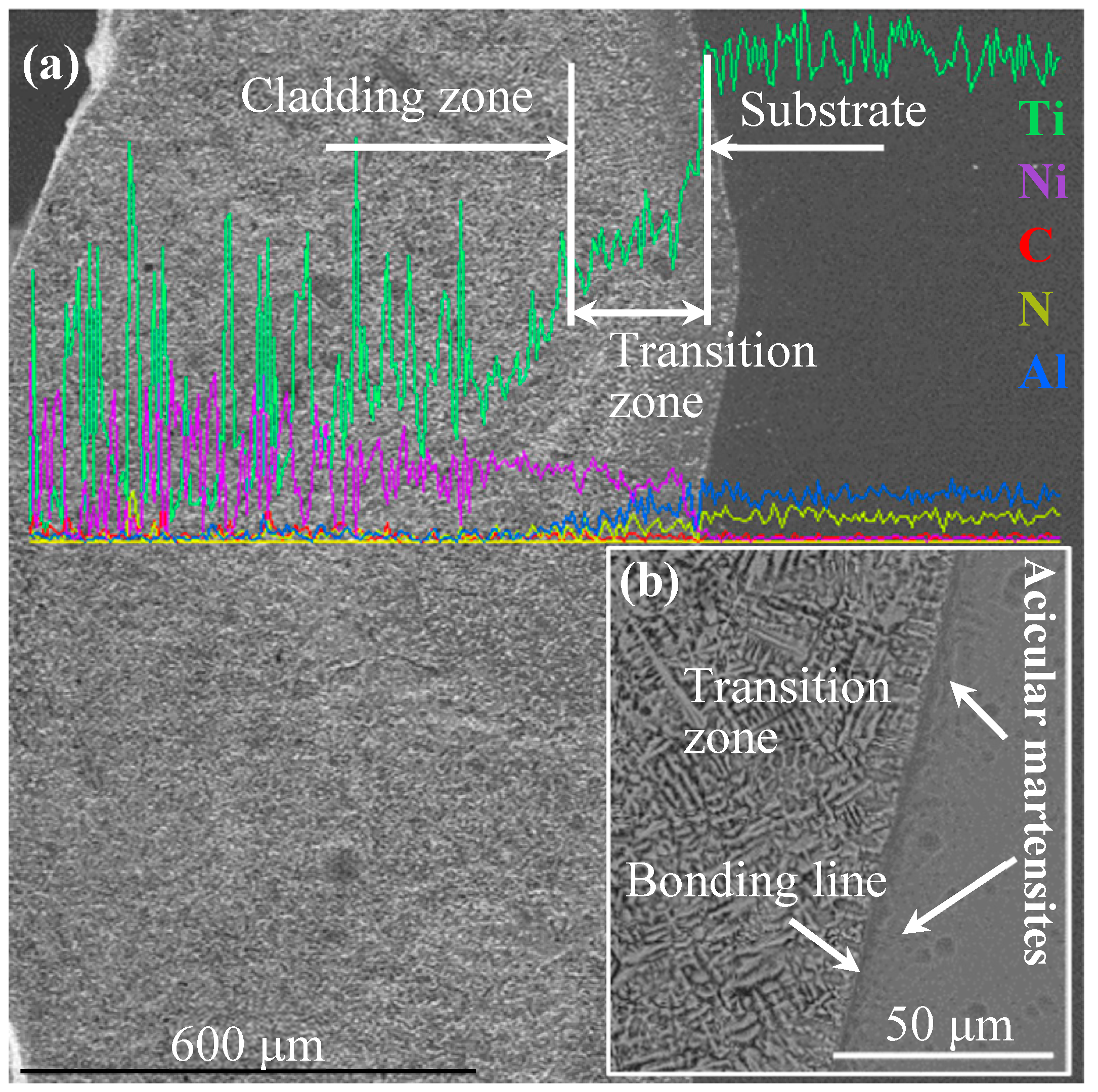
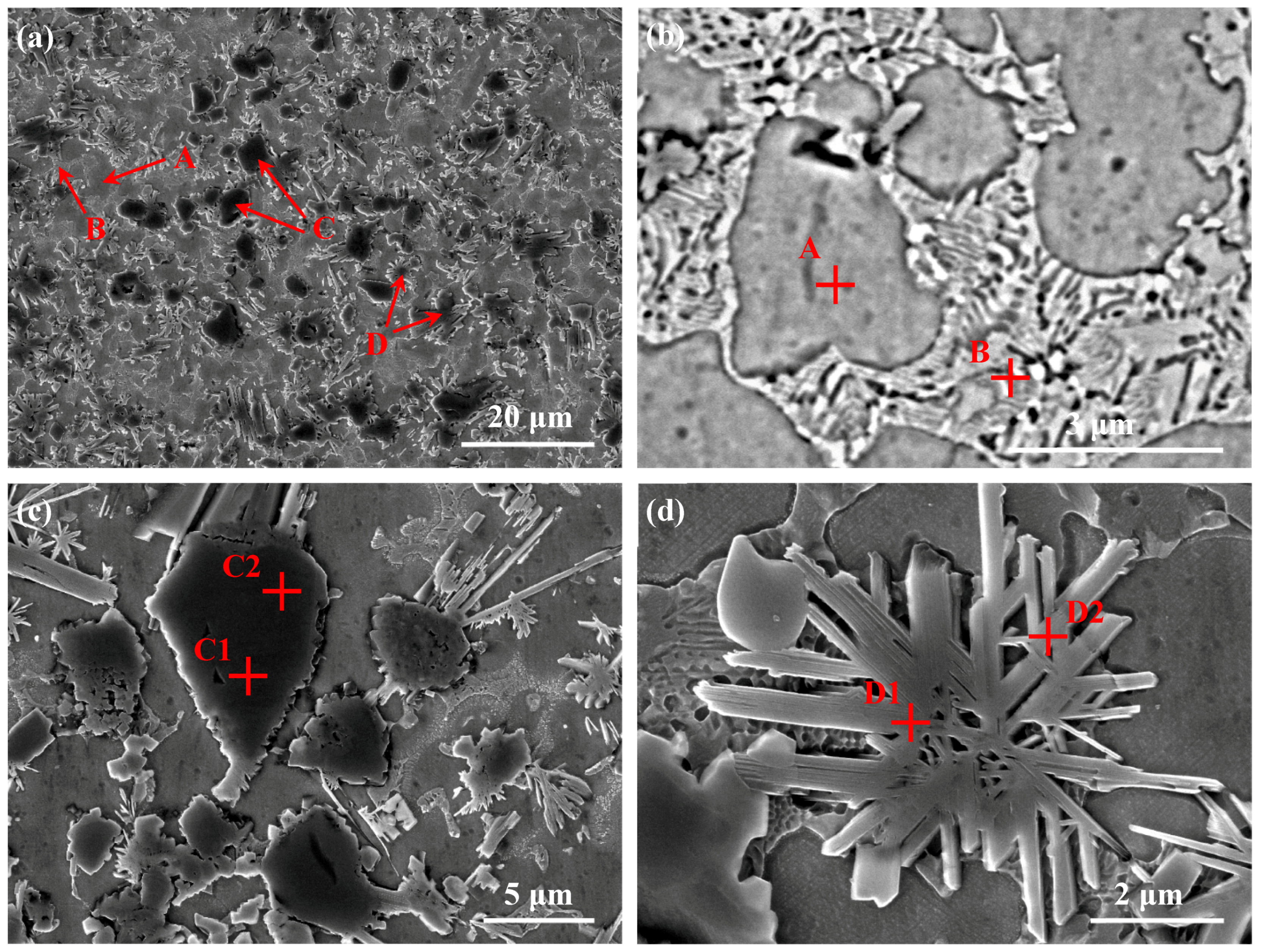
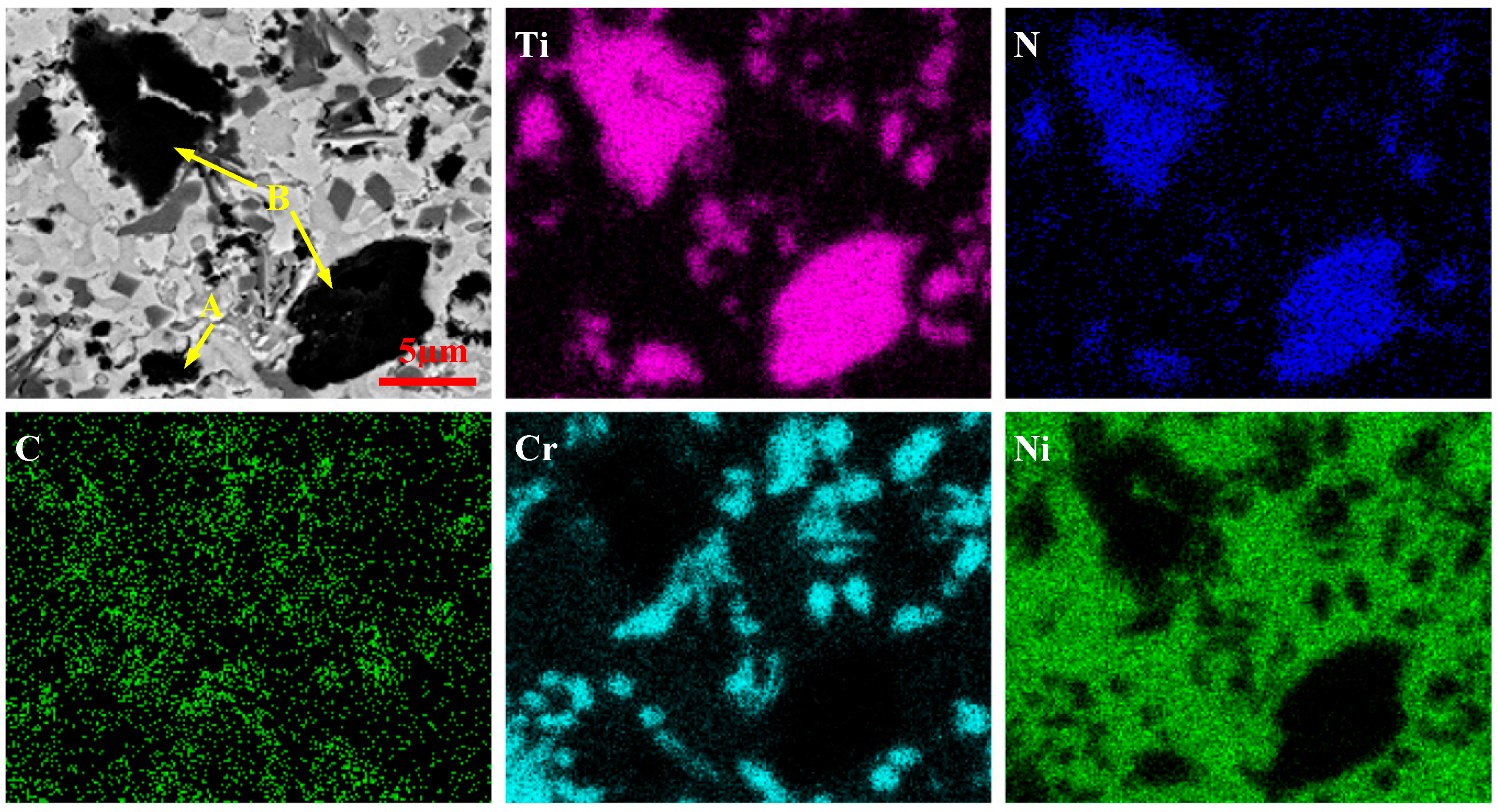
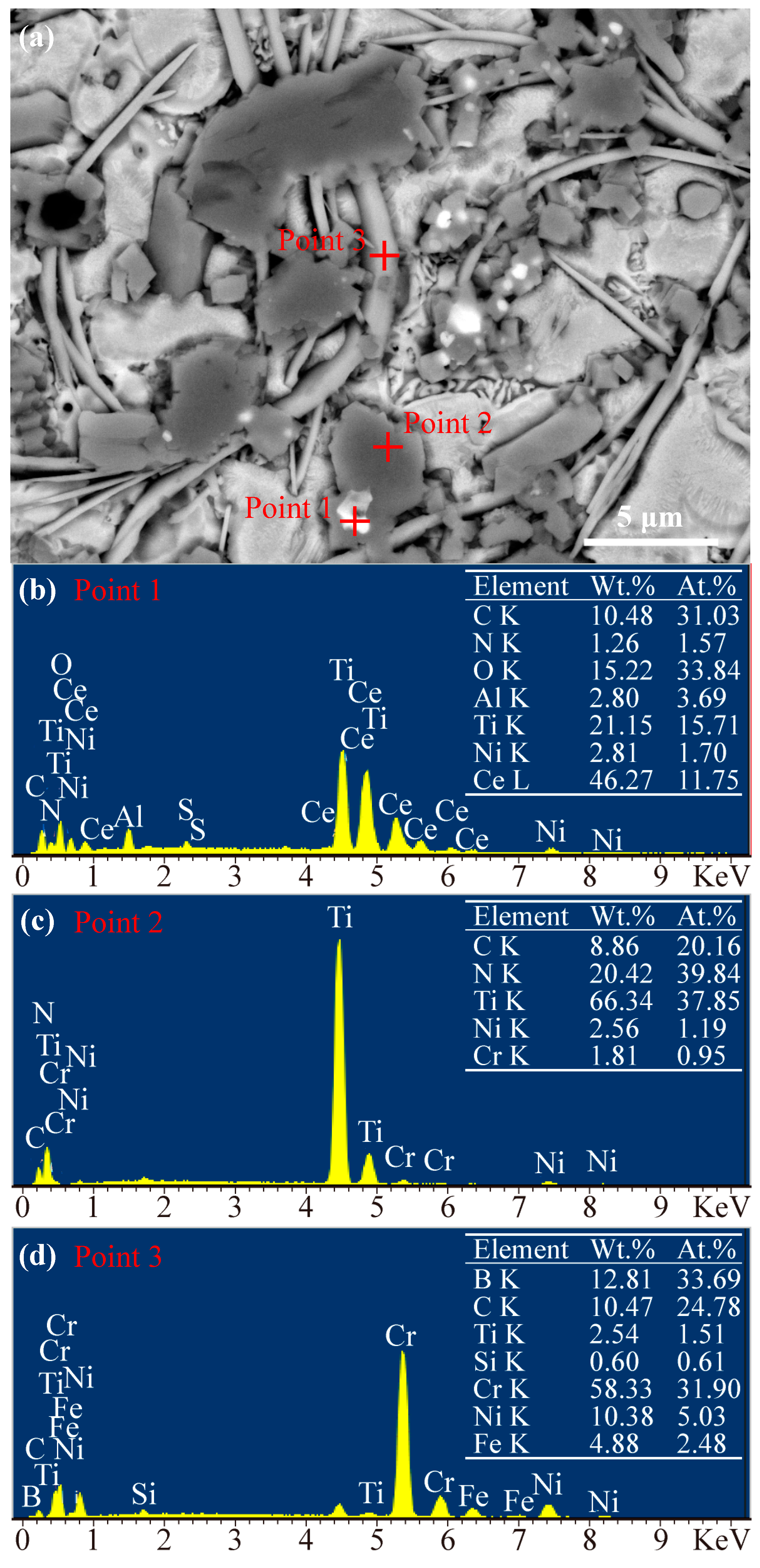
3.2. Microstructure Analysis of the Coatings
3.2.1. Microstructure of the Coatings Fabricated by Pre-Placed Powders without CeO2 Additive
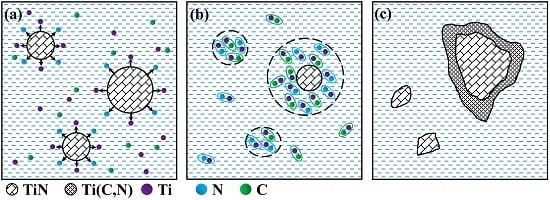
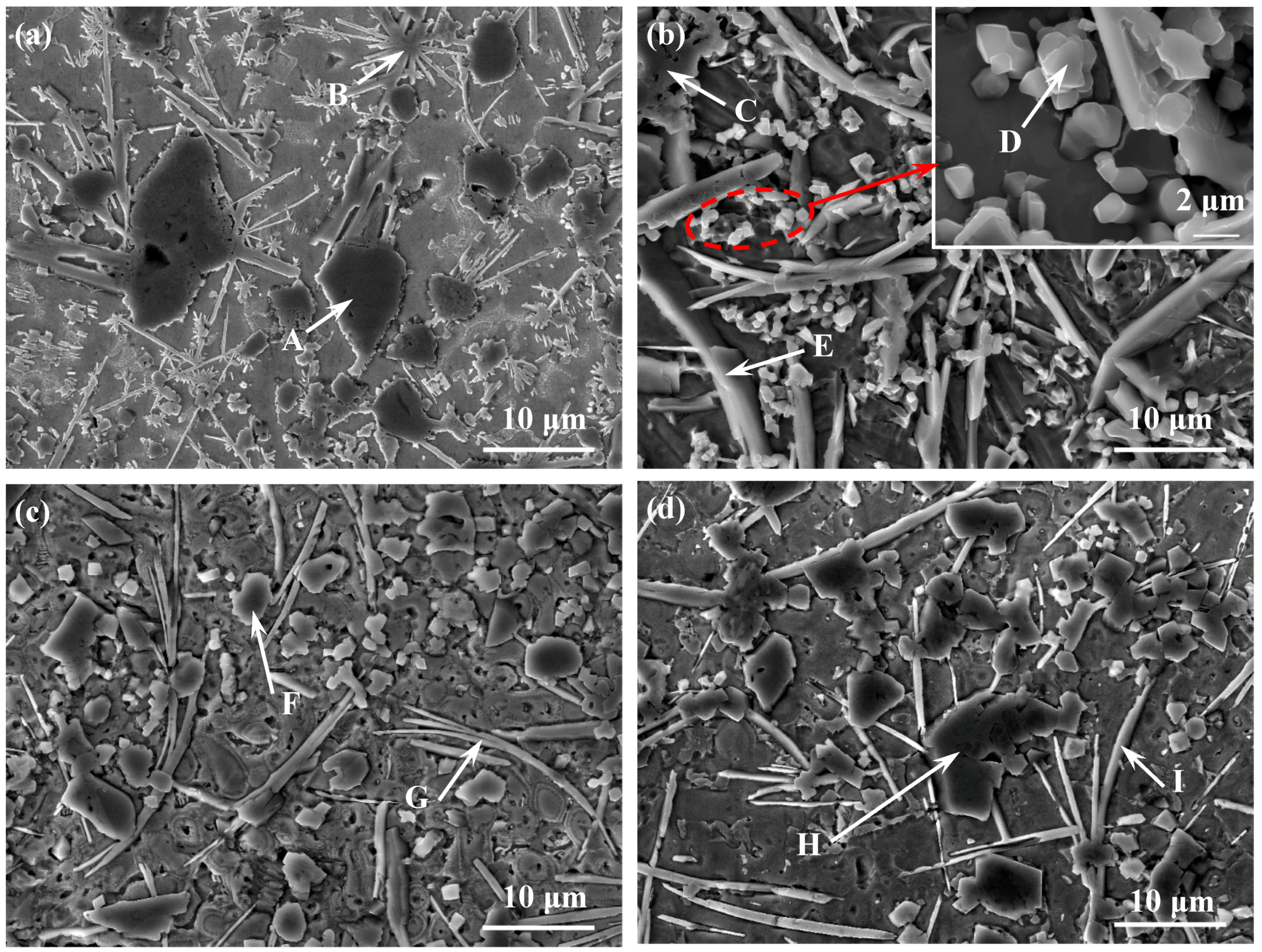
3.2.2. Influence of CeO2 Nanoparticles on the Microstructure of Coatings
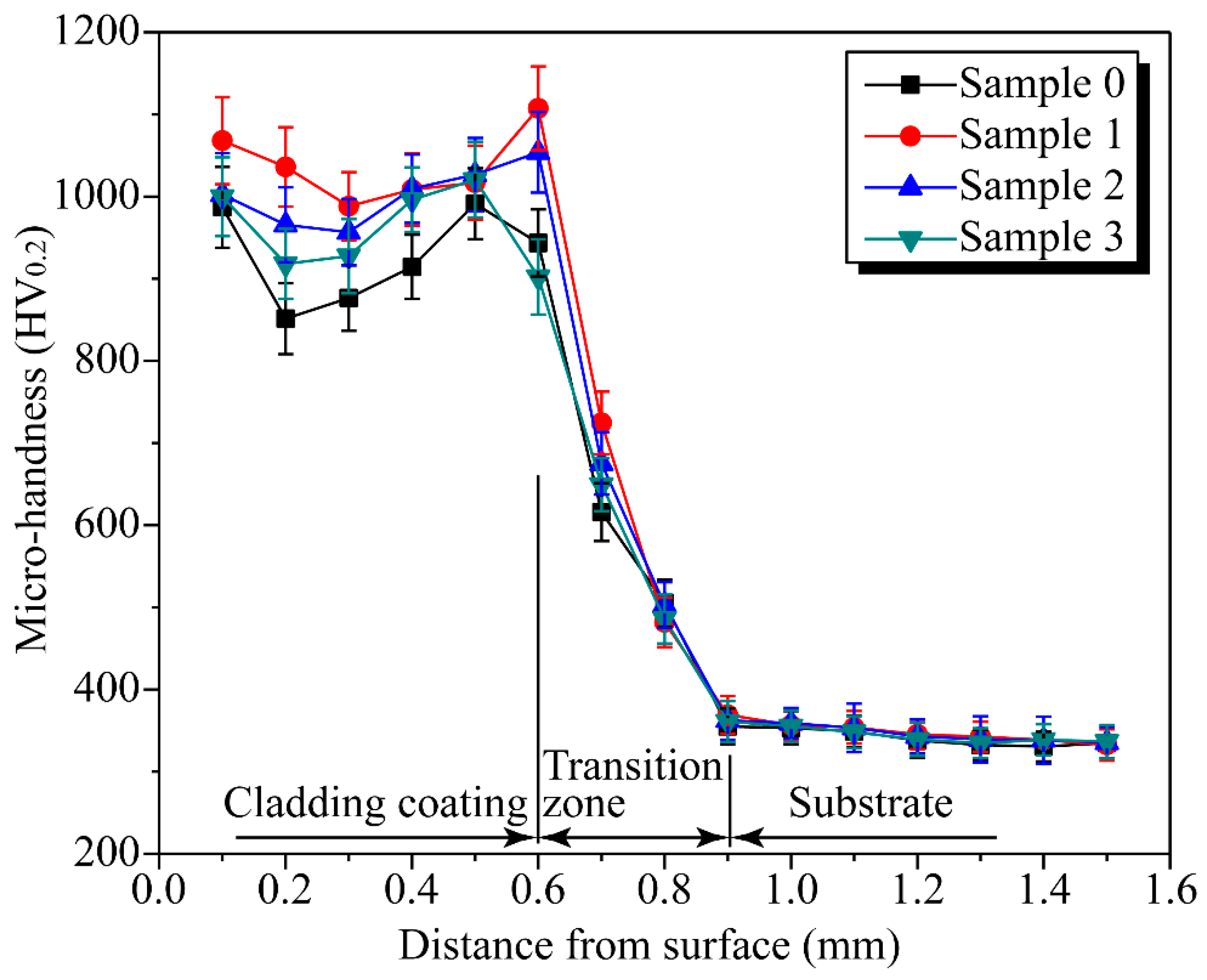
3.3. Micro-Hardness and Wear Resistance of the Coatings
4. Conclusions
- In-situ synthesized Ti(C,N) reinforced Ni-based composite coatings are fabricated on Ti6Al4V substrates by laser cladding using Ni60 alloy, C, TiN, and nano-CeO2 powders as the pre-placed materials. The composite coatings metallurgically bonded with the substrates are mainly composed of γ-Ni, Ni3Ti, Ni4B3, Ti(C,N), TiC, TiN, Cr2B, and Cr7C3 phases.
- The formation mechanisms of Ti(C,N) particles as reinforced phases in the coating are that the large Ti(C,N) particles form around TiN particles, and the small Ti(C,N) particles form by independent nucleation.
- The microstructures of the coatings fabricated by pre-placed powders with different contents of CeO2 additive are obviously improved, which is mainly related to the heterogeneous nucleation of CeO2 nanoparticles.
Author Contributions
Funding
Conflicts of Interest
References
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and applications of titanium alloys: A brief review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Peters, M.; Kumpfert, J.; Ward, C.H.; Leyens, C. Titanium Alloys for Aerospace Applications. Adv. Eng. Mater. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Sachdev, A.K.; Kulkarni, K.; Fang, Z.Z.; Yang, R.; Girshov, V. Titanium for Automotive Applications: Challenges and Opportunities in Materials and Processing. JOM 2012, 64, 553–565. [Google Scholar] [CrossRef]
- Budinski, K.G. Tribological properties of titanium alloys. Wear 1991, 151, 203–217. [Google Scholar] [CrossRef]
- Mao, Y.S.; Wang, L.; Chen, K.M.; Wang, S.Q.; Cui, X.H. Tribo-layer and its role in dry sliding wear of Ti-6Al-4V alloy. Wear 2013, 297, 1032–1039. [Google Scholar] [CrossRef]
- Lin, Y.H.; Yao, J.H.; Lei, Y.P.; Fu, H.G.; Wang, L. Microstructure and properties of TiB2-TiB reinforced titanium matrix composite coating by laser cladding. Opt. Laser. Eng. 2016, 86, 216–227. [Google Scholar] [CrossRef]
- Candel, J.J.; Amigó, V.; Ramos, J.A.; Busquets, D. Sliding wear resistance of TiCp reinforced titanium composite coating produced by laser cladding. Surf. Coat. Technol. 2010, 204, 3161–6166. [Google Scholar] [CrossRef]
- Li, J.N.; Chen, C.Z.; Squartini, T.; He, Q.S. A study on wear resistance and microcrack of the Ti3Al/TiAl+TiC ceramic layer deposited by laser cladding on Ti-6Al-4V alloy. Appl. Surf. Sci. 2010, 257, 1550–1555. [Google Scholar] [CrossRef]
- Wang, F.; Chen, C.Z.; Yu, H.J. Research status of laser cladding on titanium and its alloys: A review. Mater. Des. 2014, 58, 412–425. [Google Scholar] [CrossRef]
- Chao, M.J.; Wang, W.L.; Liang, E.J.; Ouyang, D.X. Microstructure and wear resistance of TaC reinforced Ni-based coating by laser cladding. Surf. Coat. Technol. 2008, 202, 1918–1922. [Google Scholar] [CrossRef]
- Shi, C.; Lei, J.B.; Zhou, S.F.; Dai, X.Q.; Zhang, L.C. Microstructure and mechanical properties of carbon fibers strengthened Ni-based coatings by laser cladding: The effect of of carbon fiber contents. J. Alloy. Compd. 2018, 744, 146–155. [Google Scholar] [CrossRef]
- Dong, G.; Yan, B.; Deng, Q.L.; Yu, T. Microstructure and Wear Resistance of in situ NbC Particles Reinforced Ni-based Alloy Composite Coating by Laser Cladding. J. Wuhan Univ. Technol. 2012, 27, 231–237. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.J.; Wang, H.P.; Li, M.P. Microstructure and mechanical properties of Ni-based composite coatings reinforced by in situ synthesized TiB2+TiC by laser cladding. Int. J. Min. Met. Mater. 2013, 20, 57–64. [Google Scholar] [CrossRef]
- Fernández, M.R.; García, A.; Cuetos, J.M.; González, R.; Noriega, A.; Cadenas, M. Effect of actual WC content on the reciprocating wear of a laser cladding NiCrBSi alloy reinforced with WC. Wear 2014, 324–325, 80–89. [Google Scholar] [CrossRef]
- Sun, R.L.; Lei, Y.W.; Niu, W. Laser clad TiC reinforced NiCrBSi composite coatings on Ti–6Al–4V alloy using a CW CO2 laser. Surf. Coat. Technol. 2009, 203, 1395–1399. [Google Scholar] [CrossRef]
- Forn, A.; Picas, J.A.; Fuentes, G.G.; Elizalde, E. Mechanical and tribological properties of TiCxN1−x, wear resistant coatings. Int. J. Refract. Met. Hard Mater. 2001, 19, 507–513. [Google Scholar] [CrossRef]
- Li, J.N.; Chen, C.Z.; Zhang, C.F. Effect of nano-CeO2 on microstructure properties of TiC/TiN+nTiCN reinforced composite coating. Bull. Mater. Sci. 2013, 36, 399–404. [Google Scholar]
- Yang, Y.L.; Zhang, D.; Yan, W.; Zheng, Y.R. Microstructure and wear properties of TiCN/Ti coatings on titanium alloy by laser cladding. Opt. Laser. Eng. 2010, 48, 119–124. [Google Scholar] [CrossRef]
- Peng, Y.; Miao, H.; Peng, Z. Development of TiCN-based cermets: Mechanical properties and wear mechanism. Int. J. Refract. Met. Hard Mater. 2013, 39, 78–89. [Google Scholar] [CrossRef]
- Qi, Y.T.; Cao, Z.X.; Sheng, L.Y.; Cao, R.P. Microstructure of Fe-Based Alloy Composite Coatings Reinforced by Ti(C0.3N0.7) Particles through Laser Cladding Technology. J. Iron Steel Res. Int. 2013, 20, 78–82. [Google Scholar] [CrossRef]
- Li, J.N.; Chen, C.Z.; Wang, D.G.; Li, W. Microstructures and wear properties of YPSZ/CeO2 reinforced composites deposited by laser cladding. Compos. Part B Eng. 2012, 43, 896–901. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, Q.B.; Sun, M.L.; Wei, X.G.; Zhu, Y.M. Rare earth elements modification of laser-clad nickel-based alloy coatings. Appl. Surf. Sci. 2001, 174, 191–200. [Google Scholar] [CrossRef]
- Zhang, S.H.; Li, M.X.; Tong, Y.C.; Yoon, J.H.; Lee, C.G.; He, Y.Z. Laser clad Ni-base alloy added nano- and micron-size CeO2 composites. Opt. Laser Technol. 2008, 40, 716–722. [Google Scholar] [CrossRef]
- Li, J.; G, S.; W, X.; Suo, H. Physical and surface performance of laser clad Ni based coating on a TA15-2 alloy. Chin. J. Laser. 2013, 40, 1103008. [Google Scholar]
- Qi, Y.; Wang, W. Formation mechanism of in-situ Ti(C0.3N0.7) reinforced phase through laser cladding technology. Appl. Laser 2016, 36, 511–515. [Google Scholar]
- Yu, T.; Deng, Q.L.; Gang, D.; Yang, J.G. Effects of Ta on microstructure and microhardness of Ni based laser clad coating. Appl. Surf. Sci. 2011, 257, 5098–5103. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Chen, C.Z.; Liu, J.N.; Zhao, L.J. Microstructures and properties of TiN reinforced Co-based composite coatings modified with Y2O3 by laser cladding on Ti-6Al-4V alloy. J. Alloy. Compd. 2015, 650, 178–184. [Google Scholar] [CrossRef]
- Liu, N.; Yin, W.H.; Zhu, L.W. Effect of TiC/TiN powder size on microstructure and properties of Ti(C,N)-based cermets. Mat. Sci. Eng. A 2007, 445–446, 707–716. [Google Scholar] [CrossRef]
- Sharma, S.P.; Dwivedi, D.K.; Jain, P.K. Effect of CeO2 addition on the microstructure, hardness and abrasive wear behavior of flame sprayed Ni-based coatings. Proc. Inst. Mech. Eng. J. 2008, 222, 8925–8933. [Google Scholar]
- Li, M.X.; He, Y.Z.; Yuan, X.M. Effect of nano-Y2O3 on microstructure of laser cladding cobalt-based alloy coatings. Appl. Surf. Sci. 2006, 252, 2882–2887. [Google Scholar]
- Cheng, X.Y.; Feng, Y.S.; He, J. Influence of CeO2 on Microstructure and microhardness of TiC4 coating Produced by Laser-Cladding. Mater. Mech. Eng. 2010, 34, 20–23. [Google Scholar]
- Cai, Y.C.; Luo, Z.; Chen, Y.; Ao, S.S. Influence of CeO2 on tribological behaviour of TiC/ Fe-based composite coating. Surf. Eng. 2017, 33, 1–8. [Google Scholar] [CrossRef]
- Bai, L.L.; Li, J.; Chen, J.L.; Song, R.; Shao, J.Z.; Qu, C.C. Effect of the content of B4C on microstructural evolution and wear behaviors of the laser-clad coatings fabricated on Ti6Al4V. Opt. Laser Technol. 2016, 76, 33–45. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S.; Stemp, M.; Barril, S. Third body effects and material fluxes in tribocorrosion systems involving a sliding contact. Wear 2004, 256, 517–524. [Google Scholar] [CrossRef]






| Cr | Fe | W | Si | B | C | Ni |
|---|---|---|---|---|---|---|
| 15.5 | 15 | 3 | 4 | 3.5 | 0.65 | Bal. |
| Sample Group No. | Powder Ingredients (wt %) | |||
|---|---|---|---|---|
| Ni60 | C | TiN | CeO2 | |
| Sample 0 | 88 | 2 | 10 | 0 |
| Sample 1 | 87 | 2 | 10 | 1 |
| Sample 2 | 86 | 2 | 10 | 2 |
| Sample 3 | 85 | 2 | 10 | 3 |
| Area | Elements (at %) | ||||||
|---|---|---|---|---|---|---|---|
| Ti | N | C | Cr | B | Ni | Fe | |
| A | 3.02 | - | 12.24 | 8.13 | - | 65.71 | 10.90 |
| B | 3.99 | - | 15.45 | 7.29 | 8.66 | 62.12 | 2.49 |
| C1 | 37.16 | 50.47 | 12.37 | - | - | - | - |
| C2 | 33.82 | 16.14 | 42.95 | 1.48 | - | 5.61 | - |
| D1 | 1.04 | - | 22.54 | 23.18 | 51.13 | 1.85 | 0.26 |
| D2 | 1.84 | - | 37.36 | 16.58 | 23.55 | 17.47 | 3.20 |
| Region | Elements (at %) | ||||||
|---|---|---|---|---|---|---|---|
| Ti | N | C | Cr | B | Ni | Fe | |
| A | 37.16 | 50.47 | 12.37 | - | - | - | - |
| B | 1.84 | - | 37.36 | 16.58 | 23.55 | 17.47 | 3.20 |
| C | 29.69 | 52.82 | 14.76 | 0.72 | - | 1.76 | 0.25 |
| D | 43.81 | 8.58 | 42.59 | 3.91 | - | 1.11 | - |
| E | 2.34 | - | 31.33 | 21.61 | 42.54 | 1.22 | 0.96 |
| F | 34.79 | 40.84 | 21.59 | 0.96 | - | 1.82 | - |
| G | 1.51 | - | 24.78 | 31.90 | 33.69 | 5.03 | 2.48 |
| H | 40.48 | 26.34 | 33.18 | - | - | - | - |
| I | 2.70 | - | 25.26 | 17.26 | 42.96 | 8.04 | 3.78 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Wu, F.; Wang, H.; Liu, D. Laser Cladding In-Situ Ti(C,N) Particles Reinforced Ni-Based Composite Coatings Modified with CeO2 Nanoparticles. Metals 2018, 8, 601. https://doi.org/10.3390/met8080601
Chen T, Wu F, Wang H, Liu D. Laser Cladding In-Situ Ti(C,N) Particles Reinforced Ni-Based Composite Coatings Modified with CeO2 Nanoparticles. Metals. 2018; 8(8):601. https://doi.org/10.3390/met8080601
Chicago/Turabian StyleChen, Tao, Fan Wu, Haojun Wang, and Defu Liu. 2018. "Laser Cladding In-Situ Ti(C,N) Particles Reinforced Ni-Based Composite Coatings Modified with CeO2 Nanoparticles" Metals 8, no. 8: 601. https://doi.org/10.3390/met8080601