Metallic Glass Structures for Mechanical-Energy-Dissipation Purpose: A Review
Abstract
:1. Introduction
2. MG Foams
3. MG Honeycombs
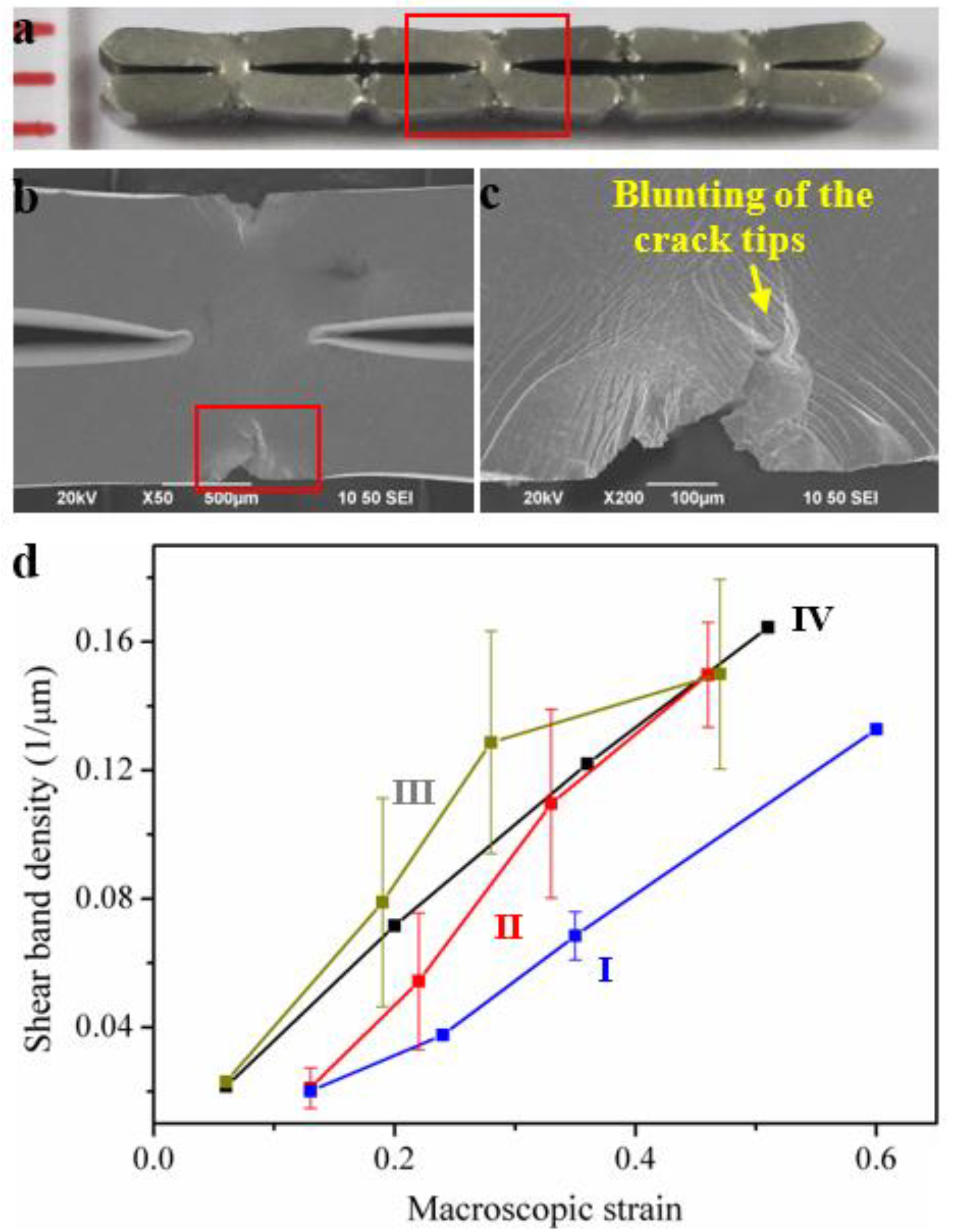
4. Cellular MGs with Macroscopic Cellular Structures
5. Microscopic MG Lattice Structures
6. Kirigami MG Structures
7. Summary and Outlook
- (1)
- The development of 3D MG honeycomb structures is based on the thermoplastic forming and joining technique, where only thin sheet materials are used [67]. It is still challenging to develop honeycomb structures with more complex geometries and a wide range of relative densities.
- (2)
- Due to the limited sample dimensions, cellular MGs with macroscopic cellular structures and saw-tooth-like MG structures have only one layer of structure [12,73]. However, more layers of cellular structures are usually required for practical applications. The use of additive manufacturing (3D printing) may be helpful for developing cellular MGs with larger sample dimensions [92,93,94]. The 3D printing technique also has the advantage of fabricating cellular structures with complex geometries, which could further be used to optimize macroscopic cellular structures for better energy-absorption performance.
- (3)
- Although up-to-date hollow microscopic MG lattice structures have relatively-smaller elastic strain energy recovery than lattice structures that make use of ceramic and crystalline metals [18,84], the high strength and large elastic limit of MGs at the nanoscale make micro MG lattice structures have good potential for high elastic performance. Further efforts need to be devoted to optimize the processing parameters or lattice structures to achieve better elastic energy storability.
- (4)
- Kirigami MG structures have demonstrated large elastic energy storability and ultra-small strain energy loss for a longer cycle life. However, how to use the kirigami structures to develop optoelectronic devices or wearing devices is still challenging. Because of the unique properties, it is expected more functional devices using MGs will be developed in future, such as large elasticity, light weight, wearable sensors and optoelectronic devices [19,89,91].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plummer, J.; Johnson, W.L. Is metallic glass poised to come of age? Nat. Mater. 2015, 14, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. Rep. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Yavari, A.R.; Lewandowski, J.J.; Eckert, J. Mechanical properties of bulk metallic glasses. MRS Bull. 2007, 32, 635–638. [Google Scholar] [CrossRef]
- Chen, M.W. Mechanical behavior of metallic glasses: Microscopic understanding of strength and ductility. Annu. Rev. Mater. Res. 2008, 38, 445–469. [Google Scholar] [CrossRef]
- Trexler, M.M.; Thadhani, N.N. Mechanical properties of bulk metallic glasses. Prog. Mater. Sci. 2010, 55, 759–839. [Google Scholar] [CrossRef]
- Schuh, C.A.; Hufnagel, T.C.; Ramamurty, U. Mechanical behavior of amorphous alloys. Acta Mater. 2007, 55, 4067–4109. [Google Scholar] [CrossRef]
- Brothers, A.H.; Dunand, D.C. Plasticity and damage in cellular amorphous metals. Acta Mater. 2005, 53, 4427–4440. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.H.; Dai, L.H. Energy absorption mechanism of open-cell Zr-based bulk metallic glass foam. Scr. Mater. 2012, 66, 721–724. [Google Scholar] [CrossRef]
- Sarac, B.; Ketkaew, J.; Popnoe, D.O.; Schroers, J. Honeycomb structures of bulk metallic glasses. Adv. Funct. Mater. 2012, 22, 3161–3169. [Google Scholar] [CrossRef]
- Sarac, B.; Schroers, J. From brittle to ductile: Density optimization for Zr-BMG cellular structures. Scr. Mater. 2013, 68, 921–924. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Z.; Robinson, H.M.; Schroers, J. Flaw tolerance vs. performance: A tradeoff in metallic glass cellular structures. Acta Mater. 2014, 73, 259–274. [Google Scholar] [CrossRef]
- Chen, S.H.; Chan, K.C.; Wu, F.F.; Xia, L. Pronounced energy absorption capacity of cellular bulk metallic glasses. Appl. Phys. Lett. 2014, 104, 111907. [Google Scholar] [CrossRef]
- Chen, S.H.; Chan, K.C.; Wu, F.F.; Xia, L. Achieving high energy absorption capacity in cellular bulk metallic glasses. Sci. Rep. 2015, 5, 10302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brothers, A.H.; Scheunemann, R.; DeFouw, J.D.; Dunand, D.C. Processing and structure of open-celled amorphous metal foams. Scr. Mater. 2005, 52, 335–339. [Google Scholar] [CrossRef]
- O’Brien, K.; Suchowski, H.; Rho, J.; Salandrino, A.; Kante, B.; Yin, X.B.; Zhang, X. Predicting nonlinear properties of metamaterials from the linear response. Nat. Mater. 2015, 14, 379–383. [Google Scholar] [CrossRef]
- Paulose, J.; Chen, B.G.G.; Vitelli, V. Topological modes bound to dislocations in mechanical metamaterials. Nat. Phys. 2015, 11, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Jafary-Zadeh, M.; Chen, D.Z.; Zhang, Y.W.; Greer, J.R. Size Effect Suppresses brittle failure in hollow Cu60Zr40 metallic glass nanolattices deformed at cryogenic temperatures. Nano Lett. 2015, 15, 5673–5681. [Google Scholar] [CrossRef] [PubMed]
- Liontas, R.; Greer, J.R. 3D nano-architected metallic glass: Size effect suppresses catastrophic failure. Acta Mater. 2017, 133, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.H.; Chan, K.C.; Yue, T.M.; Wu, F.F. Highly stretchable kirigami metallic glass structures with ultra-small strain energy loss. Scr. Mater. 2018, 142, 83–87. [Google Scholar] [CrossRef]
- Greer, A.L.; Cheng, Y.Q.; Ma, E. Shear bands in metallic glasses. Mater. Sci. Eng. Rep. 2013, 74, 71–132. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Louzguina-Luzgina, L.V.; Churyumov, A.Y. Mechanical properties and deformation behavior of bulk metallic glasses. Metals 2013, 3, 1–22. [Google Scholar] [CrossRef]
- Maaß, R.; Löffler, J.F. Shear-band dynamics in metallic glasses. Adv. Funct. Mater. 2015, 25, 2353–2368. [Google Scholar] [CrossRef]
- Sun, B.A.; Wang, W.H. The fracture of bulk metallic glasses. Prog. Mater. Sci. 2015, 74, 211–307. [Google Scholar] [CrossRef]
- Hufnagel, T.C.; Schuh, C.A.; Falk, M.L. Deformation of metallic glasses: Recent developments in theory, simulations, and experiments. Acta Mater. 2016, 109, 375–393. [Google Scholar] [CrossRef] [Green Version]
- Madge, S.V. Toughness of bulk metallic glasses. Metals 2015, 5, 1279–1305. [Google Scholar] [CrossRef]
- Ma, E.; Cheng, Y.Q. Atomic-level structure and structure-property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Gong, P.; Deng, L.; Jin, J.S.; Wang, S.B.; Wang, X.Y.; Yao, K.F. Review on the research and development of Ti-based bulk metallic glasses. Metals 2016, 6, 264. [Google Scholar] [CrossRef]
- Khan, M.M.; Nemati, A.; Rahman, Z.U.; Shah, U.H.; Asgar, H.; Haider, W. Recent advancements in bulk metallic glasses and their applications: A review. Crit. Rev. Solid State Mater. Sci. 2018, 43, 233–268. [Google Scholar] [CrossRef]
- Jafary-Zadeh, M.; Kumar, G.P.; Branicio, P.S.; Seifi, M.; Lewandowski, J.J.; Cui, F. A critical review on metallic glasses as structural materials for cardiovascular stent applications. J. Funct. Biomater. 2018, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.G.; Hutchinson, J.W.; Ashby, M.F. Multifunctionality of cellular metal systems. Prog. Mater. Sci. 1998, 43, 171–221. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, T.; Itoh, M.; Akiyama, S.; Kitahara, A. ALPORAS aluminum foam: Production process, properties, and applications. Adv. Eng. Mater. 2000, 2, 179–183. [Google Scholar] [CrossRef]
- Wen, C.E.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T. Processing of biocompatible porous Ti and Mg. Scr. Mater. 2001, 45, 1147–1153. [Google Scholar] [CrossRef]
- Lefebvre, L.P.; Banhart, J.; Dunand, D.C. Porous metals and metallic foams: Current status and recent developments. Adv. Eng. Mater. 2008, 10, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Moreno, F. Commercial applications of metal foams: Their properties and production. Materials 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Schroers, J. Processing of bulk metallic glass. Adv. Mater. 2010, 22, 1566–1597. [Google Scholar] [CrossRef] [PubMed]
- Schroers, J.; Veazey, C.; Johnson, W.L. Amorphous metallic foam. Appl. Phys. Lett. 2003, 82, 370–372. [Google Scholar] [CrossRef]
- Demetriou, M.D.; Schramm, J.P.; Veazey, C.; Johnson, W.L.; Hanan, J.C.; Phelps, N.B. High porosity metallic glass foam: A powder metallurgy route. Appl. Phys. Lett. 2007, 91, 161903. [Google Scholar] [CrossRef] [Green Version]
- Wada, T.; Inoue, A. Fabrication, thermal stability and mechanical properties of porous bulk glassy Pd-Cu-Ni-P alloys. Mater. Trans. 2003, 44, 2228–2231. [Google Scholar] [CrossRef]
- Wada, T.; Inoue, A. Formation of porous Pd-based bulk glassy alloys by a high hydrogen pressure melting-water quenching method and their mechanical properties. Mater. Trans. 2004, 45, 2761–2765. [Google Scholar] [CrossRef]
- Wada, T.; Kinaka, M.; Inoue, A. Effect of volume fraction and geometry of pores on mechanical properties of porous bulk glassy Pd42.5Cu30Ni7.5P20 alloys. J. Mater. Res. 2006, 21, 1041–1047. [Google Scholar] [CrossRef]
- Wada, T.; Inoue, A.; Greer, A.L. Enhancement of room-temperature plasticity in a bulk metallic glass by finely dispersed porosity. Appl. Phys. Lett. 2005, 86, 251907. [Google Scholar] [CrossRef]
- Cox, M.E.; Dunand, D.C. Anisotropic mechanical properties of amorphous Zr-based foams with aligned, elongated pores. Acta Mater. 2013, 61, 5937–5948. [Google Scholar] [CrossRef]
- Brothers, A.H.; Dunand, D.C. Syntactic bulk metallic glass foam. Appl. Phys. Lett. 2004, 84, 1108–1110. [Google Scholar] [CrossRef]
- Wada, T.; Wang, X.M.; Kimura, H.; Inoue, A. Supercooled liquid foaming of a Zr-Al-Cu-Ag bulk metallic glass containing pressurized helium pores. Mater. Lett. 2009, 63, 858–860. [Google Scholar] [CrossRef]
- Lee, M.H.; Sordelet, D.J. Synthesis of bulk metallic glass foam by powder extrusion with a fugitive second phase. Appl. Phys. Lett. 2006, 89, 021921. [Google Scholar] [CrossRef]
- Scudino, S.; Kim, J.Y.; Prashanth, K.G.; Lee, M.H.; Kim, B.S.; Kuhn, U.; Eckert, J. Production of customized hybrid porous structures by powder metallurgy of Ni59Zr20Ti16Si2Sn3 glassy powders. J. Mater. Res. 2013, 28, 2490–2498. [Google Scholar] [CrossRef]
- Demetriou, M.D.; Duan, G.; Veazey, C.; De Blauwe, K.; Johnson, W.L. Amorphous Fe-based metal foam. Scr. Mater. 2007, 57, 9–12. [Google Scholar] [CrossRef]
- Wada, T.; Takenaka, K.; Nishiyama, N.; Inoue, A. Formation and mechanical properties of porous Pd-Pt-Cu-P bulk glassy alloys. Mater. Trans. 2005, 46, 2777–2780. [Google Scholar] [CrossRef]
- Demetriou, M.D.; Veazey, C.; Harmon, J.S.; Schramm, J.P.; Johnson, W.L. Stochastic metallic-glass cellular structures exhibiting benchmark strength. Phys. Rev. Lett. 2008, 101, 145702. [Google Scholar] [CrossRef] [PubMed]
- Brothers, A.H.; Dunand, D.C. Ductile bulk metallic glass foams. Adv. Mater. 2005, 17, 484–486. [Google Scholar] [CrossRef]
- Schramm, J.P.; Demetriou, M.D.; Johnson, W.L.; Poon, B.; Ravichandran, G.; Rittel, D. Effect of strain rate on the yielding mechanism of amorphous metal foam. Appl. Phys. Lett. 2010, 96, 021906. [Google Scholar] [CrossRef] [Green Version]
- Demetriou, M.D.; Hanan, J.C.; Veazey, C.; Di Michiel, M.; Lenoir, N.; Ustundag, E.; Johnson, W.L. Yielding of metallic glass foam by percolation of an elastic buckling instability. Adv. Mater. 2007, 19, 1957–1962. [Google Scholar] [CrossRef]
- Hofmann, D.C. Shape memory bulk metallic glass composites. Science 2010, 329, 1294–1295. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, D.C.; Suh, J.Y.; Wiest, A.; Duan, G.; Lind, M.L.; Demetriou, M.D.; Johnson, W.L. Designing metallic glass matrix composites with high toughness and tensile ductility. Nature 2008, 451, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiao, Y.H.; Chen, G.L.; Liu, C.T.; Lu, Z.P. Bulk metallic glass composites with transformation-mediated work-hardening and ductility. Adv. Mater. 2010, 22, 2770–2773. [Google Scholar] [CrossRef] [PubMed]
- Brothers, A.H.; Dunand, D.C.; Zheng, Q.; Xu, J. Amorphous Mg-based metal foams with ductile hollow spheres. J. Appl. Phys. 2007, 102, 023508. [Google Scholar] [CrossRef]
- Brothers, A.H.; Mangrich, B.; Cox, M.; Dunand, D.C. Effect of crystalline metallic particles on the compressive behavior of a cellular amorphous metal. Scr. Mater. 2011, 64, 1031–1034. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.J.; Wang, H.; Zhou, D.Q.; Wu, Y.; Lu, Z.P. Formation mechanism and characterization of nanoporous silver with tunable porosity and promising capacitive performance by chemical dealloying of glassy precursor. Acta Mater. 2016, 105, 367–377. [Google Scholar] [CrossRef]
- Yu, J.S.; Ding, Y.; Xu, C.X.; Inoue, A.; Sakurai, T.; Chen, M.W. Nanoporous metals by dealloying multicomponent metallic glasses. Chem. Mater. 2008, 20, 4548–4550. [Google Scholar] [CrossRef]
- Li, R.; Chan, K.C.; Liu, X.J.; Zhang, X.H.; Liu, L.; Li, T.; Lu, Z.P. Synthesis of well-aligned CuO nanowire array integrated with nanoporous CuO network for oxidative degradation of methylene blue. Corros. Sci. 2017, 126, 37–43. [Google Scholar] [CrossRef]
- Romano, L.; Impellizzeri, G.; Bosco, L.; Ruffino, F.; Miritello, M.; Grimaldi, M.G. Nanoporosity induced by ion implantation in deposited amorphous Ge thin films. J. Appl. Phys. 2012, 111, 113515. [Google Scholar] [CrossRef]
- Grillo, R.; Torrisi, V.; Ruffino, F. Nanoporous Au: An experimental study on the porosity of dealloyed AuAg leafs. Superlattices Microstruct. 2016, 100, 780–791. [Google Scholar] [CrossRef]
- Jayakumar, B.; Hanan, J.C. Modeling the axial mechanical response of amorphous Fe45Ni45Mo7B3 honeycombs. Metall. Mater. Trans. A 2012, 43, 2669–2675. [Google Scholar] [CrossRef]
- Schroers, J.; Pham, Q.; Peker, A.; Paton, N.; Curtis, R.V. Blow molding of bulk metallic glass. Scr. Mater. 2007, 57, 341–344. [Google Scholar] [CrossRef]
- Duan, G.; Wiest, A.; Lind, M.L.; Li, J.; Rhim, W.K.; Johnson, W.L. Bulk metallic glass with benchmark thermoplastic processability. Adv. Mater. 2007, 19, 4272–4275. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, X.; Wang, W.H. Metallic glass mold insert for hot embossing of polymers. J. Appl. Phys. 2012, 112, 024506. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, W.; Carstensen, J.; Ketkaew, J.; Mota, R.M.O.; Guest, J.K.; Schroers, J. 3D metallic glass cellular structures. Acta Mater. 2016, 105, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.H.; Chan, K.C.; Xia, L. Deformation behavior of bulk metallic glass structural elements. Mater. Sci. Eng. A 2014, 606, 196–204. [Google Scholar] [CrossRef]
- Chen, S.H.; Chan, K.C.; Xia, L. Deformation behavior of a Zr-based bulk metallic glass under a complex stress state. Intermetallics 2013, 43, 38–44. [Google Scholar] [CrossRef]
- Chen, S.H.; Chan, K.C.; Xia, L. Fracture morphologies of Zr-based bulk metallic glasses under different stress states. Adv. Eng. Mater. 2015, 17, 366–373. [Google Scholar] [CrossRef]
- Chen, S.H. Deformation Behaviour of Bulk Metallic Glasses under Complex Stress States. Ph.D. Thesis, The Hong Kong Polytechnic University, Hong Kong, China, September 2014. [Google Scholar]
- Bei, H.; Xie, S.; George, E.P. Softening caused by profuse shear banding in a bulk metallic glass. Phys. Rev. Lett. 2006, 96, 105503. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chan, K.C.; Wang, G.; Yi, J. Saw-tooth-like bulk metallic glass structures with greatly enhanced energy-absorption performance. J. Alloy. Compd. 2016, 661, 49–54. [Google Scholar] [CrossRef]
- Wu, F.F.; Chan, K.C.; Jiang, S.S.; Chen, S.H.; Wang, G. Bulk metallic glass composite with good tensile ductility, high strength and large elastic strain limit. Sci. Rep. 2014, 4, 5302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, J.; Tang, M.B.; Kim, K.B.; Theissmann, R.; Baier, F.; Wang, W.H.; Eckert, J. “Work-hardenable” ductile bulk metallic glass. Phys. Rev. Lett. 2005, 94, 205501. [Google Scholar] [CrossRef] [PubMed]
- Schramm, J.P.; Hofmann, D.C.; Demetriou, M.D.; Johnson, W.L. Metallic-glass-matrix composite structures with benchmark mechanical performance. Appl. Phys. Lett. 2010, 97, 241910. [Google Scholar] [CrossRef] [Green Version]
- Schaedler, T.A.; Jacobsen, A.J.; Torrents, A.; Sorensen, A.E.; Lian, J.; Greer, J.R.; Valdevit, L.; Carter, W.B. Ultralight metallic microlattices. Science 2011, 334, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Meza, L.R.; Das, S.; Greer, J.R. Strong, lightweight, and recoverable three-dimensional ceramic nanolattices. Science 2014, 345, 1322–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, D.C.; Meza, L.R.; Greer, F.; Greer, J.R. Fabrication and deformation of three-dimensional hollow ceramic nanostructures. Nat. Mater. 2013, 12, 893–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Yan, P.F.; Wang, Y.B.; Tan, J.; Zhang, Z.F.; Sui, M.L.; Ma, E. Tensile ductility and necking of metallic glass. Nat. Mater. 2007, 6, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.C.; Greer, J.R. Transition from a strong-yet-brittle to a stronger-and-ductile state by size reduction of metallic glasses. Nat. Mater. 2010, 9, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.S.; Cheng, Y.Q.; Yue, Y.H.; Zhang, L.; Zhang, Z.; Han, X.D.; Ma, E. Uniform tensile elongation in framed submicron metallic glass specimen in the limit of suppressed shear banding. Acta Mater. 2011, 59, 6511–6518. [Google Scholar] [CrossRef]
- Liontas, R.; Jafary-Zadeh, M.; Zeng, Q.S.; Zhang, Y.W.; Mao, W.L.; Greer, J.R. Substantial tensile ductility in sputtered Zr-Ni-Al nano-sized metallic glass. Acta Mater. 2016, 118, 270–285. [Google Scholar] [CrossRef] [Green Version]
- Rys, J.; Valdevit, L.; Schaedler, T.A.; Jacobsen, A.J.; Carter, W.B.; Greer, J.R. Fabrication and Deformation of Metallic Glass Micro-Lattices. Adv. Eng. Mater. 2014, 16, 889–896. [Google Scholar] [CrossRef]
- Hu, Q.; Fu, M.W.; Zeng, X.R.; Ma, C.L. Thin-walled Ti41.5Zr2.5Hf5Cu42.5Ni7.5Si1 bulk metallic glass tubes: Promising energy absorbers and lightweight structures. J. Alloy. Compd. 2013, 546, 180–184. [Google Scholar] [CrossRef]
- Shyu, T.C.; Damasceno, P.F.; Dodd, P.M.; Lamoureux, A.; Xu, L.Z.; Shlian, M.; Shtein, M.; Glotzer, S.C.; Kotov, N.A. A kirigami approach to engineering elasticity in nanocomposites through patterned defects. Nat. Mater. 2015, 14, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Isobe, M.; Okumura, K. Initial rigid response and softening transition of highly stretchable kirigami sheet materials. Sci. Rep. 2016, 6, 24758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadpoor, A.A. Mechanical meta-materials. Mater. Horiz. 2016, 3, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Sha, Z.D.; She, C.M.; Xu, G.K.; Pei, Q.X.; Liu, Z.S.; Wang, T.J.; Gao, H.J. Metallic glass-based chiral nanolattice: Light weight, auxeticity, and superior mechanical properties. Mater. Today 2017, 20, 569–576. [Google Scholar] [CrossRef]
- Lamoureux, A.; Lee, K.; Shlian, M.; Forrest, S.R.; Shtein, M. Dynamic kirigami structures for integrated solar tracking. Nat. Commun. 2015, 6, 8092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xian, H.J.; Cao, C.R.; Shi, J.A.; Zhu, X.S.; Hu, Y.C.; Huang, Y.F.; Meng, S.; Gu, L.; Liu, Y.H.; Bai, H.Y.; et al. Flexible strain sensors with high performance based on metallic glass thin film. Appl. Phys. Lett. 2017, 111, 121906. [Google Scholar] [CrossRef]
- Pauly, S.; Lober, L.; Petters, R.; Stoica, M.; Scudino, S.; Kuhn, U.; Eckert, J. Processing metallic glasses by selective laser melting. Mater. Today 2013, 16, 37–41. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Li, Y.Q.; Chen, C.; Tsai, H.L. 3D printing of large, complex metallic glass structures. Mater. Des. 2017, 117, 213–222. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.J.; Xing, W.; Ouyang, D.; Liu, L. 3D printing of Fe-based bulk metallic glass composites with combined high strength and fracture toughness. Mater. Des. 2018, 143, 285–296. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.H.; Cheng, H.Y.; Chan, K.C.; Wang, G. Metallic Glass Structures for Mechanical-Energy-Dissipation Purpose: A Review. Metals 2018, 8, 689. https://doi.org/10.3390/met8090689
Chen SH, Cheng HY, Chan KC, Wang G. Metallic Glass Structures for Mechanical-Energy-Dissipation Purpose: A Review. Metals. 2018; 8(9):689. https://doi.org/10.3390/met8090689
Chicago/Turabian StyleChen, S. H., H. Y. Cheng, K. C. Chan, and G. Wang. 2018. "Metallic Glass Structures for Mechanical-Energy-Dissipation Purpose: A Review" Metals 8, no. 9: 689. https://doi.org/10.3390/met8090689
APA StyleChen, S. H., Cheng, H. Y., Chan, K. C., & Wang, G. (2018). Metallic Glass Structures for Mechanical-Energy-Dissipation Purpose: A Review. Metals, 8(9), 689. https://doi.org/10.3390/met8090689




