Study of Formed Oxides in IN718 Alloy during the Fabrication by Selective Laser Melting and Electron Beam Melting
Abstract
:1. Introduction
2. Materials and Experimental Procedure
3. Results
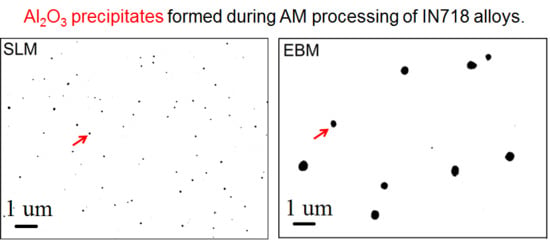
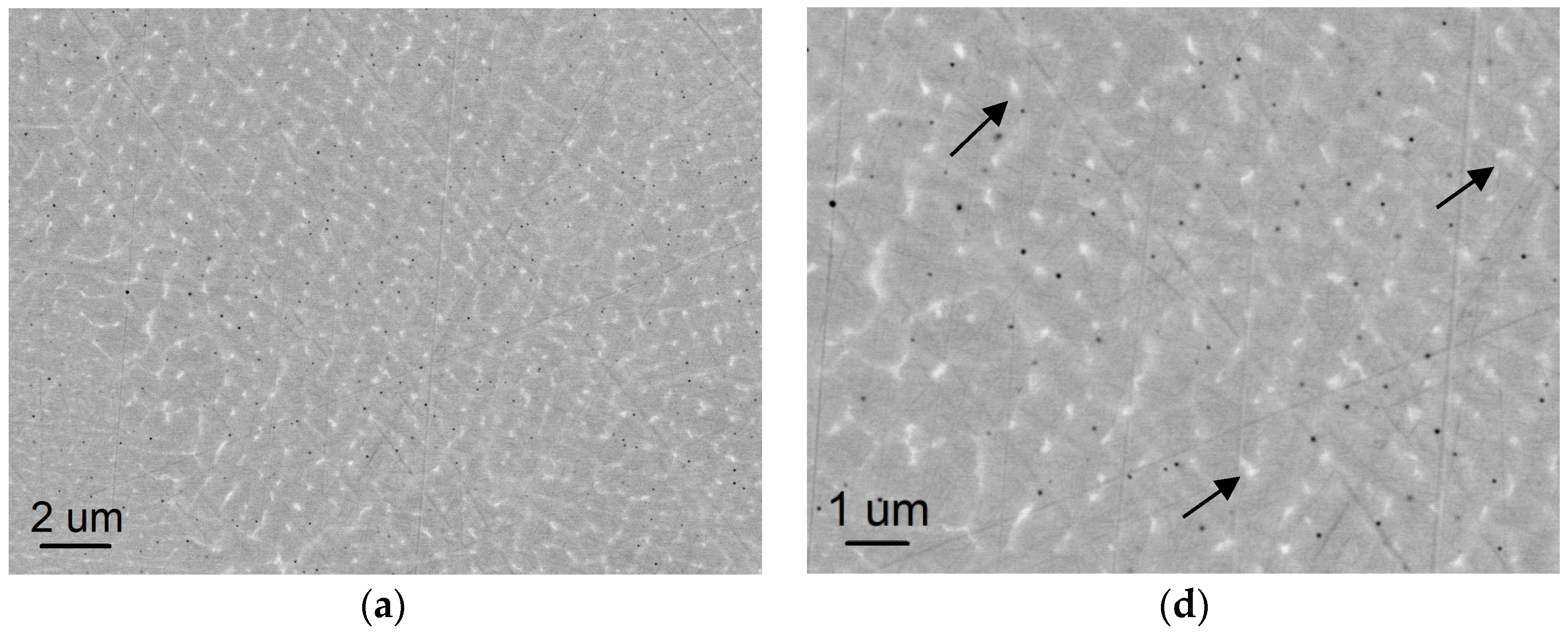
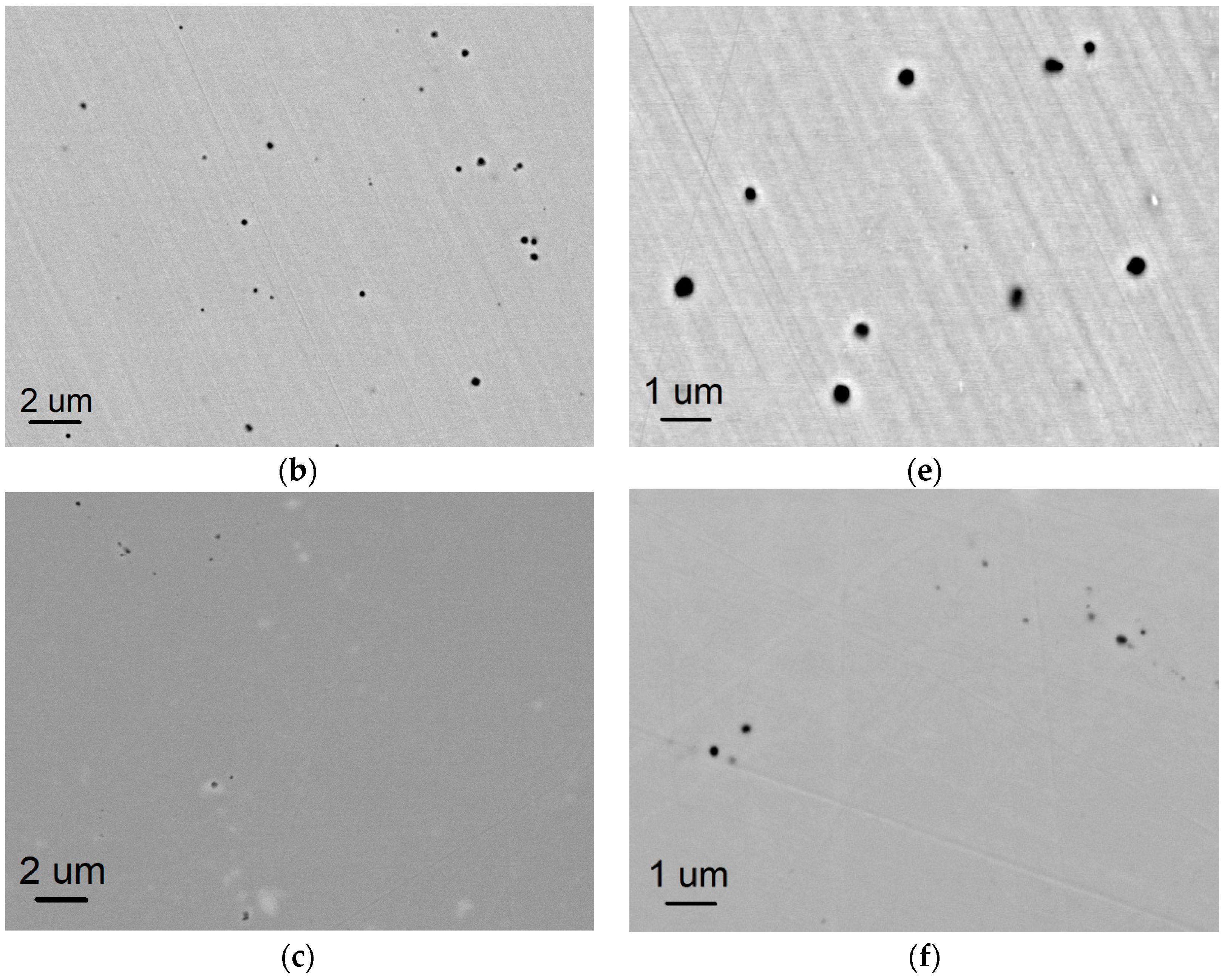
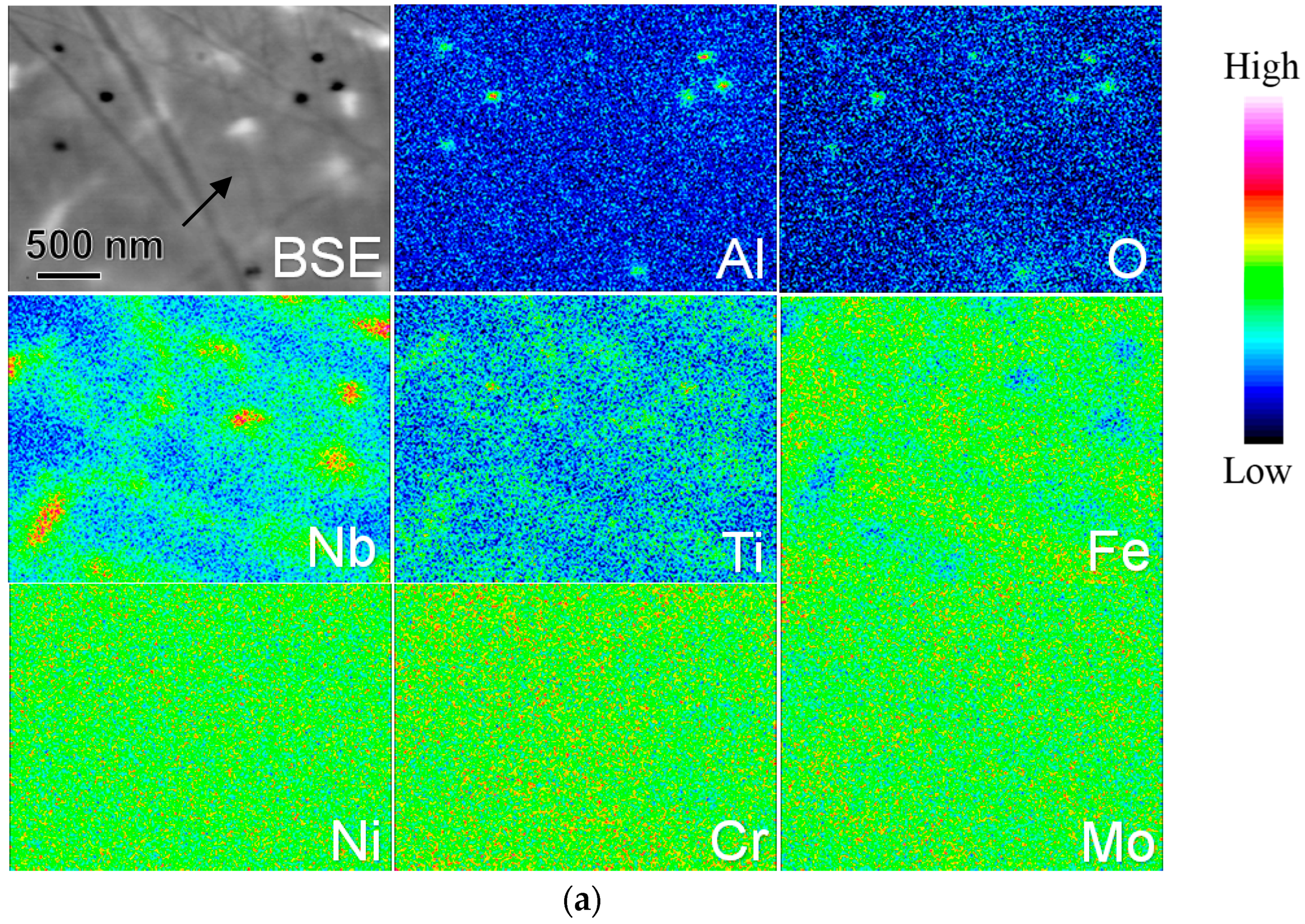
3.1. Microstructures of AM-Fabricated Alloys
3.2. Oxidation Behavior of Raw Powders
3.3. Microstructures of SPS-Fabricated Alloys
4. Discussion
4.1. Microstructures of AM- and SPS-Fabricated Alloys
4.2. Calculation of the Introduced Oxygen Content in AM Process
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Korner, C. Additive manufacturing of metallic components by selective electron beam melting-a review. Int. Mater. Rev. 2016, 61, 361–377. [Google Scholar] [CrossRef]
- Murr, L.E.; Martinez, E.; Amato, K.N.; Gaytan, S.M.; Hernandez, J.; Ramirez, D.A.; Shindo, P.W.; Medina, F.; Wicker, R.B. Fabrication of metal and alloy components by additive manufacturing: Examples of 3D materials science. J. Mater. Res. Technol. 2012, 1, 42–54. [Google Scholar] [CrossRef]
- Gokuldoss, P.K.; Kolla, S.; Eckert, J. Additive manufacturing processes: Selective laser melting, electron beam melting and binder jetting-selection guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.B.; Gu, D.D. Selective laser melting additive manufacturing of Inconel 718 superalloy parts: Densification, microstructure and properties. J. Alloys Compd. 2014, 585, 713–721. [Google Scholar] [CrossRef]
- Kuo, Y.L.; Kakehi, K. Influence of powder surface contamination in the Ni-based superalloy alloy718 fabricated by selective laser melting and hot isostatic pressing. Metals 2017, 7, 367. [Google Scholar] [CrossRef]
- Bai, Q.; Lin, J.; Jiang, J.; Dean, T.A.; Zou, J.; Tian, G. A study of direct forging process for powder superalloys. Mater. Sci. Eng. A 2015, 621, 68–75. [Google Scholar] [CrossRef]
- Rao, G.A.; Srinivas, M.; Sarma, D.S. Effect of solution treatment temperature on microstructure and mechanical properties of hot isostatically pressed superalloy Inconel 718. Mater. Sci. Technol. 2004, 20, 1161–1170. [Google Scholar] [CrossRef]
- Vilaro, T.; Colin, C.; Bartout, J.D.; Naze, L.; Sennour, M. Microstructural and mechanical approaches of the selective laser melting process applied to a nickel-based superalloy. Mater. Sci. Eng. A 2012, 534, 446–451. [Google Scholar] [CrossRef]
- Tucho, W.M.; Cuvillier, P.; Kverneland, A.S.; Hansen, V. Microstructure and hardness studies of Inconel 718 manufactured by selective laser melting before and after solution heat treatment. Mater. Sci. Eng. A 2017, 689, 220–232. [Google Scholar] [CrossRef]
- Song, B.; Zhao, X.; Li, S.; Han, C.J.; Wei, Q.S.; Wen, S.F.; Liu, J.; Shi, Y.S. Different in microstructure and properties between selective laser melting and traditional manufacturing for fabrication of metal parts: A review. Front. Mech. Eng. 2015, 10, 111–125. [Google Scholar] [CrossRef]
- Trosch, T.; Strobner, J.; Volkl, R.; Glatzel, U. Microstructure and mechanical properties of selective laser melted Inconel 718 compared to forging and casting. Mater. Lett. 2016, 164, 428–431. [Google Scholar] [CrossRef]
- Amato, K.N.; Gaytan, S.M.; Murr, L.E.; Martinez, E.; Shindo, P.W.; Hernandez, J.; Collins, S.; Medina, F. Microstructures and mechanical behavior of Inconel 718fabricated by selective laser melting. Acta Mater. 2012, 60, 2229–2239. [Google Scholar] [CrossRef]
- Wang, X.Q.; Keya, T.; Chou, K. Build height effect on the Inconel 718 parts fabricated by selective laser melting. Procedia Manuf. 2016, 5, 1006–1017. [Google Scholar] [CrossRef]
- Korner, C.; Helmer, H.; Bauereib, A.; Singer, R.F. Tailoring the grain structure of IN718 during selective electron beam melting. In Proceedings of the Eurosuperalloys 2014, MATEC Web of Conferences, Giens, France, 12–16 May 2014; Volume 14. Article number 08001. [Google Scholar]
- Jia, Q.B.; Gu, D.D. Selective laser melting additive manufactured Inconel 718 superalloy parts: High-temperature oxidation property and its mechanisms. Opt. Laser Technol. 2014, 62, 161–171. [Google Scholar] [CrossRef]
- Wang, Z.M.; Guan, K.; Gao, M.; Li, X.Y.; Chen, X.F.; Zeng, X.Y. The microstructure and mechanical properties of deposited IN718 by selective laser melting. J. Alloys Compd. 2012, 513, 518–523. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Cao, X.; Wanjara, P.; Medraj, M. Oxide films in laser additive manufactured Inconel 718. Acta Mater. 2013, 61, 6562–6576. [Google Scholar] [CrossRef]
- Ardila, L.C.; Garciandia, F.; Gonzalez-Diaz, J.B.; Alvarez, P.; Echeverria, A.; Petite, M.M.; Deffley, R.; Ochoa, J. Effect of IN718 recycled powder reuse on properties of parts manufactured by means of selective laser melting. Phys. Procedia 2014, 56, 99–107. [Google Scholar] [CrossRef]
- Kowalski, M.; Spencer, P.J. Thermodynamic reevaluation of the C-O, Fe-O and Ni-O systems: Remodelling of the liquid, BCC and FCC phases. Calphad 1995, 19, 229–243. [Google Scholar] [CrossRef]
- Perusin, S.; Monceau, D.; Andrieu, E. Investigations on the diffusion of oxygen in nickel at 1000 °C by SIMS analysis. J. Electrochem. Soc. 2005, 152, 390–397. [Google Scholar] [CrossRef]
- Sames, W.J.; Unocic, K.A.; Dehoff, R.R.; Lolla, T.; Babu, S.S. Thermal effects on microstructural heterogeneity of Inconel 718 materials fabricated by electron beam melting. J. Mater. Res. 2014, 29, 1920–1930. [Google Scholar] [CrossRef]
- Mostafa, A.; Rubio, I.P.; Brailovski, V.; Jahazi, M.; Medraj, M. Structure, texture and phases in 3D printed IN718 alloy subjected to homogenization and HIP treatments. Metals 2017, 7, 196. [Google Scholar] [CrossRef]
- Parimi, L.L.; Ravi, G.A.; Clark, D.; Attallah, M.M. Microstructure and texture development in direct laser fabricated IN718. Mater. Charact. 2014, 89, 102–111. [Google Scholar] [CrossRef]
- Benn, R.C.; Salva, R.P. Additive manufactured Inconel alloy 718. In Proceedings of the 7th International Syposium on Superalloy 718 and Derivatives, TMS, Pittsburgh, PA, USA, 10–13 October 2010; pp. 455–469. [Google Scholar]
- Rao, G.A.; Srinivas, M.; Sarma, D.S. Influence of modified processing on structure and properties of hot isostatically pressed superalloy Inconel 718. Mater. Sci. Eng. A 2006, 418, 282–291. [Google Scholar] [CrossRef]
- Xie, X.S.; Xu, C.M.; Wang, G.L.; Dong, J.X.; Cao, W.D.; Kennedy, R. TTT diagram of a newly developed nickel-based superalloy-Allvac 718plusTM. In Proceedings of the Superalloys 718, 625, 706 and Derivatives 2005, TMS, Pittsburgh, PA, USA, 2–5 October 2005; pp. 193–202. [Google Scholar]
- Srinivasan, D.; Lawless, L.U.; Ott, E.A. Experimental determination of TTT diagram for alloy 718plus. In Proceedings of the Superalloys 2012: 12th International Symposium on Superalloys, TMS, Seven Springs, PA, USA, 9–13 September 2012; pp. 759–768. [Google Scholar]
- Azadian, S.; Wei, L.Y.; Warren, R. Delta phase precipitation in Inconel 718. Mater. Charact. 2004, 53, 7–16. [Google Scholar] [CrossRef]
- Dehmas, M.; Lacaze, J.; Niang, A.; Viguier, B. TEM study of high-temperature precipitation of Delta phase in Inconel 718. Adv. Mater. Sci. Eng. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.C.; Guo, Q.Y.; Li, C.; Mei, Y.P.; Zou, X.S.; Huang, Y.; Li, H.J. Recent progress on evolution of precipitates on Inconel 718 superalloy. Acta Metall. Sin. 2016, 52, 1259–1266. [Google Scholar]
- He, Y.D.; Li, Z.W.; Qi, H.B.; Gao, W. Standard free energy change of formation per unit volume: A new parameter for evaluating nucleation and growth of oxides, sulphides, carbides and nitrides. Mater. Res. Innov. 1997, 1, 157–160. [Google Scholar] [CrossRef]
- Stratton, P. Ellingham diagrams-their use and misuse. IHTSE 2013, 7, 70–73. [Google Scholar] [CrossRef]
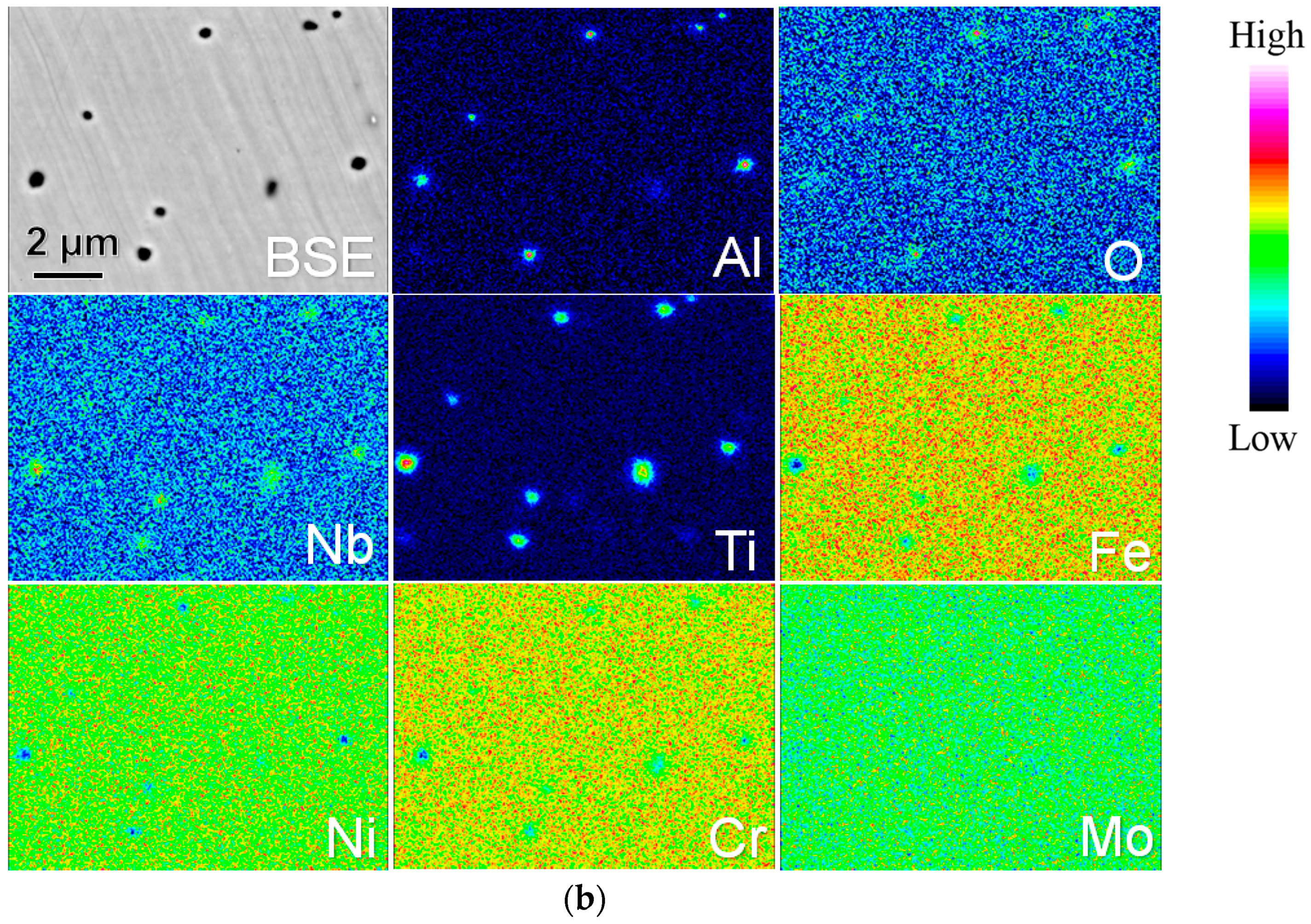
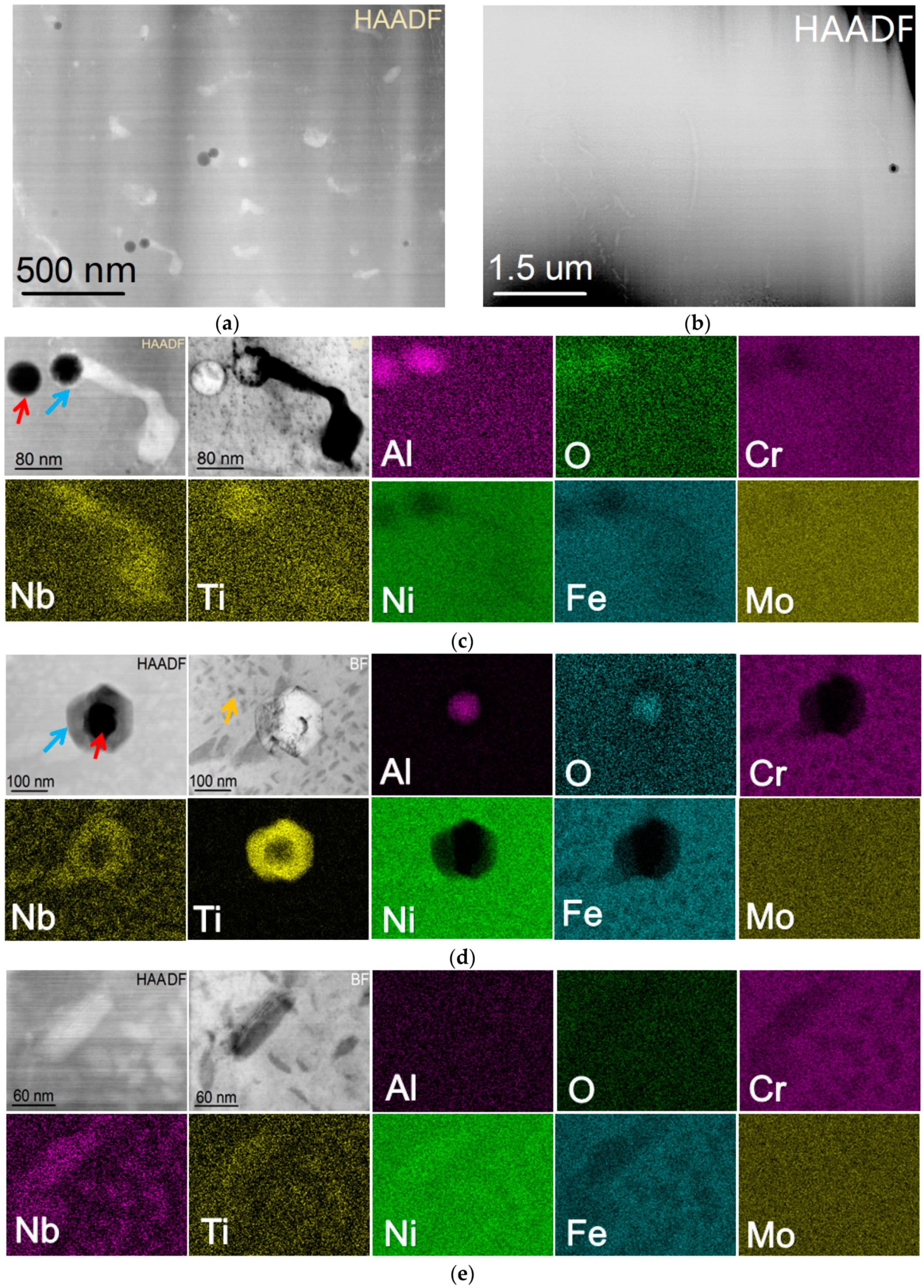
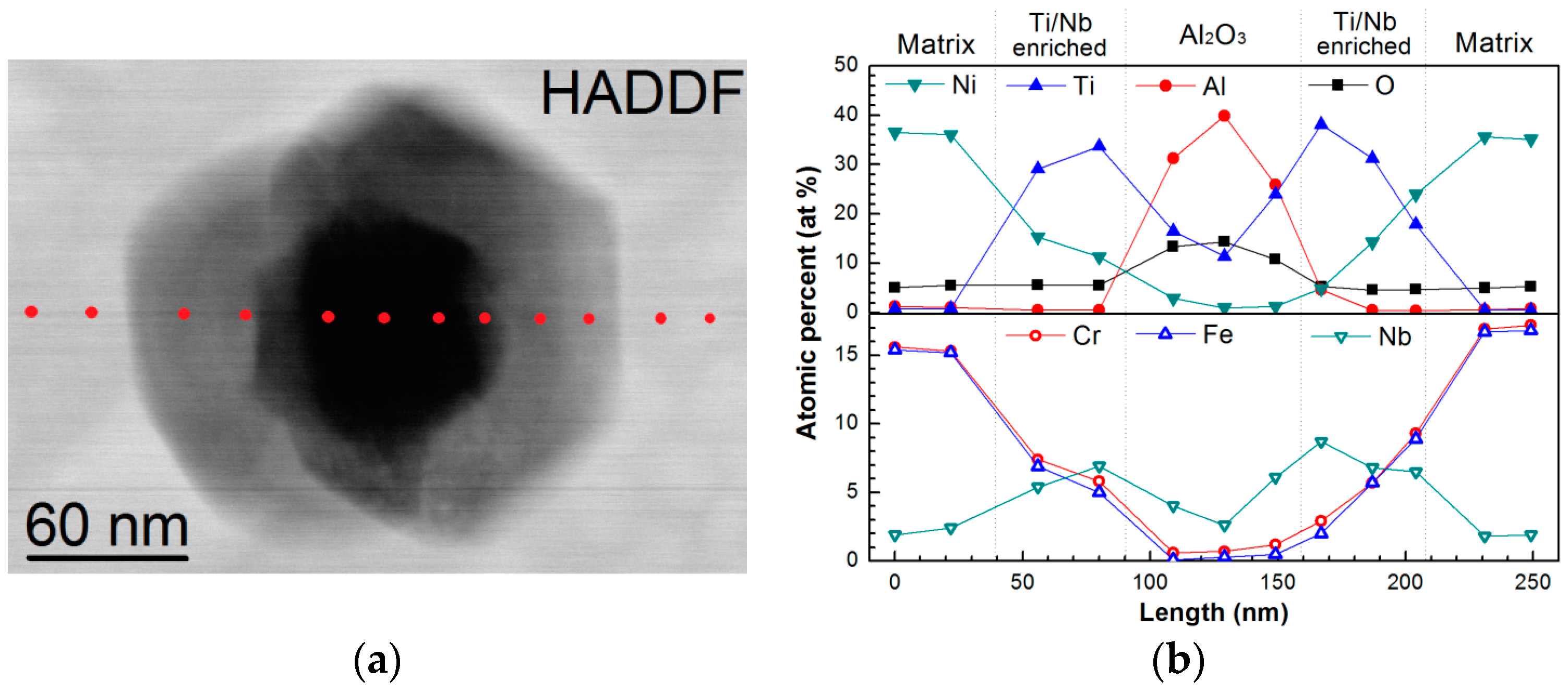


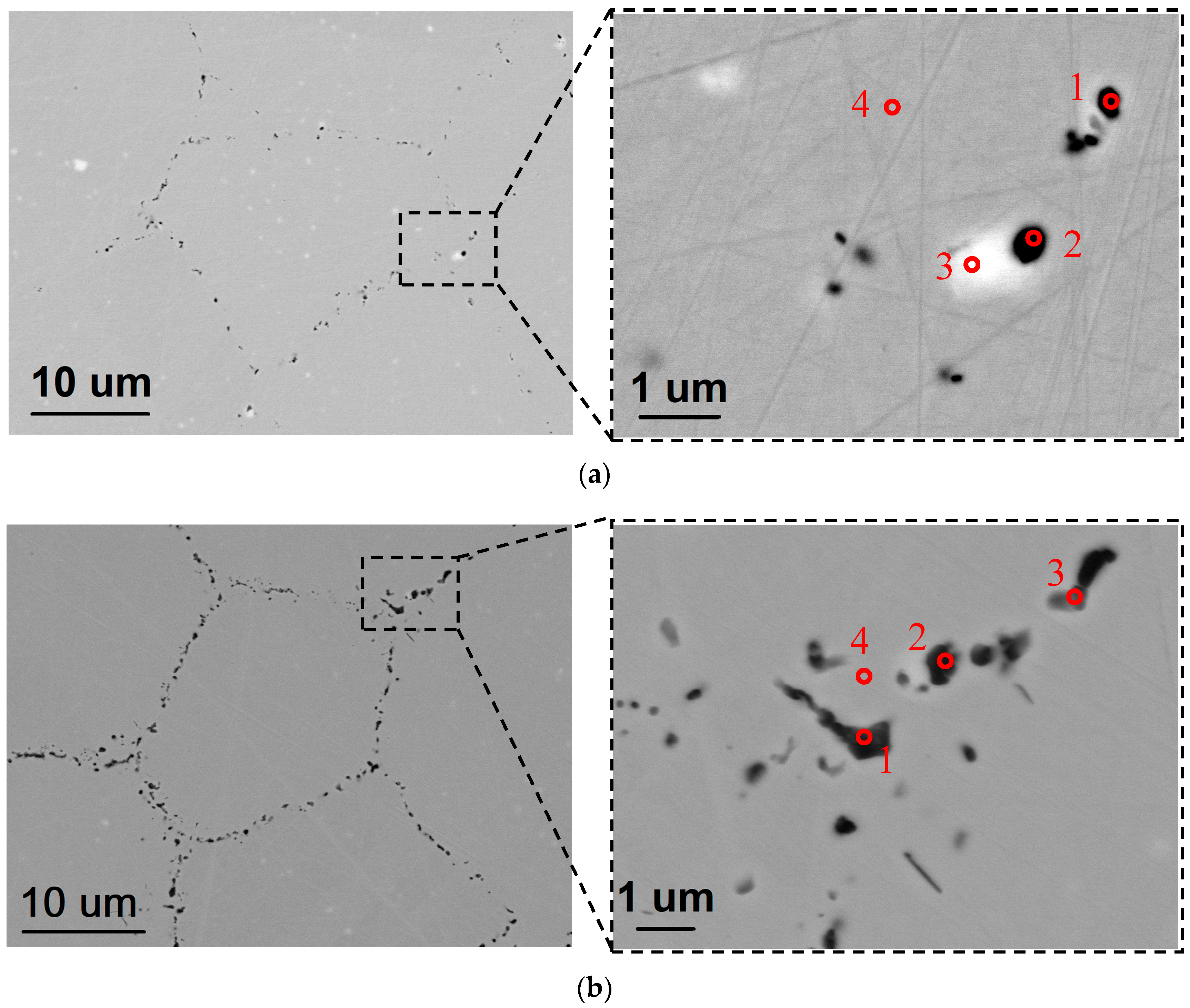
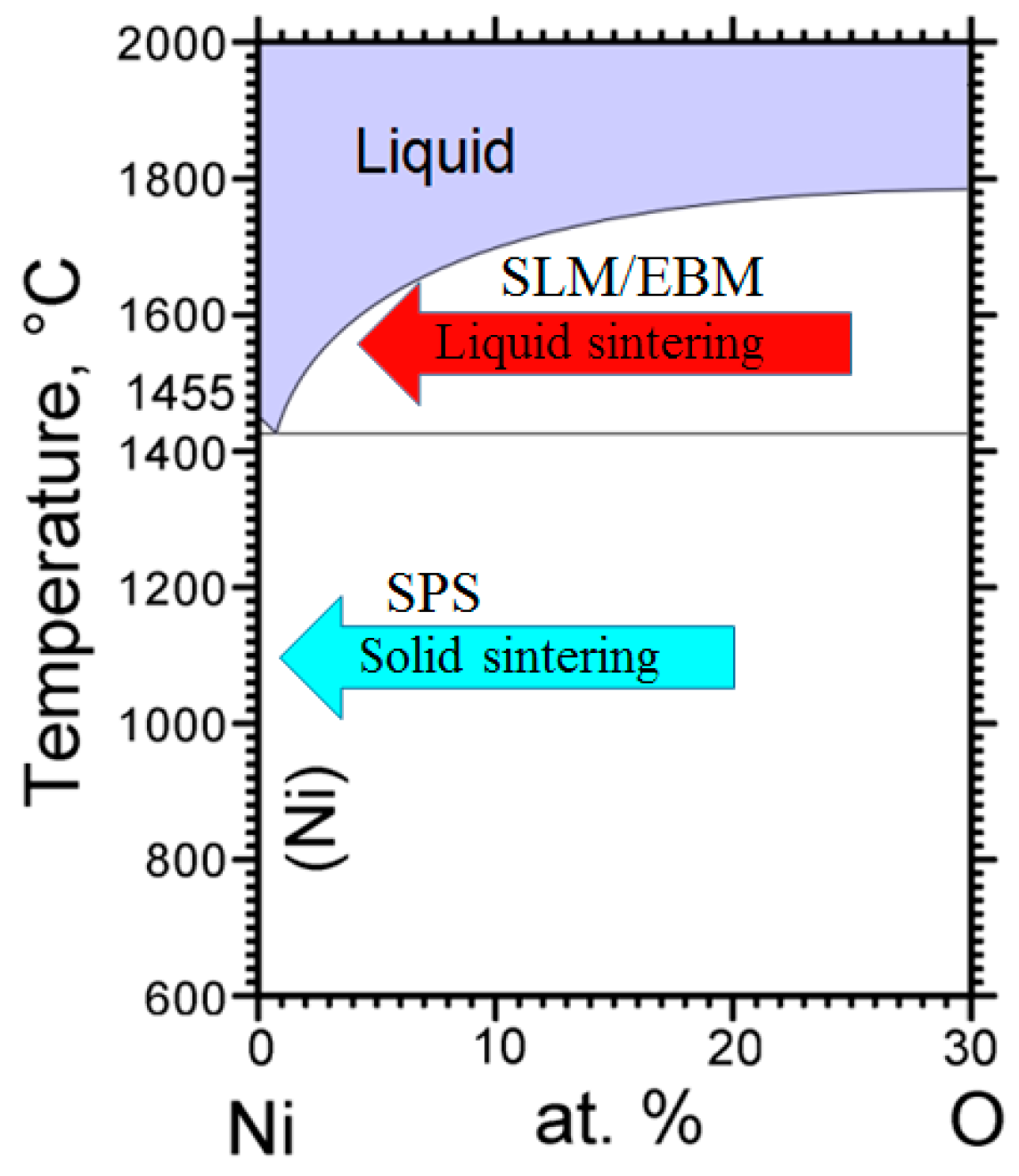
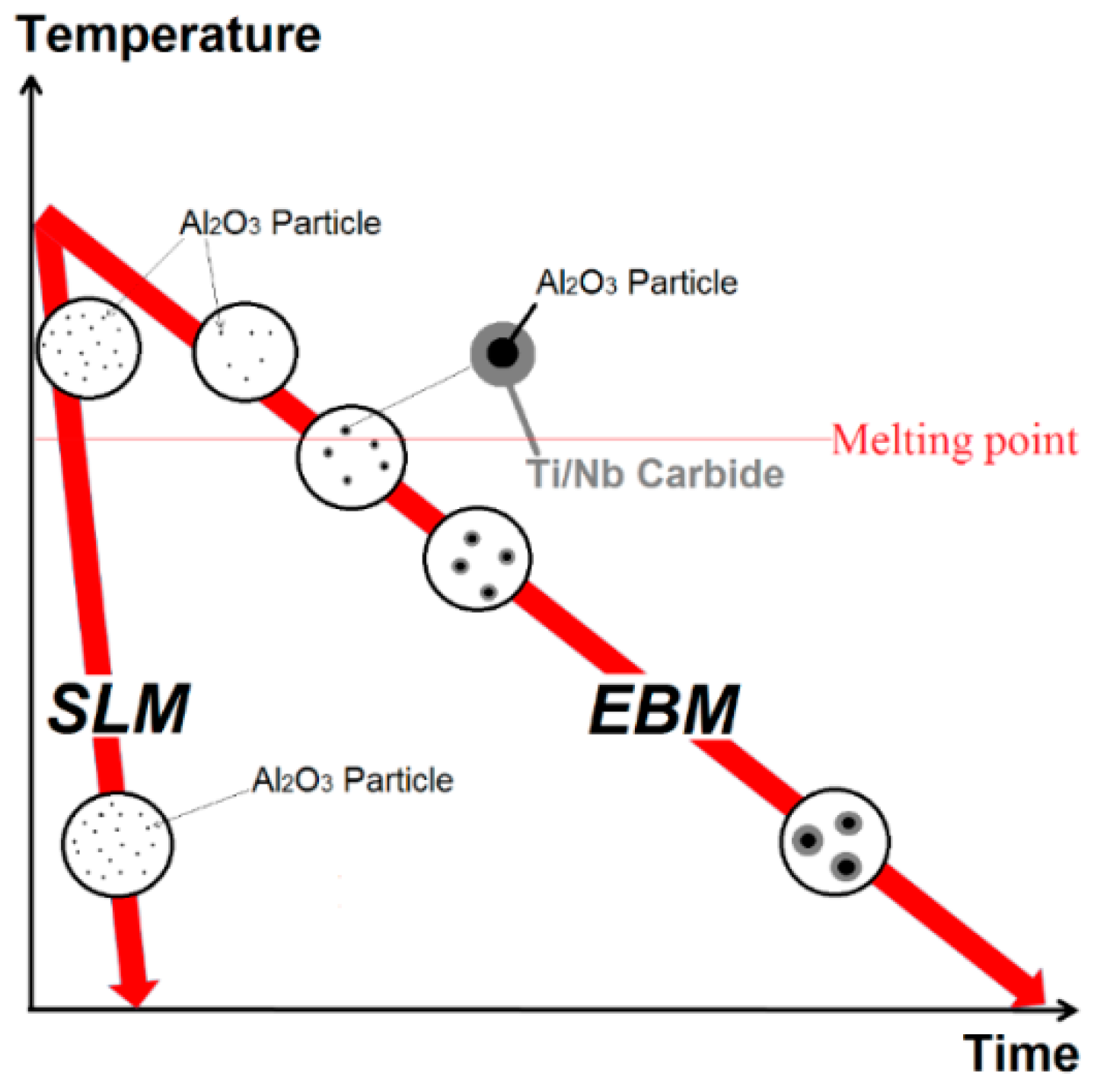


| Powders | Ni | Cr | Fe | Nb | Mo | Ti | Al | Co | O |
|---|---|---|---|---|---|---|---|---|---|
| IN718 SLM | Bal. | 19.63 | 18.21 | 5.05 | 2.85 | 1.10 | 0.46 | 0.03 | 0.019 |
| IN718 EBM | Bal. | 19.30 | 18.34 | 4.89 | 3.04 | 1.07 | 0.54 | 0.01 | 0.014 |
| IN718 SPS | Bal. | 18.92 | 18.09 | 5.21 | 3.02 | 0.90 | 0.62 | 0.02 | 0.016 |
| No. | Ni | Fe | Cr | Al | O | Mo | Nb | Ti |
|---|---|---|---|---|---|---|---|---|
| 1 | 9.2 | 9.2 | 6.1 | 0.1 | 72.5 | 2.7 | 0 | 0.2 |
| 2 | 7.7 | 9.5 | 6.6 | 0.2 | 73.5 | 2.2 | 0 | 0.1 |
| 3 | 0.1 | 0.2 | 19.9 | 0.9 | 74.8 | 2.0 | 1.5 | 0.5 |
| 4 | 0.7 | 0.8 | 25.0 | 0.6 | 69.6 | 2.2 | 0.7 | 0.6 |
| 5 | 9.9 | 2.9 | 6.4 | 2.3 | 67.9 | 3.4 | 5.3 | 1.9 |
| 6 | 48.8 | 11.8 | 9.0 | 0.4 | 20.5 | 7.2 | 1.9 | 0.5 |
| 7 | 44.0 | 11.0 | 6.2 | 1.0 | 27.7 | 7.3 | 2.4 | 0.5 |
| 8 | 38.7 | 12.8 | 15.0 | 1.2 | 23.7 | 6.4 | 1.7 | 0.4 |
| No. | Specimens | Cr | Nb | Ti | Ni | Al | O | Fe | Mo |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Vacuum | 22.2 | 3.3 | 1.1 | 40.0 | 3.9 | 3.0 | 19.9 | 1.9 |
| 2 | 17.0 | 11.2 | 3.6 | 25.4 | 10.5 | 18.8 | 12.0 | 1.5 | |
| 3 | 17.5 | 28.3 | 7.5 | 30.7 | 1.2 | 0 | 13.5 | 1.4 | |
| 4 | 21.6 | 3.1 | 1.2 | 47.3 | 1.4 | 0 | 19.3 | 2.0 | |
| 1 | Air | 7.6 | 1.2 | 1.0 | 9.6 | 29.1 | 44.6 | 6.0 | 0.5 |
| 2 | 8.0 | 2.4 | 0.9 | 8.3 | 26.6 | 46.4 | 6.7 | 0.6 | |
| 3 | 14.5 | 1.6 | 0.8 | 30.1 | 12.2 | 26.3 | 12.5 | 1.1 | |
| 4 | 21.8 | 2.5 | 1.0 | 52.1 | 0.7 | 0 | 18.5 | 1.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Hayashi, S.; Kakehi, K.; Kuo, Y.-L. Study of Formed Oxides in IN718 Alloy during the Fabrication by Selective Laser Melting and Electron Beam Melting. Metals 2019, 9, 19. https://doi.org/10.3390/met9010019
Yu H, Hayashi S, Kakehi K, Kuo Y-L. Study of Formed Oxides in IN718 Alloy during the Fabrication by Selective Laser Melting and Electron Beam Melting. Metals. 2019; 9(1):19. https://doi.org/10.3390/met9010019
Chicago/Turabian StyleYu, Hao, Shigenari Hayashi, Koji Kakehi, and Yen-Ling Kuo. 2019. "Study of Formed Oxides in IN718 Alloy during the Fabrication by Selective Laser Melting and Electron Beam Melting" Metals 9, no. 1: 19. https://doi.org/10.3390/met9010019