Material scientists have highlighted the remarkable properties of Sc as an alloying element for Al(-Mg) for a long time [
1,
2,
3,
4]. It is proven to have a positive impact on the strength even at small concentrations below 0.5 wt.-%, as well as a raising effect on the recrystallization temperature, which directly leads to enhanced weldability and hot cracking behavior of the material. For Al alloys, the properties are linked to an intermetallic compound Al
Sc that shows little misfit with the Al matrix. However, extraction of Sc from its fluoridic or oxidic form to form a metal phase is challenging due to its high affinity to oxygen and halogenides, its high melting point, and also its low density. Special processes that have been used for the extraction are usually inefficient, costly, unscalable or are associated with low yields. Electrochemical processes may be applied, most notably as molten salt electrolysis. The principles of Hall–Heroult and the FCC process are under investigation for the application in Sc metallurgy. Low-temperature electrolysis in ionic liquids are of high potential but are not applicable for large production quantities, rather for applications such as coatings. Processing via carbothermic reduction does not provide a fully reduced product [
5]. By employing the techniques of vacuum induction melting (VIM), the parameters are easy to manipulate, reaction times are fast, and the input material may be versatile. The application of reduction processes via VIM is unusual but has been performed before [
6].
In 1937, the extraction of Sc metal from Sc precursors was first reported by Fischer et al. [
7]. Motivated by the interest in the physical and chemical properties of the rare earth element, calciothermy was applied to reduce ScF
. At this point in time, tantalum crucibles or sheets were not available; instead, oxidic crucibles were used, which led to limited success in the product purity. The principles were later advanced by Spedding et al. [
8], who used Ta crucibles for the reduction processes and subsequent refining steps, which resulted in 10 g Sc with minor impurities.
At the same time, research on the in situ production of Al-Sc master alloys by aluminothermic reduction of Sc salts has been studied by numerous research with increasing numbers of publications within the last few years. Most research has been carried out on master alloy production in the range of 2–10% Sc. The approach for most experimental campaigns is to dissolve ScF
in a suitable salt slag and to induce the reduction by bringing it in contact with Al [
9]. The strong advantage of this technology is the high Sc conversion yield (up to 91% for Al-Sc 2%) as well as the prevention of harmful off-gases. On the other hand, only low Sc concentrations may be incorporated in the Al melt and the resulting slag residue phase has little economic value. By using pure ScF
and Al as input material, higher Sc contents in the Al matrix are achievable. Data in the literature indicate that the maximum Sc content is governed by the process temperature—at a maximum temperature of 1040
C, Sc content reached 10% [
10]. However, processing with this system leads to the formation of fluoridic gases, mainly AlF and AlF
. A thorough analysis of the sublimates is given by Sokolova et al. [
11]. Used refractory materials are usually graphite [
9,
10] or Ta [
8,
12]. MgO refractory material was used at the beginning of the research on Sc reduction [
7]. Furnaces used for the reduction step are induction furnaces in open atmosphere [
9], a retort type reactor [
10], and resistance heating furnaces [
11,
12]. Hydrofluoric acid is used for ScF
synthesis in the classical approach. Since the handling of HF is implicating safety issues and an additional processing step is involved, the Sc extraction from Sc
O
is desired. Thus, metallothermic reduction of the oxide is thermochemically unfeasible for pure substances on a technological basis. Therefore, different approaches are described in the literature that mostly comprise in situ conversion of the oxide to fluoride. Harata et al. [
12] used a mixture of Al and Ca as reducing agent and produced an alloy with roughly 9 wt.-% Sc in the Al matrix, indicating a complete conversion of the oxide into the metallic form.
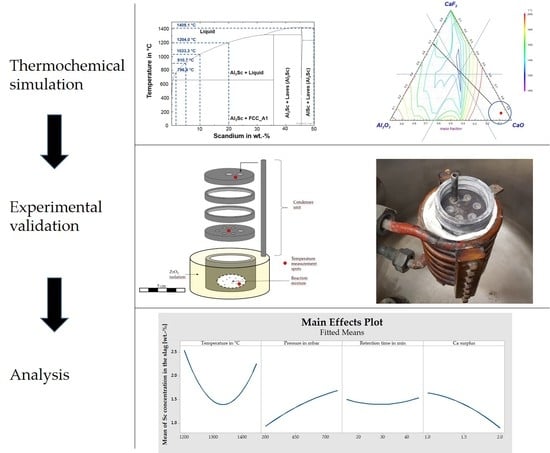
A novel approach for in situ extraction of Al-Sc master alloys is presented in the present paper. By combining the reduction of Al and Sc in one step, the alloying of Sc to Al melts, which are often paired with Sc losses in a dross phase, is surpassed. The by-product, a slag phase with a liquidus temperature of <1400 C, enables an all-liquid system that is treatable in conventional vacuum induction furnaces. The use of a Ta crucible allows fast heating rates and stability against the formed liquid phases. Reduction mechanism and pathways are proposed as a result of a thermogravimetric analysis and factorial design of experiment based VIM experiments.