Effect of Deposition Parameters on Microstructure of the Ti-Mg Immiscible Alloy Thin Film Deposited by Multi-Arc Ion Plating
Abstract
:1. Introduction
2. Experimental Part
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ali, M.; Hussein, M.A.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloys Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Balog, M.; Ibrahim, A.M.H.; Krizik, P.; Bajana, O.; Klimova, A.; Catic, A.; Schauperl, Z. Bioactive Ti + Mg composites fabricated by powder metallurgy: The relation between the microstructure and mechanical properties. J. Mech. Behav. Biomed. Mater. 2019, 90, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. J. Magnes. Alloy 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Esen, Z.; Dikici, B.; Duygulu, O.; Dericioglu, A.F. Titanium-magnesium based composites: Mechanical properties and in-vitro corrosion response in Ringer’s solution. Mater. Sci. Eng. A 2013, 573, 119–126. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Luo, T.; Song, M.; Wu, H.; Xiao, J.; Tan, Y.N.; Cheng, M.; Chen, B.; Niu, X.R.; et al. Powder metallurgical low-modulus Ti-Mg alloys for biomedical applications. Mater. Sci. Eng. C 2015, 56, 241–250. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, L.J.; An, Q.; Geng, L.; Wang, X.J.; Wang, S. Study on titanium-magnesium composites with bicontinuous structure fabricated by powder metallurgy and ultrasonic infiltration. J. Mech. Behav. Biomed. Mater. 2018, 81, 10–15. [Google Scholar] [CrossRef]
- Jiang, G.; Li, Q.; Wang, C.; Dong, J.; He, G. Fabrication of graded porous titanium–magnesium composite for load-bearing biomedical applications. Mater. Des. 2015, 67, 354–359. [Google Scholar] [CrossRef]
- Ouyang, S.; Huang, Q.; Liu, Y.; Ouyang, Z.X.; Ling, L.X. Powder metallurgical Ti-Mg metal-metal composites facilitate osteoconduction and osseointegration for orthopedic application. Bioact. Mater. 2019, 4, 37–42. [Google Scholar] [CrossRef]
- Ma, E. Alloys created between immiscible elements. Prog. Mater. Sci. 2005, 50, 413–509. [Google Scholar] [CrossRef]
- Gremaud, R.; Baldi, A.; Gonzalez-Silveira, M.; Dam, B.; Griessen, R. Chemical short-range order and lattice deformations in MgyTi1-yHx thin films probed by hydrogenography. Phys. Rev. B 2008, 77, 144204. [Google Scholar] [CrossRef]
- Murray, J.L. The Mg-Ti (Magnesium-Titanium) System. Bull. Alloy Phase Diagrams 1986, 7, 245–248. [Google Scholar] [CrossRef]
- Qi, Y.; Contreras, K.G.; Jung, H.D.; Kim, H.E.; Lapovok, R.; Estrin, Y. Ultrafine-grained porous titanium and porous titanium/magnesium composites fabricated by space holder-enabled severe plastic deformation. Mater. Sci. Eng. C 2016, 59, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Alfreider, M.; Jeong, J.; Esterl, R.; Oh, S.H.; Kiener, D. Synthesis and Mechanical Characterisation of an Ultra-Fine Grained Ti-Mg Composite. Materials (Basel) 2016, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.A.; Bruce, R.W.; Feng, J.; Fliflet, A.W. Consolidation of Blended Titanium/Magnesium Powders by Microwave Processing. Key Eng. Mater. 2013, 551, 73–85. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Froes, F.H. Nanocrystalline Titanium-Magnesium Alloys through Mechanical Alloying. J. Mater. Res. 1990, 5, 1880–1886. [Google Scholar] [CrossRef]
- Liang, G.; Schulz, R. Synthesis of Mg-Ti alloy by mechanical alloying. J. Mater. Sci. 2003, 38, 1179–1184. [Google Scholar] [CrossRef]
- Wardclose, C.M.; Partridge, P.G. The Production of Titanium Magnesium Alloys by Vapor Quenching. Mater. Lett. 1991, 11, 295–300. [Google Scholar] [CrossRef]
- Wei, X.S.; Xu, W.; Xia, K. Metastable orthorhombic phases at ambient pressure in mechanically milled pure Ti and Ti-Mg. Scr. Mater. 2014, 93, 32–35. [Google Scholar] [CrossRef]
- Asano, K.; Enoki, H.; Akiba, E. Synthesis process of Mg-Ti BCC alloys by means of ball milling. J. Alloys Compd. 2009, 486, 115–123. [Google Scholar] [CrossRef]
- Cai, X.C.; Song, J.; Yang, T.T.; Peng, Q.M.; Huang, J.Y.; Shen, T.D. A bulk nanocrystalline Mg-Ti alloy with high thermal stability and strength. Mater. Lett. 2018, 210, 121–123. [Google Scholar] [CrossRef]
- Song, G.-L.; Haddad, D. The topography of magnetron sputter-deposited Mg-Ti alloy thin films. Mater. Chem. Phys. 2011, 125, 548–552. [Google Scholar] [CrossRef]
- Leegwater, H.; Schut, H.; Egger, W.; Baldi, A.; Dam, B. Divacancies and the hydrogenation of Mg-Ti films with short range chemical order. Appl. Phys. Lett. 2010, 96, 121902. [Google Scholar] [CrossRef]
- Hwang, S.; Lim, S.H.; Han, S. Highly adhesive and bioactive Ti-Mg alloy thin film on polyether ether ketone formed by PIII & D technique. Appl. Surf. Sci. 2019, 471, 878–886. [Google Scholar]
- Guan, X.; Wang, Y.; Zhang, G.; Jiang, X.; Wang, L.P.; Xue, Q.J. Microstructures and properties of Zr/CrN multilayer coatings fabricated by multi-arc ion plating. Tribol. Int. 2017, 106, 78–87. [Google Scholar] [CrossRef]
- Bai, X.; Li, J.; Zhu, L. Structure and properties of TiSiN/Cu multilayer coatings deposited on Ti6Al4V prepared by arc ion plating. Surf. Coat. Technol. 2019, 372, 16–25. [Google Scholar] [CrossRef]
- Boxman, R.L.; Goldsmith, S. Macroparticle contamination in cathodic arc coatings: generation, transport and control. Surf. Coat. Technol. 1992, 52, 39–50. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, X.F.; Qiu, C.J.; Yang, X.; Liu, Y.H.; Wang, X.J. High temperature and high pressure flowing water corrosion resistance of multi-arc ion plating Cr/TiAlN and Cr/TiAlSiN coatings. Mater. Res. Express 2019, 6, 086449. [Google Scholar] [CrossRef]
- Harris, S.G.; Doyle, E.D.; Wong, Y.C.; Munroe, P.R.; Cairney, J.M.; Long, J.M. Reducing the macroparticle content of cathodic arc evaporated TiN coatings. Surf. Coat. Technol. 2004, 183, 283–294. [Google Scholar] [CrossRef]
- Cai, F.; Chen, M.H.; Li, M.X.; Zhang, S.H. Influence of negative bias voltage on microstructure and property of Al-Ti-N films deposited by multi-arc ion plating. Ceram. Int. 2017, 43, 3774–3783. [Google Scholar] [CrossRef]
- Sanchette, F.; Ducros, C.; Schmitt, T.; Steyer, P.; Billard, A. Nanostructured hard coatings deposited by cathodic arc deposition: From concepts to applications. Surf. Coat. Technol. 2011, 205, 5444–5453. [Google Scholar] [CrossRef]
- Mei, H.J.; Zhao, S.S.; Chen, W.; Wang, Q.M.; Liang, H.F. Microstructure and residual stress of TiN films deposited at low temperature by arc ion plating. T. Nonferr. Metal Soc. 2018, 28, 1368–1376. [Google Scholar] [CrossRef]
- Kimblin, C.W. Erosion and ionization in the cathode spot regions of vacuum arcs. J. Appl. Phys. 1973, 44, 3074–3081. [Google Scholar] [CrossRef]
- Asano, K.; Enoki, H.; Akiba, E. Synthesis of HCP, FCC and BCC structure alloys in the Mg-Ti binary system by means of ball milling. J. Alloys Compd. 2009, 480, 558–563. [Google Scholar] [CrossRef]
- Gohil, S.; Banerjee, R.; Bose, S.; Ayyub, P. Influence of synthesis conditions on the nanostructure of immiscible copper–silver alloy thin films. Scr. Mater. 2008, 58, 842–845. [Google Scholar] [CrossRef]
- Cai, F.; Zhang, S.H.; Li, J.L.; Chen, Z.; Li, M.X.; Wang, L. Effect of nitrogen partial pressure on Al-Ti-N films deposited by arc ion plating. Appl. Surf. Sci. 2011, 258, 1819–1825. [Google Scholar] [CrossRef]
- Li, L.; Lv, G.H.; Yang, S.Z. Effects of nitrogen partial pressure in Ta-N films grown by the cathodic vacuum arc technique. J. Phys. D: Appl. Phys. 2013, 46, 285202. [Google Scholar] [CrossRef]
- Lu, J.Q.; Yoon, J.H.; Cho, T.Y.; Joo, Y.K.; Lee, C.G. Effects of pressure on metal atom transport and plasma properties during arc ion plating of TiAlN. Met. Mater. Int. 2007, 13, 123–128. [Google Scholar] [CrossRef]
- Fu, B.; Thompson, G.B. In situ growth stresses during the phase separation of immiscible FeCu thin films. Appl. Surf. Sci. 2010, 257, 1500–1505. [Google Scholar] [CrossRef]
- Nag, S.; Mahdak, K.C.; Devaraj, A.; Gohil, S.; Ayyub, P.; Banerjee, R. Phase separation in immiscible silver-copper alloy thin films. J. Mater. Sci. 2009, 44, 3393–3401. [Google Scholar] [CrossRef]

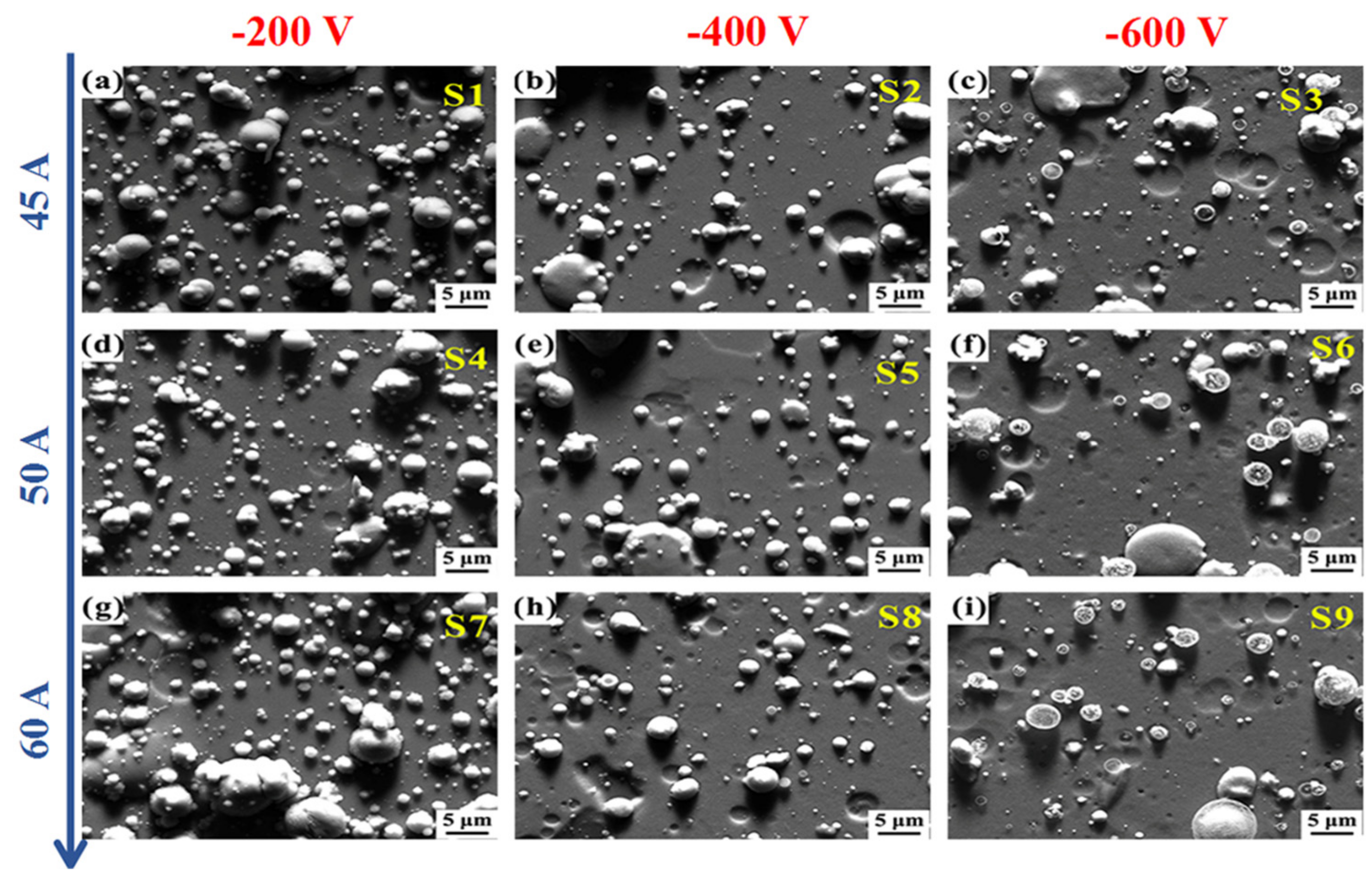


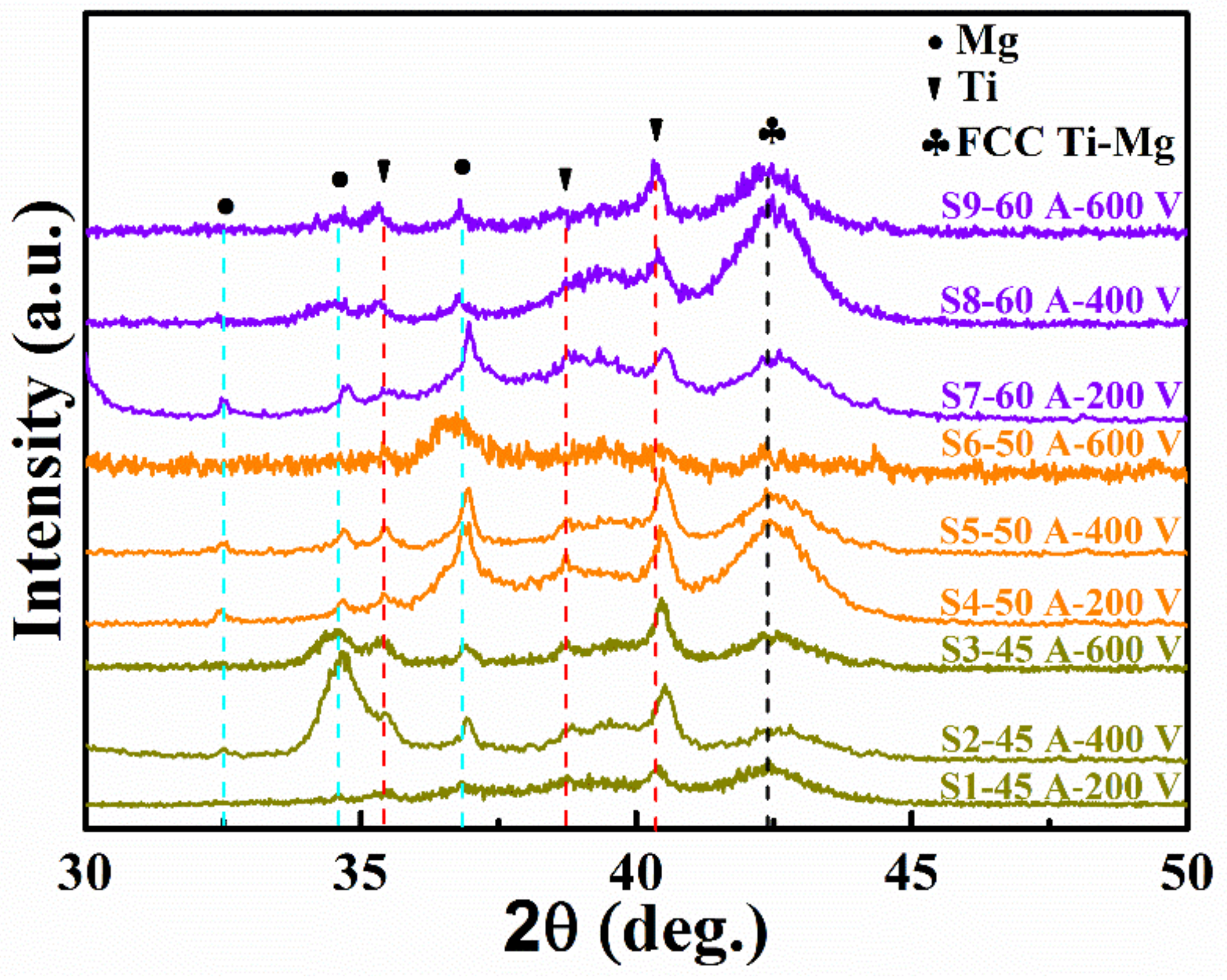





| Sample | Mg Target Current (A) | Bias Voltage (V) | Pressure (Pa) | Thickness (nm) | Deposition Rate (nm/min) | Ti (at. %) | Mg (at. %) |
|---|---|---|---|---|---|---|---|
| S1 | 45 | −200 | 0.8 | 1214 | 13.5 | 86.83 ± 0.73 | 13.17 ± 0.72 |
| S2 | 45 | −400 | 0.8 | 1298 | 14.4 | 65.23 ± 0.33 | 34.77 ± 0.31 |
| S3 | 45 | −600 | 0.8 | 1045 | 11.6 | 57.11 ± 0.09 | 42.89 ± 0.11 |
| S4 | 50 | −200 | 0.8 | 1317 | 14.6 | 61.08 ± 0.53 | 38.92 ± 0.53 |
| S5 | 50 | −400 | 0.8 | 1400 | 15.6 | 40.67 ± 0.51 | 59.33 ± 0.52 |
| S6 | 50 | −600 | 0.8 | 1231 | 13.7 | 50.22 ± 0.16 | 49.78 ± 0.19 |
| S7 | 60 | −200 | 0.8 | 1366 | 15.2 | 56.48 ± 0.28 | 43.52 ± 0.25 |
| S8 | 60 | −400 | 0.8 | 1400 | 15.6 | 22.35 ± 0.44 | 77.65 ± 0.45 |
| S9 | 60 | −600 | 0.8 | 1062 | 11.8 | 34.89 ± 0.62 | 65.11 ± 0.65 |
| S10 | 45 | −200 | 3.2 | 1248 | 13.9 | 43.85 ± 0.04 | 56.15 ± 0.09 |
| S11 | 45 | −200 | 5.5 | 1450 | 16.1 | 54.94 ± 0.35 | 45.06 ± 0.33 |
| S12 | 50 | −200 | 3.2 | 1366 | 15.2 | 28.93 ± 0.23 | 71.07 ± 0.24 |
| S13 | 50 | −200 | 5.5 | 1619 | 18.0 | 19.03 ± 0.14 | 80.97 ± 0.14 |
| S14 | 60 | −200 | 3.2 | 1821 | 20.2 | 11.50 ± 0.16 | 88.50 ± 0.19 |
| S15 | 60 | −200 | 5.5 | 1568 | 17.4 | 18.39 ± 0.27 | 81.61 ± 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Dong, Y.; Qu, D.; Ma, T.; Shen, J. Effect of Deposition Parameters on Microstructure of the Ti-Mg Immiscible Alloy Thin Film Deposited by Multi-Arc Ion Plating. Metals 2019, 9, 1229. https://doi.org/10.3390/met9111229
Wei X, Dong Y, Qu D, Ma T, Shen J. Effect of Deposition Parameters on Microstructure of the Ti-Mg Immiscible Alloy Thin Film Deposited by Multi-Arc Ion Plating. Metals. 2019; 9(11):1229. https://doi.org/10.3390/met9111229
Chicago/Turabian StyleWei, Xianshun, Yue Dong, Dongdong Qu, Tiancai Ma, and Jun Shen. 2019. "Effect of Deposition Parameters on Microstructure of the Ti-Mg Immiscible Alloy Thin Film Deposited by Multi-Arc Ion Plating" Metals 9, no. 11: 1229. https://doi.org/10.3390/met9111229





