Electron Microscopy Investigations of А356 Alloy Modified with Nanoparticles
Abstract
:1. Introduction
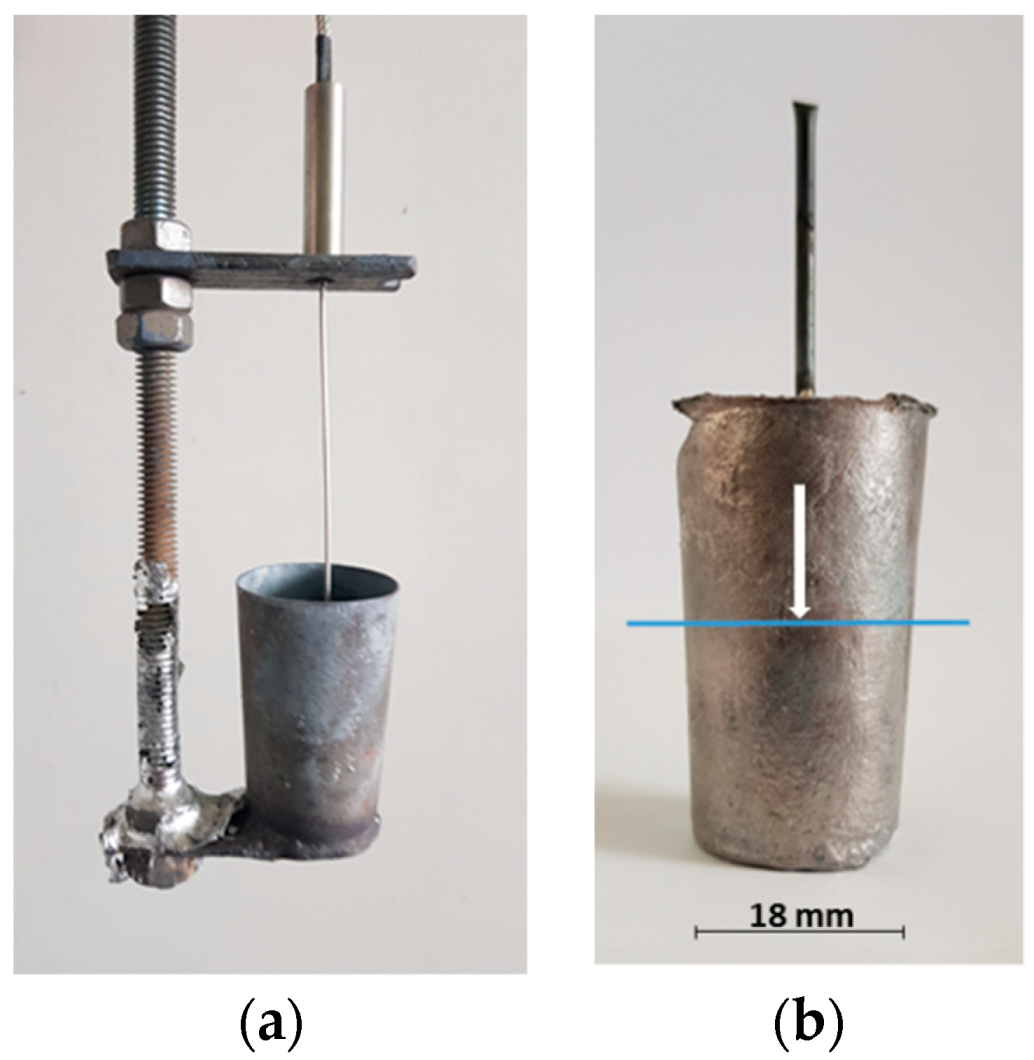
2. Materials and Methods
3. Results
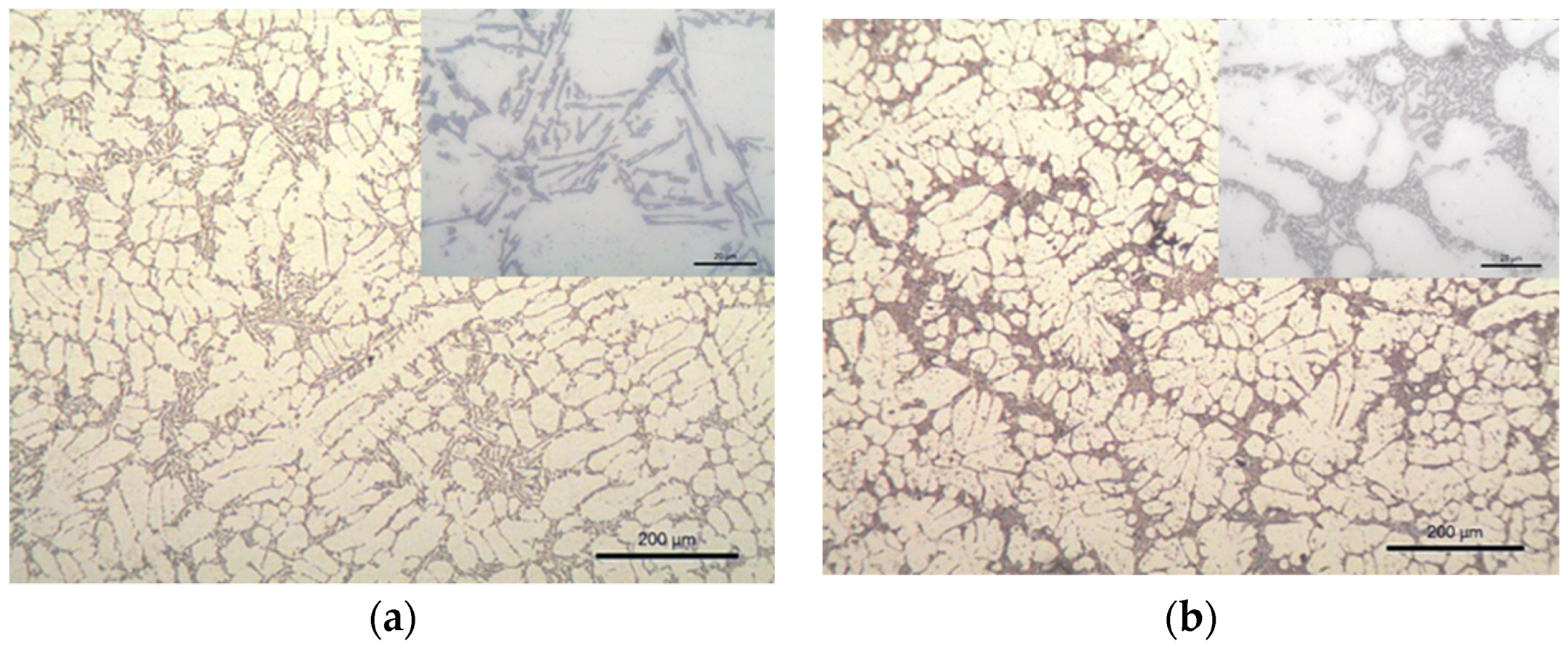
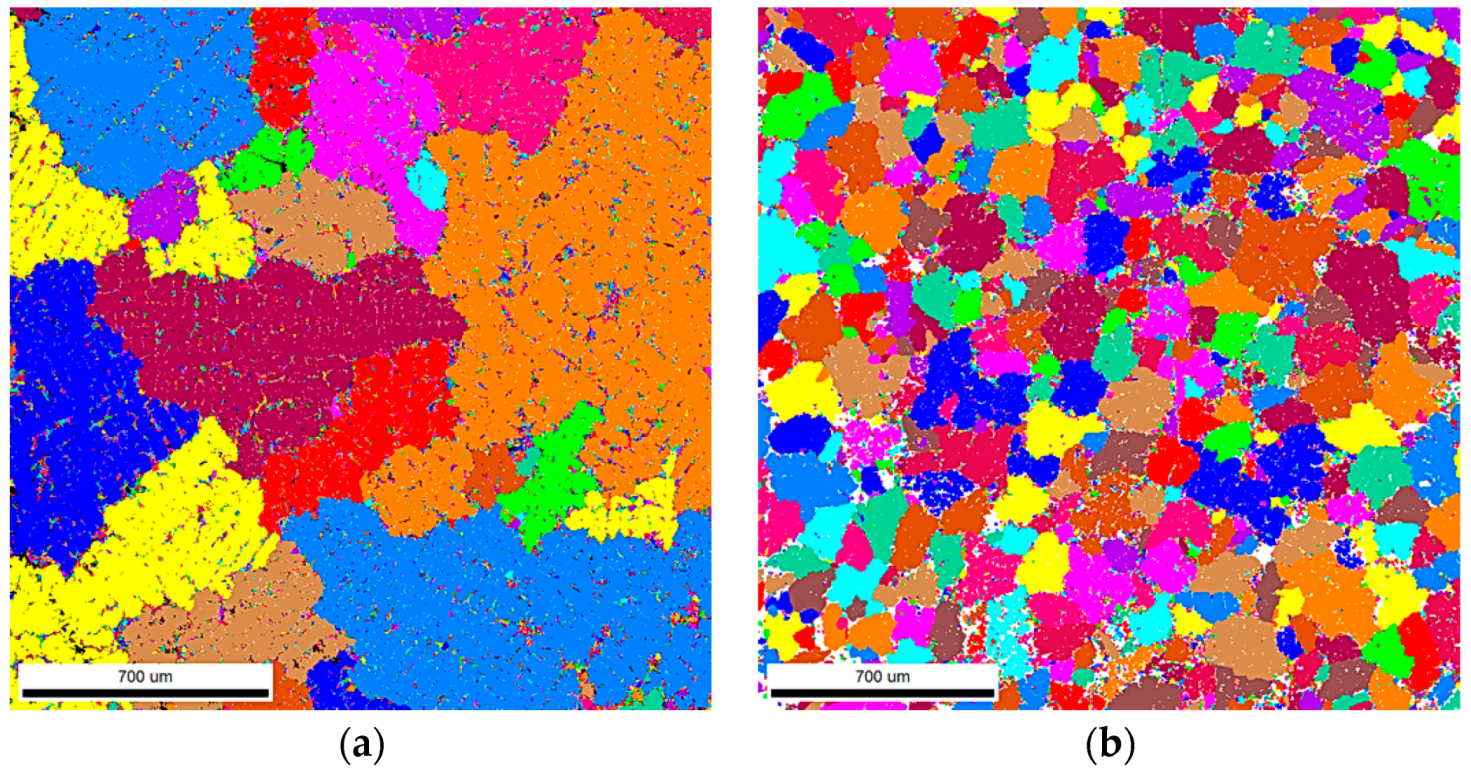
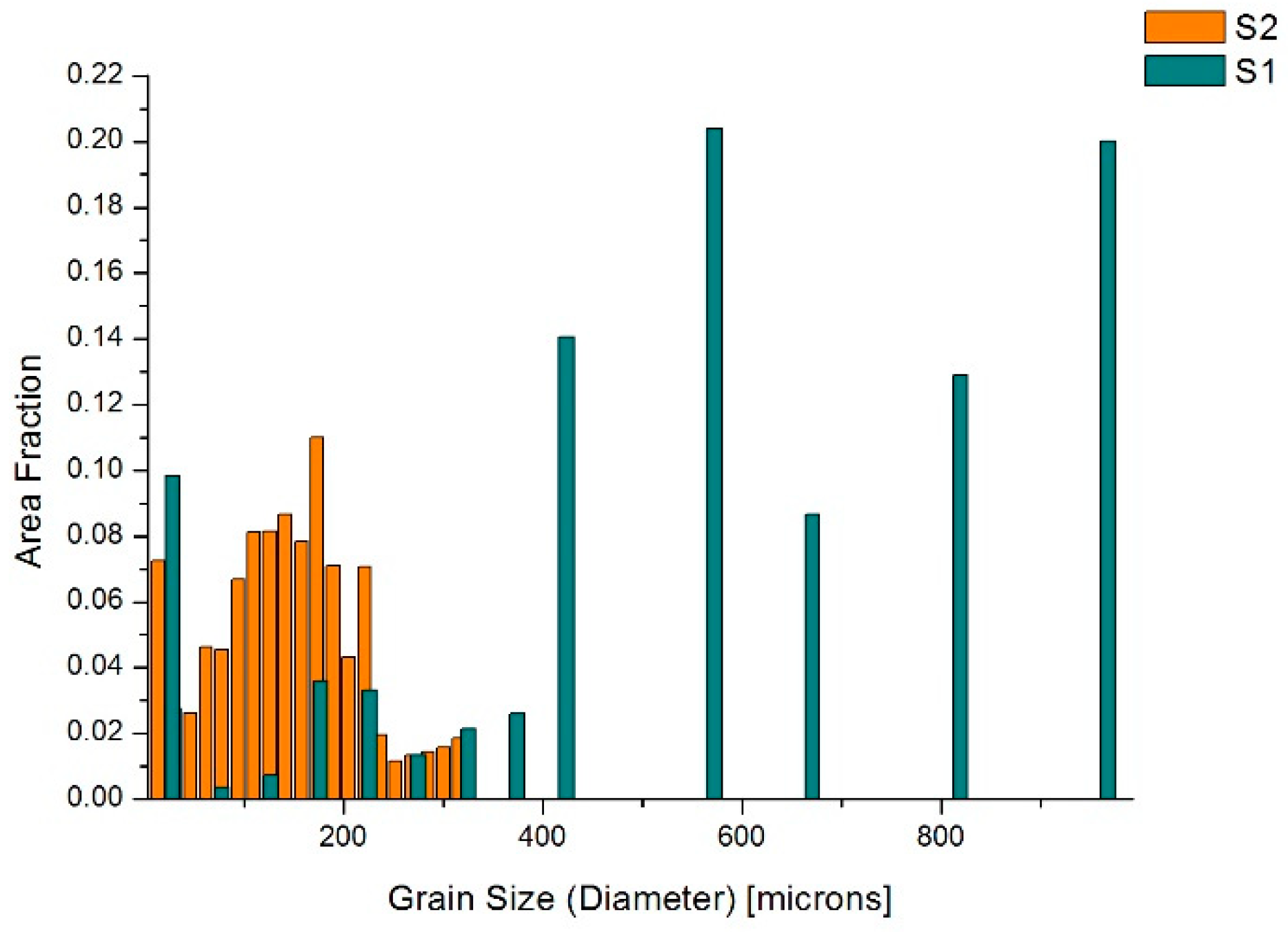
3.1. Samples S1 and S2 (Modification with SiC Nanoparticles)
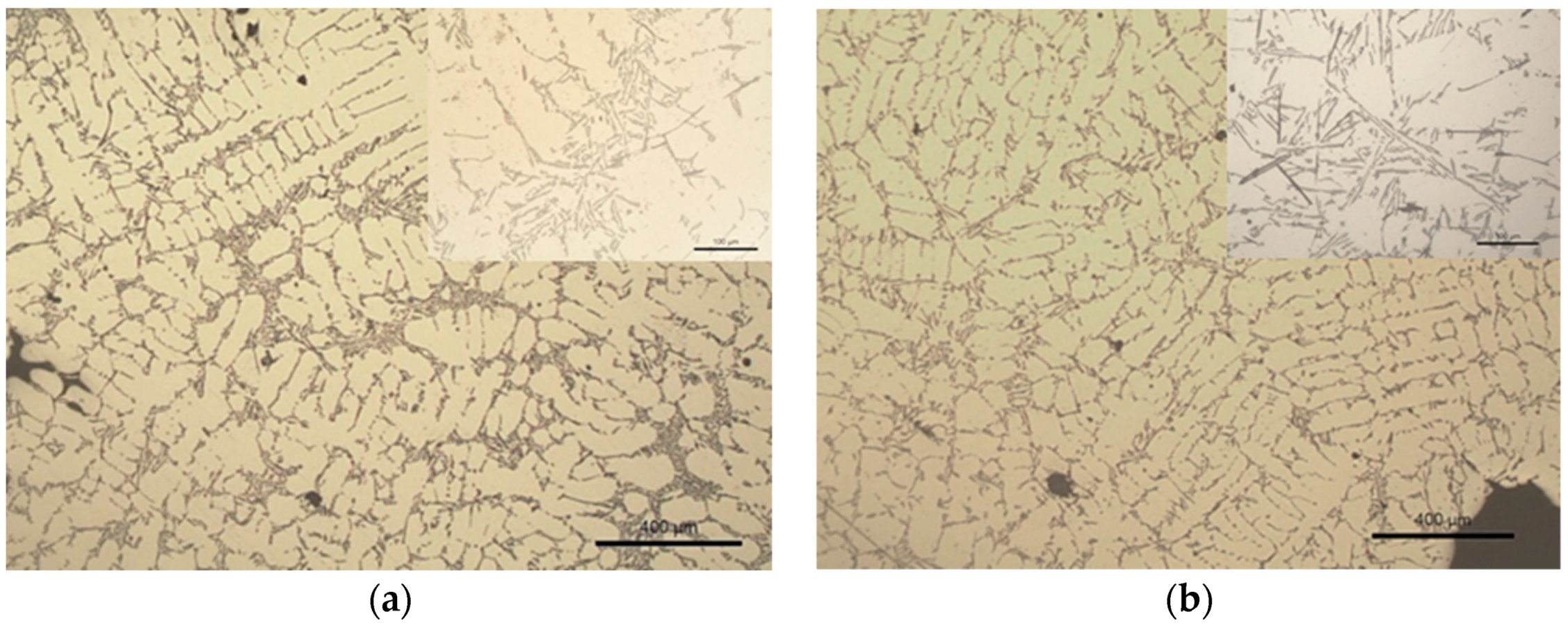
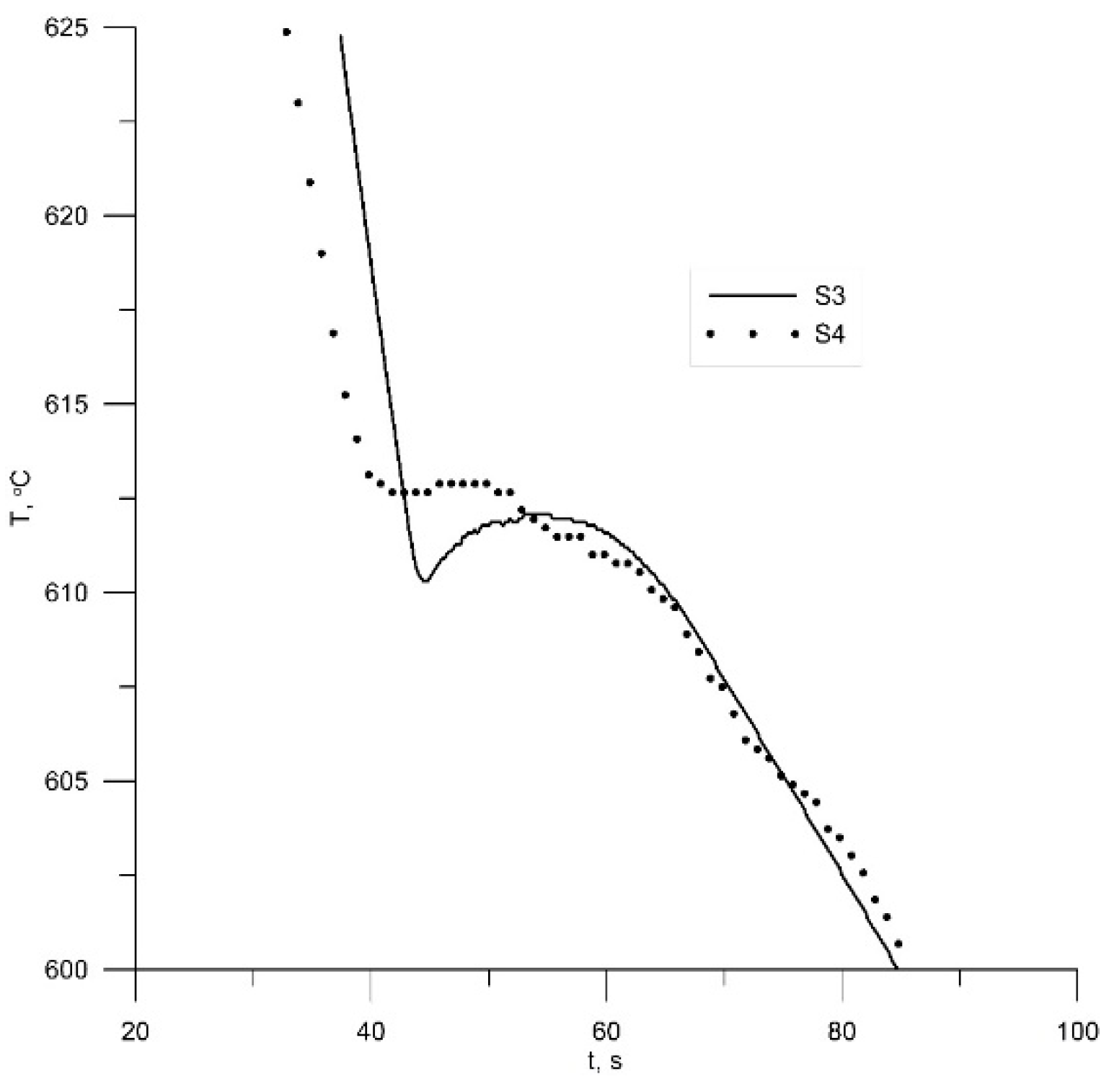
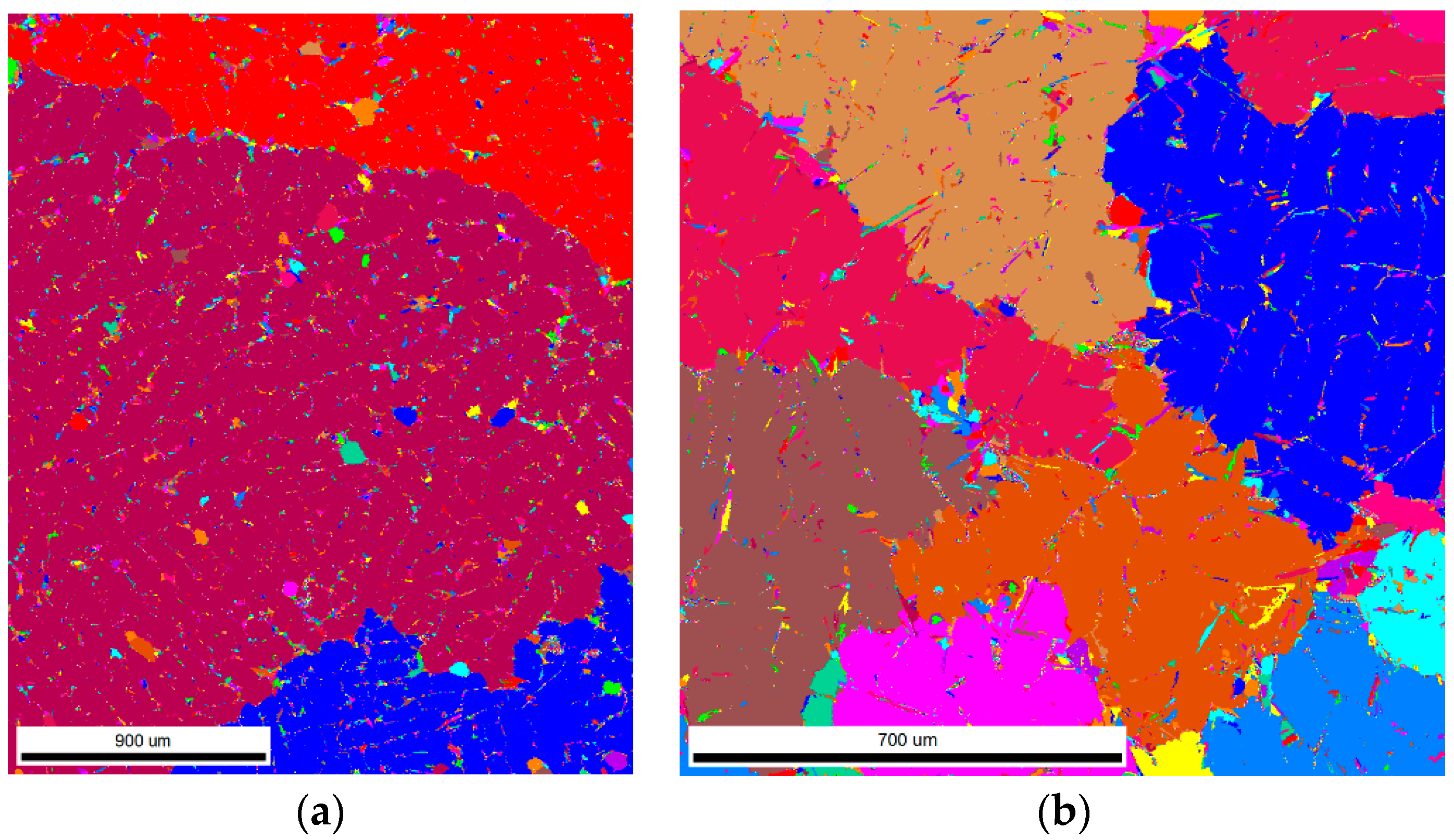
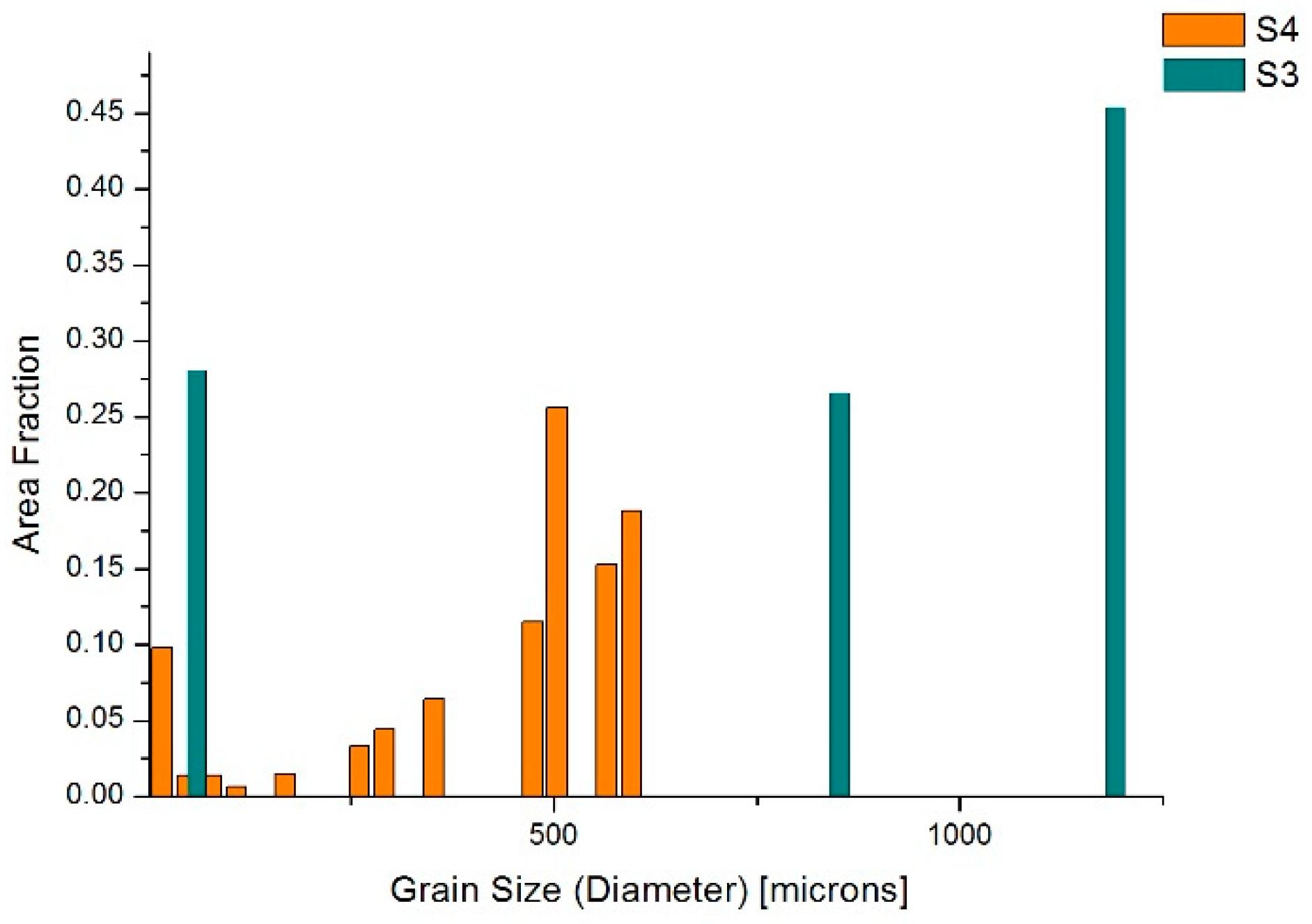
3.2. Samples S3 and S4 (Modification with TiCN Nanoparticles)
3.3. STEM Analysis of Sample S2 (Modification with SiC Nanoparticles)
4. Discussion
4.1. Samples S1 and S2—LM and EBSD Characterization
4.2. Samples S3 and S4—LM, Thermal Analyses and EBSD Characterization
4.3. STEM Analysis of Sample S2
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Polmear, I.; StJohn, D.; Nie, J.F.; Qian, M. Light Alloys: Metallurgy of the Light Metals; Butterworth&Heinemann: Oxford, UK, 2017. [Google Scholar]
- Gruzleski, G.; Closset, J. The Treatment of Liquid AL-Si Alloys; AFS: Des Plaines, IL, USA, 1990; Volume 97, pp. 107–126. [Google Scholar]
- Krushenko, G.G.; Torshilova, S.I.; Krushenko, S.G. Complex modification of hypoeutectic Al-Si alloys. Cast. Prod. 1984, 2, 32. (In Russian) [Google Scholar]
- Lu, J.; Chen, B.; Liu, X.; Yang, F.; Robinson, I. 3D microstructure reconstruction of casting aluminum alloy based on serial block-face scanning electron microscopy. J. Alloy. Compd. 2019, 778, 721–730. [Google Scholar] [CrossRef]
- Rao, J.; Zhang, J.; Liu, R.; Zheng, J.; Yin, D. Modification of eutectic Si and the microstructure in an Al-7Si alloy with barium addition. Mater. Sci. Eng. A 2018, 728, 72–79. [Google Scholar] [CrossRef]
- Amirkhanlou, S.; Shouxun, J. Casting lightweight stiff aluminum alloys: A review. Crit. Rev. Solid State Mater. Sci. 2019, 1–16. [Google Scholar] [CrossRef]
- Lee, M.S.; Terry, B.S. Effects of processing parameters on aluminide morphology in aluminum grain refining master alloys. Mater. Sci. Technol. 1991, 7, 608–612. [Google Scholar] [CrossRef]
- Rao, A.A.; Murty, B.S.; Chakraborty, M. The role of zirconium and impurities in grain refinement of aluminium with AI-Ti-B. Mater. Sci. Technol. 1997, 13, 769–777. [Google Scholar] [CrossRef]
- Nogita, K.; Dahle, A.K. Eutectic growth mode in strontium, antimony and phosphorous modified Al–Si foundry alloys. Mater. Trans. 2001, 42, 393–396. [Google Scholar] [CrossRef]
- Lu, S.-Z.; Hellawell, A. The mechanism of silicon modification in aluminum–silicon alloys: Impurity induced twinning. Metall. Trans. A 1987, 18, 1721–1733. [Google Scholar] [CrossRef]
- Abdollahi, A.; Gruzleski, J.E. An evaluation of calcium as a eutectic modifier in A357 alloy. Int. J. Cast Met. Res. 1998, 11, 145–155. [Google Scholar] [CrossRef]
- Szajnar, J.; Wróbel, T. Inoculation of aluminum with titanium and boron addition. J. Achiev. Mater. Manuf. Eng. 2007, 23, 51–54. [Google Scholar]
- Kwon, Y.D.; Lee, Z.H. The effect of grain refining and oxide inclusion on the fluidity of Al-4.5Cu-0.6Mn and A356 Alloys. Mater. Sci. Eng. 2003, 360, 372–376. [Google Scholar] [CrossRef]
- Knuutinen, A.; Nogita, K.; McDonald, S.D.; Dahle, A.K. Modification of Al–Si alloys with Ba, Ca, Y and Yb. J. Light Met. 2001, 1, 229–240. [Google Scholar] [CrossRef]
- Sritharan, T.; Li, H. Influence of titanium to boron ratio on the ability to grain refine aluminum-silicon alloys. Mater. Process. Technol. 1997, 63, 585–589. [Google Scholar] [CrossRef]
- Spittle, J.A.; Sadli, S. Effect of alloy variables on grain refinement of binary aluminum alloys with Al–Ti–B. Mater. Sci. Technol. 1995, 11, 533–537. [Google Scholar] [CrossRef]
- Spittle, J.A.; Keeble, J.M.; Meshhedani, A.I. The Grain Refinement of Al-Si Foundry Alloys; TMS Light Metals: Pittsburgh, PA, USA, 1997; pp. 795–800. [Google Scholar]
- Sigworth, G.K.; Guzowaski, M.M. Grain refining of hypo-eutectic Al–Si alloys. AFS Trans. 1985, 93, 907–912. [Google Scholar]
- Jones, G.P.; Pearson, J. Factors affecting the grain-refinement of aluminum using titanium and boron additives. Metall. Trans. B 1976, 7, 223–234. [Google Scholar] [CrossRef]
- Kori, S.A.; Murty, B.S.; Chakraborty, M. Development of an efficient grain refiner for Al–7Si alloy and its modification with strontium. Mater. Sci. Eng. A 2000, 283, 94–104. [Google Scholar] [CrossRef]
- Rao, A.K.P.; Kumar, G.S.V.; Reddy, N.S.; Venkateswarulu, K.; Kori, S.A.; Prasad, K.V.S.; Rao, A.A. Grain Refinement and Modification of Al Alloys; Department of Metallurgical and Materials Engineering, Indian Institute of Technology Madras: Chennai, India, 2002. [Google Scholar]
- Saburov, V.P. Choice of Modificators and Practice of Cast Alloys Modification; Publishing house OmPI: Omsk, Russia, 1984; p. 98. (In Russian) [Google Scholar]
- Schaffer, P.L.; Dahle, A.K. Settling behavior of different grain refiners in aluminum. Mater. Sci. Eng. A 2005, 413, 373–378. [Google Scholar] [CrossRef]
- Kalinina, N.E.; Kavatz, O.A.; Kalinin, V.T. Modification influence of mechanical and corrosion properties of cast aluminum alloys, In Hightemperature Technique Problems; Dagestan State University Publishing House: Makhachkala, Russia, 2008; pp. 57–61. (In Russian) [Google Scholar]
- Krushenko, G.G.; Joukov, M.F.; Kornilov, A.A. Method for alloys modification. J. Invent. 1981, 23. (In Russian) [Google Scholar]
- Balashov, B.A.; Krushenko, G.G.; Vasilenko, Z.A.; Anikina, B.I. Dependence of Al grains sizes of method for master alloy Al-Zr production. Non-Ferr. Met. 1989, 5, 92–93. (In Russian) [Google Scholar]
- Basavakumara, K.G.; Mukunda, P.G.; Chakraborty, M. Influence of grain refinement and modification on microstructure and mechanical properties of Al–7Si and Al–7Si–2.5Cu cast alloys. Mater. Charact. 2008, 59, 283–289. [Google Scholar] [CrossRef]
- Davies, I.G.; Dennis, J.M.; Hellawell, A. The nucleation of aluminum grains in alloys of aluminum with titanium and boron. Metall. Trans. 1970, 1, 275–280. [Google Scholar]
- Mazahery, A.; Shabani, M.O. Microstructural and abrasive wear properties of SiC reinforced aluminum-based composite produced by compocasting. Trans. Nonferrous Met. Soc. China 2013, 23, 1905–1914. [Google Scholar] [CrossRef]
- Nagaral, M.; Bharath, V.; Auradi, V. Effect of Al2O3 Particles on Mechanical and Wear Properties of 6061al Alloy Metal Matrix Composites. J. Mater. Sci. Eng. 2013, 2, 120. [Google Scholar] [CrossRef]
- Ramesh, M.; Karthikeyan, T.; Kumaravel, A.; Kumaran, S.S.; Raja, V.L. Metallurgical and Mechanical Behavior of Basalt Particulate Reinforced Aluminum (A356) Matrix Composites. Eur. J. Sci. Res. 2012, 95, 524–532. [Google Scholar]
- Shin, J.H.; Jeon, J.H.; Bae, D.H. Microstructure refining of aluminum alloys using aluminothermic reaction with ZnO nanoparticles. Mater. Lett. 2015, 151, 96–99. [Google Scholar] [CrossRef]
- Borodianskiy, K. Metallurgical processes in AlSi alloy improved by WC nanoparticles. In Proceedings of the 15-th Israeli-Russian Bi-National Workshop 2016, The Optimization of Composition, Structure and Properties of Metals, Oxides, Composites, Nano and Amorphous Materials, Ekaterinburg, Russia, 26–30 September 2016; pp. 10–18. [Google Scholar]
- Mohsen, H.; Omid, M.; Mohammadian-Semnani, H. Evaluation of microstructural and mechanical properties of A356 composite strengthened by nanocrystalline V8C7-Al2O3 particles synthesized through mechanically activated sintering. J. Alloy. Compd. 2019, 782, 995–1007. [Google Scholar]
- Ferreira, V.; Egizabal, P.; Popov, V.; de Cortázar, M.G.; Irazustabarrena, A.; López-Sabirón, A.M.; Ferreira, G. Lightweight automotive components based on nanodiamond-reinforced aluminum alloy: A technical and environmental evaluation. Diam. Relat. Mater. 2019, 92, 174–186. [Google Scholar] [CrossRef]
- Valikhov, V.; Kahidze, N.; Khrustalyov, A.; Zhukov, I.; Vorozhtsov, A. Investigation of structure, mechanical properties and crystallization of aluminum alloys containing aluminum oxide nanoparticles. MATEC Web Conf. 2018, 243, 22. [Google Scholar] [CrossRef]
- Veeraiah, G.; Santosh Kumar, M.S.S.; Vimal Varma, V.V.; Sudhakar, I. Synthesis of nano particles reinforced composites using A356 aluminium alloy as matrix for brake shoes using stir casting route. IOP Conf. Ser. Mater. Sci. Eng. 2018, 455, 012077. [Google Scholar] [CrossRef]
- Afkham, Y.; Khosroshah, R.A.; Rahimpour, S.; Aavani, C.; Brabazon, D.; Mousavian, R.T. Enhanced mechanical properties of in situ aluminum matrix composites reinforced by alumina nanoparticles. Arch. Civ. Mech. Eng. 2018, 18, 215–226. [Google Scholar] [CrossRef]
- Hashim, F.A.; Abdulkader, N.J.; Jasim, N.S. Effect of nano BN Addition on the Properties of an Aluminum Metal Matrix Composite. Eng. Technol. J. 2018, 36, 196–695. [Google Scholar] [CrossRef]
- Yijie, Z.; Ji, S. Shouxun, Reinforcement of TiB2 Nanoparticles in Aluminum Piston Alloys for High Performance at Elevated Temperature. Nanomanuf. Metrol. 2018, 1, 248–251. [Google Scholar] [CrossRef]
- Ibrahim, S.I.; Tawfeeq, A.F. The Response of Some Properties of (Al-Si-Mg) Alloy to Nano-Ceramic Materials’ Addition. J. Eng. (Eng. J.) 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Borodianskiy, K.; Kossenko, A.; Zinigrad, M. Improvement of the Mechanical Properties of Al-Si Alloys by TiC Nanoparticles. Metall. Mater. Trans. A 2013, 44, 4948–4953. [Google Scholar] [CrossRef]
- Dimitrova, R.; Kuzmanov, P.; Lazarova, R.; Manolov, V. Investigation of nanopowders application in metal casting. Adv. Mater. Res. 2013, 629, 284–291. [Google Scholar] [CrossRef]
- Kuzmanov, P.; Dimitrova, R.; Lazarova, R.; Cherepanov, A.; Popov, S.; Petrov, R.; Manolov, V. Investigation of the structure and mechanical properties of castings of alloy AlSi7Mg, cast iron GG15 and GG25 and steel GX120Mn12, modified by nanosized powders. Proc. Inst. Mech. Eng. Part N J. Nanoeng. Nanosyst. 2014, 228, 11–18. [Google Scholar] [CrossRef]
- Dimitrova, R.; Kuzmanov, P.; Stanev, S.; Lazarova, R.; Manolov, V. Investigation of metal alloys modified with nano-sized particles. J. Mater. Sci. Technol. 2012, 20, 98–116. [Google Scholar]
- Bojanova, N.; Dimitrova, R.; Velikov, A.; Stanev, S. Effect of nanomodifiers on the density and the porosity of AlSi7Mg alloy castings. J. Mach. Technol. Mater. 2013, 3, 56–59. [Google Scholar]
- Lazarova, R.; Bojanova, N.; Dimitrova, R.; Panov, I.; Manolov, V. Influence of nanoparticles introducing in the melt of aluminum alloys on castings microstructure and properties. Int. J. Met. 2016, 10, 466–476. [Google Scholar] [CrossRef]
- Kaleicheva, J.; Mishev, V.; Avdeev, G.; Karaguiozova, Z.; Dineva, B. Influence of nanoadditives on the structure and properties of austempered ductile irons. In Proceedings of the European Conference on Heat Treatment and 21st IFHTSE Congress, Munich, Germany, 12–15 May 2014. [Google Scholar]
- Gobre, V.V.; Tkatchenko, A. Scaling laws for van der Waals interactions in nanostructured materials. Nat. Commun. 2013, 4, 2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Si | Mg | Ti | Fe | Cu | Mn | Al |
|---|---|---|---|---|---|---|
| 7.50 | 0.30 | 0.02 | 0.39 | 0.05 | 0.03 | bal. |
| Sample | SDAS, µm | HV0.01 |
|---|---|---|
| S1—non-modified | 46.7 | 598.2 |
| S2—modified with 0.1 wt.% SiC+Cu | 17.7 (↓62%) | 675.7 (↑13%) |
| Sample | SDAS, µm | HV0.01 |
|---|---|---|
| S3—non-modified | 47.83 | 638.2 |
| S4—modified with 0.04 wt.% TiCN | 44.12 (↓7.8%) | 660.6 (↑3.5%) |
| Memo | C | O | Mg | Al | Si | Fe | Cu | Total (at.%) Accuracy |
|---|---|---|---|---|---|---|---|---|
| 28 | 1.33 | 6.87 | 0.18 | 11.04 | 1.31 | 0.37 | 78.9 | 100 |
| 29 | 2 | 3.71 | 0 | 12.87 | 1.45 | 0.48 | 79.49 | 100 |
| 30 | 2.37 | 11.89 | 0.23 | 12.85 | 1.68 | 0.49 | 70.49 | 100 |
| 31 | 1.43 | 12.25 | 0.42 | 56.91 | 1.73 | 0.28 | 26.98 | 100 |
| 32 | 0 | 7.6 | 0.15 | 88.85 | 1.6 | 0.24 | 1.56 | 100 |
| Base alloy | - | - | 0.3336 | 92.187 | 7.2417 | 0.1899 | 0.0214 | 99.9736 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrova, R.; Petrov, R.; Kuzmanov, P.; Velikov, А.; Manolov, V. Electron Microscopy Investigations of А356 Alloy Modified with Nanoparticles. Metals 2019, 9, 1294. https://doi.org/10.3390/met9121294
Dimitrova R, Petrov R, Kuzmanov P, Velikov А, Manolov V. Electron Microscopy Investigations of А356 Alloy Modified with Nanoparticles. Metals. 2019; 9(12):1294. https://doi.org/10.3390/met9121294
Chicago/Turabian StyleDimitrova, Rositza, Roumen Petrov, Pavel Kuzmanov, Аngel Velikov, and Valentin Manolov. 2019. "Electron Microscopy Investigations of А356 Alloy Modified with Nanoparticles" Metals 9, no. 12: 1294. https://doi.org/10.3390/met9121294





