A Study on the Wettability of Ion-Implanted Stainless and Bearing Steels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ion-Implanted Steels
2.3. Characterizations
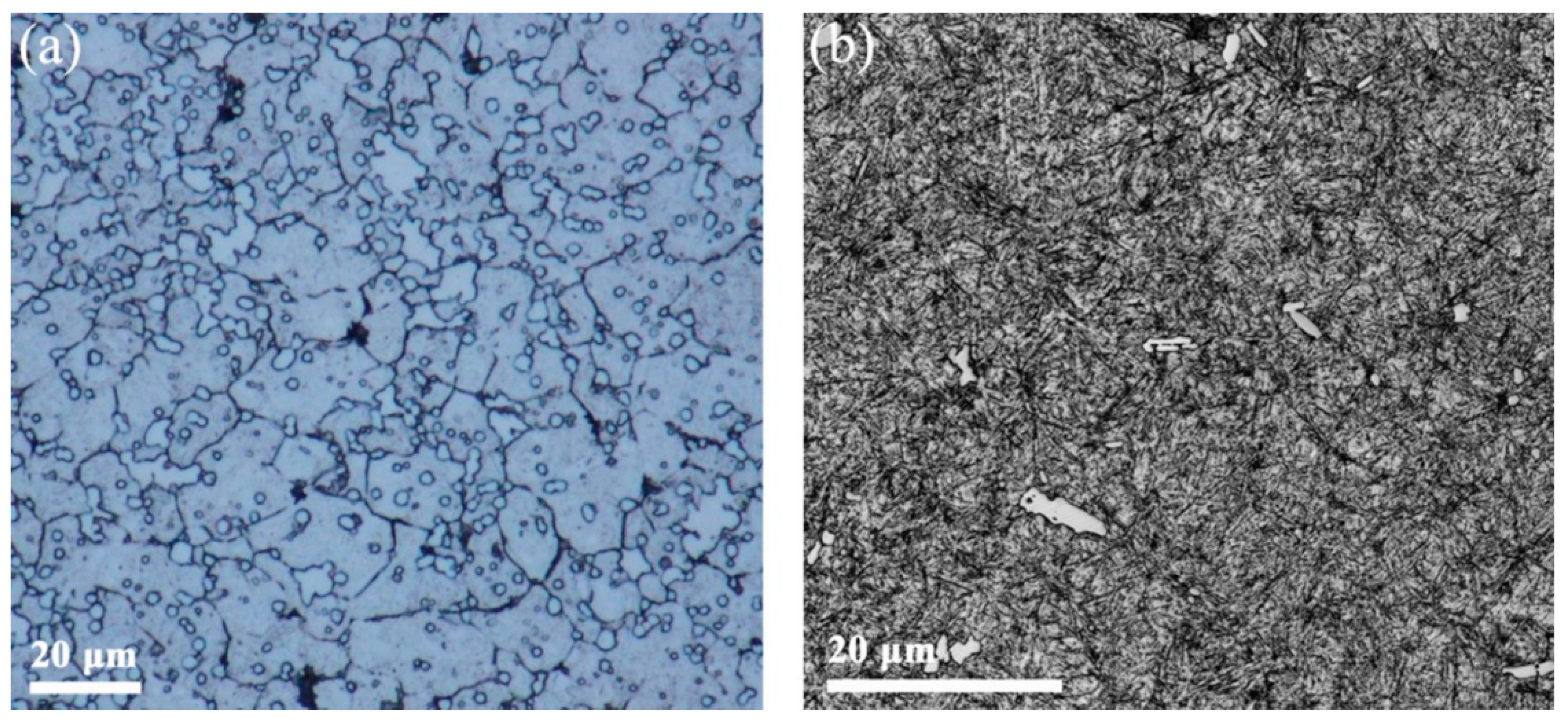
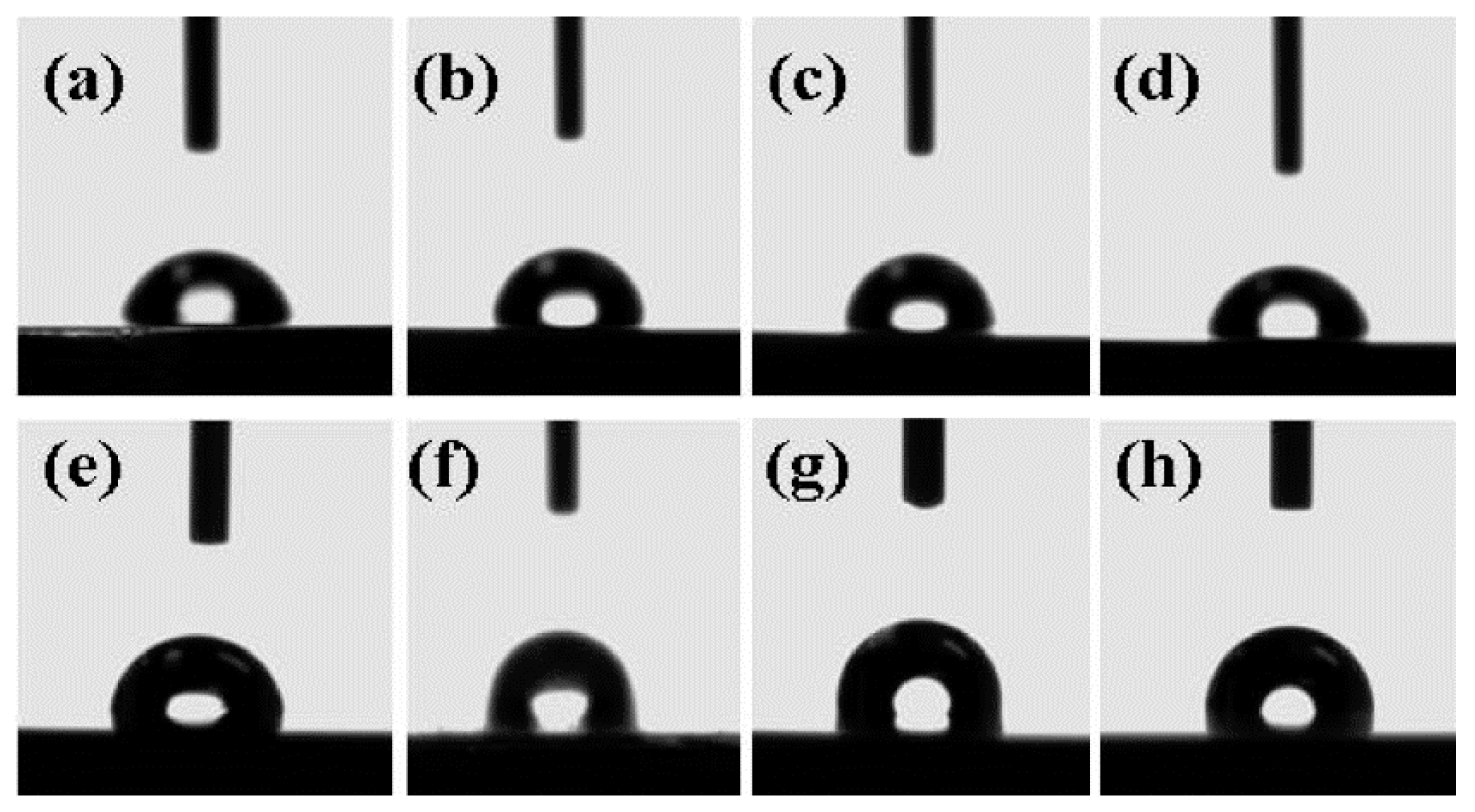
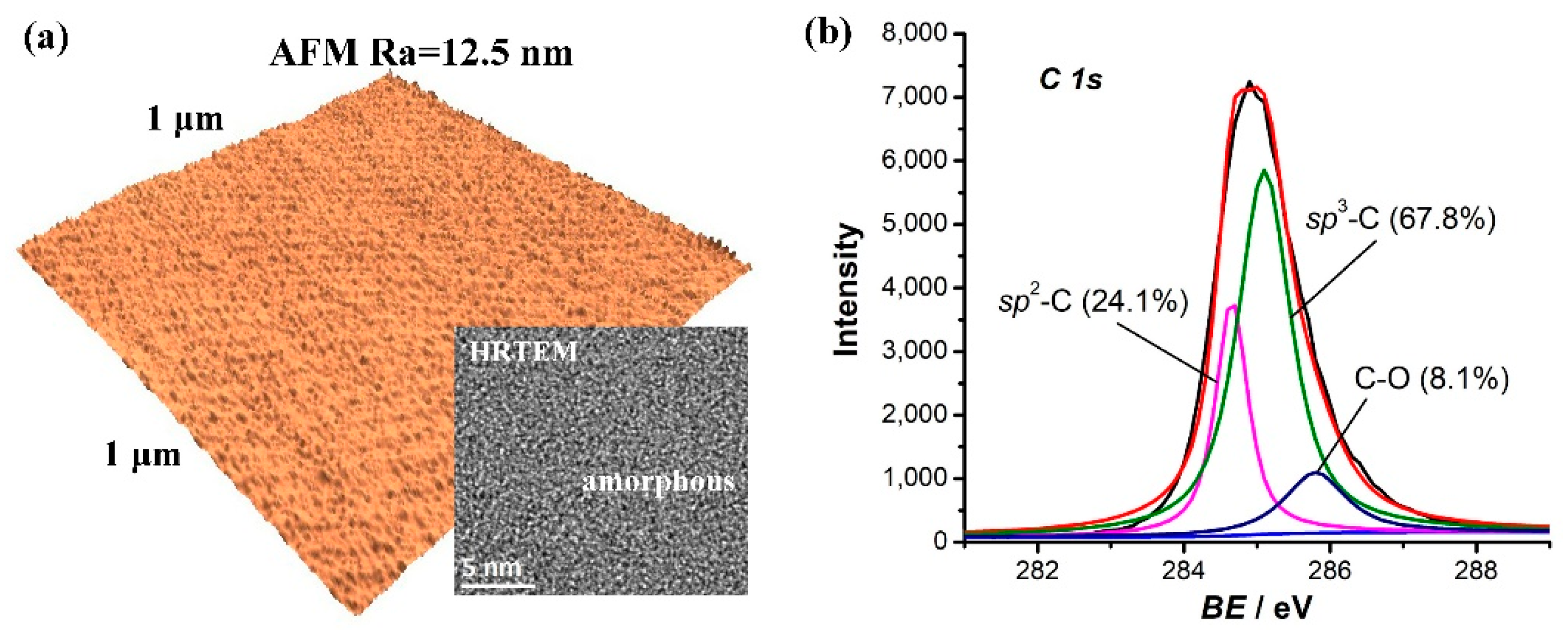
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, X.B.; Yan, W.; Xu, D.K.; Yan, M.C.; Yang, C.G.; Shan, Y.Y.; Yang, K. Microbial corrosion resistance of a novel Cu-bearing pipeline steel. J. Mater. Sci. Technol. 2018, 34, 2480–2491. [Google Scholar] [CrossRef]
- Khan, F.A. The effect of soaking on segregation and primary-carbide dissolution in ingot-cast bearing steel. Metals 2018, 8, 800. [Google Scholar] [CrossRef]
- Wu, H.X.; Ho, J.K.L.; Dong, G.N.; Zhang, D.Y. Friction reduction of pre-phosphating nanofilm on bearing steel by tricresyl phosphate pretreatment in boundary lubrication. J. Eng. Tribol. 2015, 229, 101–111. [Google Scholar] [CrossRef]
- Smidt, F.A.; Hubler, G.K. Recent advances in ion beam modification of metals. Nucl. Instrum. Meth. B 1993, 80–81, 207–216. [Google Scholar] [CrossRef]
- Cui, F.Z.; Luo, Z.S. Biomaterials modification by ion-beam processing. Surf. Coat. Technol. 1999, 112, 278–285. [Google Scholar] [CrossRef]
- Han, S.; Lee, Y.; Kim, H.; Kim, G.; Lee, J.; Yoon, J.; Kim, G. Polymer surface modification by plasma source ion implantation. Surf. Coat. Technol. 1997, 93, 261–264. [Google Scholar] [CrossRef]
- Li, D.; Zhao, J.; Gu, H.; Lu, R.; Ding, F.; Zhang, Q. Effect of ion implantation on wettability, anticoagulability and anticalcification of polyurethane. Prog. Mater. Sci. 1993, 1, 66–99. [Google Scholar] [CrossRef]
- Xu, M.; Li, Z.; Wang, G.; Wang, C.; Tian, X. Research on the microstructure and phase of interface of Al ion implanted stainless steel/Al joints. Rare Met. Mater. Eng. 2012, S2, 773–776. [Google Scholar]
- Dayss, E.; Leps, G.; Meinhardt, J. Surface modification for improved adhesion of a polymer–metal compound. Surf. Coat. Technol. 1999, 116–119, 986–990. [Google Scholar] [CrossRef]
- Karimi, M.V.; Sinha, S.K.; Kothari, D.C.; Khanna, A.K.; Tyagi, A.K. Effect of ion implantation on corrosion resistance and high temperature oxidation resistance of Ti deposited 316 stainless steel. Surf. Coat. Technol. 2002, 158–159, 609–614. [Google Scholar] [CrossRef]
- Li, X.; Yue, W.; Wang, C.; Gao, X.; Wang, S.; Liu, J. Comparing tribological behaviors of plasma nitrided and untreated bearing steel under lubrication with phosphor and sulfur-free organotungsten additive. Tribol. Int. 2012, 51, 47–53. [Google Scholar] [CrossRef]
- Nosaka, M.; Oike, M.; Kikuchi, M.; Kamijo, K.; Tajiri, M. Tribo-characteristics of self-lubricating ball bearings for the LE-7 liquid hydrogen rocket-turbopump. Tribol. Trans. 1993, 36, 432–442. [Google Scholar] [CrossRef]
- Gu, L.; Wang, L.Q.; Li, X.J.; Qi, Y.L. Study and review of liquid hydrogen/oxygen turbo-pump cryogenic bearing technology. Chin. Mech. Eng. 2002, 13, 86–89. [Google Scholar] [CrossRef]
- Yazıcıa, M.; Kovacıb, H.; Yetimc, A.F.; Çelik, A. Structural, mechanical and tribological properties of Ti and TiN coatings on 316L stainless steel. Ceram. Int. 2018, 44, 14195–14201. [Google Scholar] [CrossRef]
- Sun, T.; Wang, L.P.; Yu, Y.H.; Wang, Y.H.; Wang, X.F.; Tang, B.Y. TiN/ZrO2 multilayers synthesized on GCr15 bearing steel using plasma immersion ion implantation and deposition. Surf. Coat. Technol. 2007, 201, 6615–6618. [Google Scholar] [CrossRef]
- Azzi, M.; Paquette, M.; Szpunar, J.A.; Klemberg-Sapieha, J.E.; Martinu, L. Tribocorrosion behaviour of DLC-coated 316L stainless steel. Wear 2009, 267, 860–866. [Google Scholar] [CrossRef]
- Mezzi, A.; Kaciulis, S. Surface investigation of carbon films: From diamond to graphite. Surf. Interface Anal. 2010, 42, 1082–1084. [Google Scholar] [CrossRef]
- Toro, R.G.; Calandra, P.; Cortese, B.; de Caro, T.; Brucale, M.; Mezzi, A.; Federici, F.; Caschera, D. Argon and hydrogen plasma influence on the protective properties of diamond-like carbon films as barrier coating. Surf. Interfaces 2017, 6, 60–71. [Google Scholar] [CrossRef]
- Yu, G.Q.; Tay, B.K.; Sun, Z. Fluorinated amorphous diamond-like carbon films deposited by plasma-enhanced chemical vapor deposition. Surf. Coat. Technol. 2005, 191, 236–241. [Google Scholar] [CrossRef]
- Kwok, S.C.H.; Wang, J.; Chu, P.K. Surface energy, wettability, and blood compatibility phosphorus doped diamond-like carbon films. Diam. Relat. Mater. 2005, 14, 78–85. [Google Scholar] [CrossRef]
- Moghadam, R.Z.; Ehsani, M.H.; Dizaji, H.R.; Kameli, P.; Jannesari, M. Modification of hydrophobicity properties of diamond like carbon films. Mater. Lett. 2018, 220, 301–304. [Google Scholar] [CrossRef]
- Jin, J.; Chen, Y.; Gao, K.; Huang, X. The effect of ion implantation on tribology and hot rolling contact fatigue of Cr4Mo4Ni4V bearing steel. Appl. Surf. Sci. 2014, 305, 93–100. [Google Scholar] [CrossRef]
- Jin, J.; Xu, Z.; Wang, J.; Ming, J.; Guo, Q.; Li, L. Property of multilayer TiCN films formed by ion-beam-assisted-deposition. Nucl. Technol. 2007, 30, 1019–1022. [Google Scholar] [CrossRef]
- Xiao, D.; Xia, D.J. Effect of ultra-attenuation in surface energy decreasing. Therm. Sci. Technol. 2006, 1, 81–84. [Google Scholar] [CrossRef]
- Jin, J.; Shao, T.M. Graded microstructure and mechanical performance of Ti/N-implanted M50 steel with polyenergy. Materials 2017, 10, 1204. [Google Scholar] [CrossRef]




| Materials | Sample Number | Implantation Elements | Implantation Dose/×1017 Ions/cm2 | Implantation Energy/keV | Coating |
|---|---|---|---|---|---|
| 9Cr18 stainless steel | S0 | - | - | - | Textured |
| S1 | F+ | 0.5 | 400 | - | |
| S2 | N+ | 2 | 100 | - | |
| S3 | - | - | - | DLC | |
| S4 | F+ | 0.5 | 400 | DLC | |
| S5 | - | - | - | TiN | |
| S6 | N+ | 2 | 100 | TiN | |
| S7 | N+ + Ti+ | 2(N+) + 2(Ti+) | 100(N+) + 90(Ti+) | - | |
| M50 bearing steel | M0 | - | - | - | - |
| M1 | N+ + Ti+ | 2(N+) + 2(Ti+) | 100(N+) + 90(Ti+) | - |
| Number | S0 | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|---|
| Contact angle (°) | 88.9 | 98.8 | 96.3 | 90.5 | 96.8 | 102.1 | 100.4 | 93.3 |
| Number | S0 | S1 | S4 | M1 |
|---|---|---|---|---|
| Contact angle (°) | 12.0 | 10.2 | 15.2 | 10.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yin, X.; Jin, J. A Study on the Wettability of Ion-Implanted Stainless and Bearing Steels. Metals 2019, 9, 208. https://doi.org/10.3390/met9020208
Chen X, Yin X, Jin J. A Study on the Wettability of Ion-Implanted Stainless and Bearing Steels. Metals. 2019; 9(2):208. https://doi.org/10.3390/met9020208
Chicago/Turabian StyleChen, Xinchun, Xuan Yin, and Jie Jin. 2019. "A Study on the Wettability of Ion-Implanted Stainless and Bearing Steels" Metals 9, no. 2: 208. https://doi.org/10.3390/met9020208





