Characterization and Feasibility Studies on Complete Recovery of Rare Earths from Glass Polishing Waste
Abstract
:1. Introduction
2. Materials and Methods
3. Results
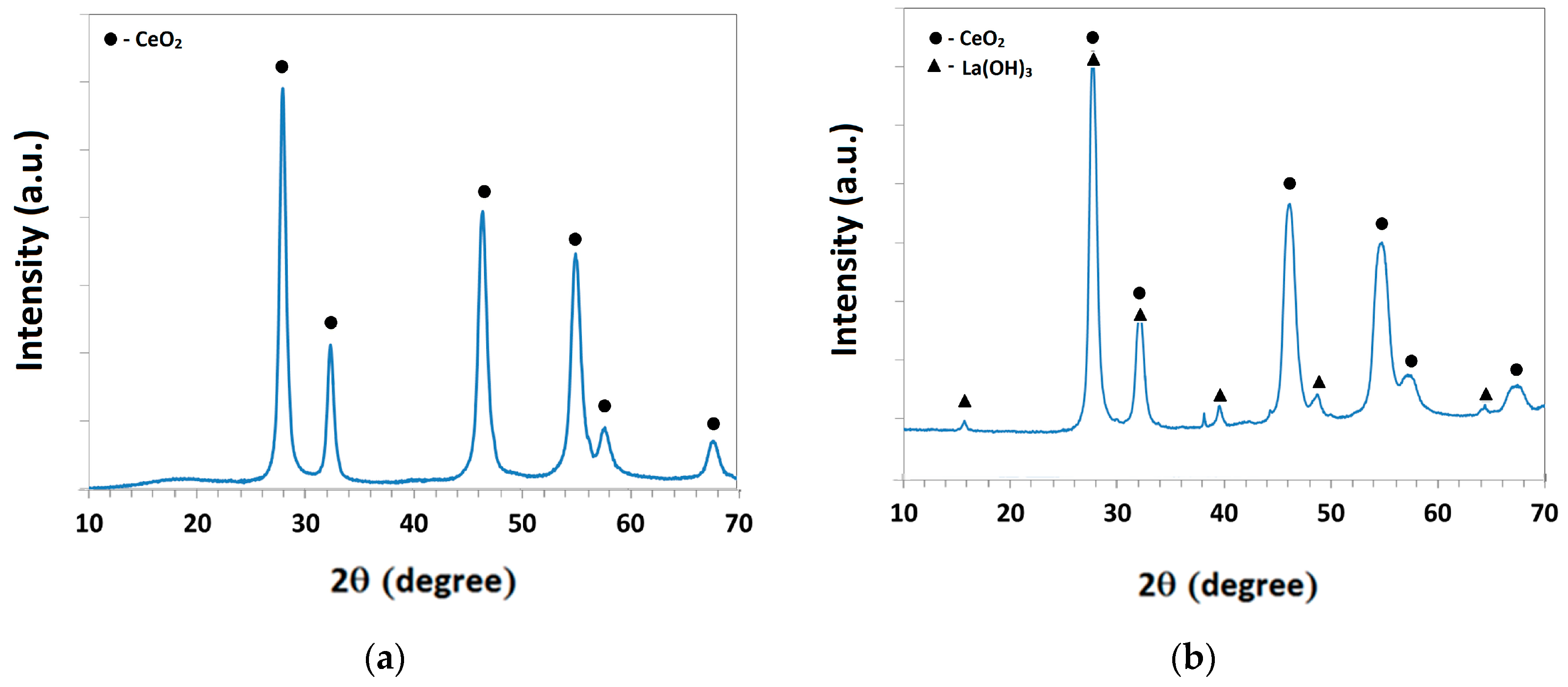
3.1. Characterisation
3.2. Ca Removal
3.3. Alkali Leaching for Al and Si Removal
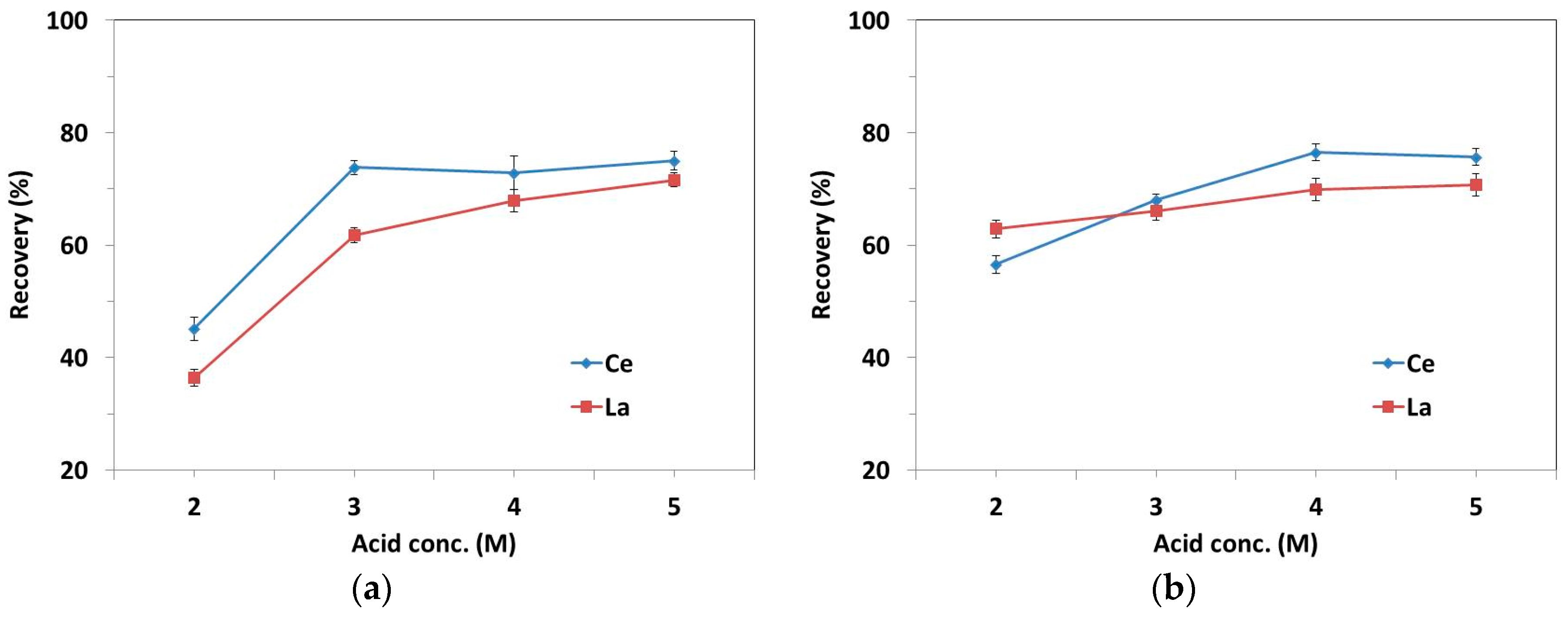
3.4. Acid-Based Process
3.5. Alkali-Based Process
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2015; ISBN 1466576383. [Google Scholar]
- Argus Media Analysing the Changing Global Rare Earths Supply and Demand Outlook. Available online: http://www.argusmedia.jp/~/media/files/pdfs/regional-specific/jp/downloads/argus-metal-pages-forum082016-rareearths.pdf/?la=en (accessed on 16 February 2017).
- Gambogi, J. USGS 2014 Minerals Yearbook: Rare Earths; USGS: Reston, VA, USA, 2016.
- Tercero Espinoza, L.; Hummen, T.; Brunot, A.; Hovestad, A.; Peña Garay, I.; Velte, D.; Smuk, L.; Todorovic, J.; Van Der Eijk, C.; Joce, C. Critical Raw Materials Substitution Profiles; Fraunhofer Institute for Systems and Innovation Research: Karlsruhe, Germany, 2015. [Google Scholar]
- Lucas, J.; Lucas, P.; Le Mercier, T.; Rollat, A.; Davenport, W. Chapter 12—Polishing with Rare Earth Oxides Mainly Cerium Oxide CeO2. In Rare Earths; Elsevier: Amsterdam, The Netherlands, 2015; pp. 191–212. ISBN 978-0-444-62735-3. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Kasai, T.; Bhushan, B. Physics and tribology of chemical mechanical planarization. J. Phys. Condens. Matter 2008, 20, 225011–225023. [Google Scholar] [CrossRef]
- Um, N.; Hirato, T. A hydrometallurgical method of energy saving type for separation of rare earth elements from rare earth polishing powder wastes with middle fraction of ceria. J. Rare Earths 2016, 34, 536–542. [Google Scholar] [CrossRef]
- Mishima, F.; Terada, T.; Akiyama, Y.; Nishijima, S. High Gradient Superconducting Magnetic Separation for Iron Removal from the Glass Polishing Waste. IEEE Trans. Appl. Supercond. 2011, 21, 2059–2062. [Google Scholar] [CrossRef]
- Borra, C.R.; Vlugt, T.J.H.; Yang, Y.; Offerman, S.E. Recovery of Cerium from Glass Polishing Waste: A Critical Review. Metals 2018, 8, 801. [Google Scholar] [CrossRef]
- Kato, K.; Yoshioka, T.; Okuwaki, A. Study for recycling of ceria-based glass polishing powder. Ind. Eng. Chem. Res. 2000, 39, 943–947. [Google Scholar] [CrossRef]
- Kato, K.; Yoshioka, T.; Okuwaki, A. Recyle of Ceria-Based Glass Polishing Powder Using NaOH Solution. Nippon Kagaku Kaishi 2000, 10, 725–732. [Google Scholar] [CrossRef]
- Moon, W.; Na, S.; Oh, H. Method for Recycling Cerium Oxide Abrasive. U.S. Patent 20110219704A1, 15 September 2011. [Google Scholar]
- Matsui, H.; Harada, D.; Takeuchi, M. Method for Recovery of Cerium Oxide. U.S. Patent 20130152483A1, 20 June 2013. [Google Scholar]
- Janoš, P.; Kuráň, P.; Ederer, J.; Šastný, M.; Vrtoch, L.; Pšenička, M.; Henych, J.; Mazanec, K.; Skoumal, M. Recovery of Cerium Dioxide from Spent Glass-Polishing Slurry and Its Utilization as a Reactive Sorbent for Fast Degradation of Toxic Organophosphates. Adv. Mater. Sci. Eng. 2015, 8. [Google Scholar] [CrossRef]
- Poscher, A.; Luidold, S.; Schnideritsch, H.; Antrekowitsch, H. Extraction of Lanthanides from Spent Polishing Agent. In Rare Earths Industry: Technological, Economic, and Environmental Implications; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 209–222. ISBN 9780128023280. [Google Scholar]
- Poscher, A.; Luidold, S.; Antrekowitsch, H. Extraction of cerium and lanthanum from spent glass polishing agent. In Materials Science & Technology 2013; London, I.M., Goode, J.R., Moldoveanu, G., Rayat, M.S., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montréal, QC, Canada, 2013; pp. 543–552. [Google Scholar]
- Henry, P.; Lamotte, S.; Bier, J. Recycling of rare earth materials at Hydrometal (Belgium). In 52nd Conference of Metallurgists (COM), Hosting by Materials Science Technology Conference (MS&T); London, I.M., Goode, J.R., Moldoveanu, G., Rayat, M.S., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montréal, QC, Canada, 2013; pp. 537–542. [Google Scholar]
- Byeon, M.S.; Kim, J.Y.; Hwang, K.T.; Kim, U.; Cho, W.S.; Kang, W.K. Recovery and purification of cerium from glass polishing slurry. In Proceedings of the 18th International Conference on Composite Materials, Jeju, Korea, 21–26 August 2011. [Google Scholar]
- Kim, J.Y.; Kim, U.S.; Byeon, M.S.; Kang, W.K.; Hwang, K.T.; Cho, W.S. Recovery of cerium from glass polishing slurry. J. Rare Earths 2011, 29, 1075–1078. [Google Scholar] [CrossRef]
- Janoš, P.; Novak, J.; Broul, M. A Procedure for Obtaining Salts of Rare Earth Elements. U.S. Patent 21,039,151, 31 October 1988. [Google Scholar]
- Terziev, A.L.; Minkova, N.L.; Todorovsky, D.S. Regeneration of waste rare earth oxides based polishing materials. Bulg. Chem. Commun. 1996, 29, 274–284. [Google Scholar]
- Ryan, K.M.; McGrath, J.P.; Farrell, R.A.; O Neill, W.M.; Barnes, C.J.; Morris, M.A. Measurements of the lattice constant of ceria when doped with lanthana and praseodymia—The possibility of local defect ordering and the observation of extensive phase separation. J. Phys. Condens. Matter 2003, 15, L49–L58. [Google Scholar] [CrossRef]
- Chi, R.; Zhang, X.; Zhu, G.; Zhou, Z.A.; Wu, Y.; Wang, C.; Yu, F. Recovery of rare earth from bastnasite by ammonium chloride roasting with fluorine deactivation. Miner. Eng. 2004, 17, 1037–1043. [Google Scholar] [CrossRef]
- Um, N.; Hirato, T. Dissolution Behavior of La2O3, Pr2O3, Nd2O3, CaO and Al2O3 in Sulfuric Acid Solutions and Study of Cerium Recovery from Rare Earth Polishing Powder Waste via Two-Stage Sulfuric. Acid Leach. Mater. Trans. 2013, 54, 713–719. [Google Scholar] [CrossRef]








| Elements | Sample A (wt.%) | Sample B (wt.%) | Analysis |
|---|---|---|---|
| Ce | 23.3 ± 0.7 | 52.2 ± 1.5 | ICP-OES |
| La | 9.1 ± 0.3 | 18.7 ± 0.6 | |
| F | 2.3 | 5.9 | WD-XRF |
| Si | 2.6 | 0.4 | |
| Al | 2.2 | - | |
| Ca | 20.8 | 0.2 | |
| Fe | 0.4 | 0. 6 | |
| P | 0.4 | 0.9 | |
| Ba | 0.2 | 0.4 | |
| Na | 0.2 | - | |
| Mg | 0.1 | - | |
| Ti | 0.1 | - | |
| Sn | 0.1 | 0.4 | |
| Ag | - | 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borra, C.R.; Vlugt, T.J.H.; Spooren, J.; Nielsen, P.; Yang, Y.; Offerman, S.E. Characterization and Feasibility Studies on Complete Recovery of Rare Earths from Glass Polishing Waste. Metals 2019, 9, 278. https://doi.org/10.3390/met9030278
Borra CR, Vlugt TJH, Spooren J, Nielsen P, Yang Y, Offerman SE. Characterization and Feasibility Studies on Complete Recovery of Rare Earths from Glass Polishing Waste. Metals. 2019; 9(3):278. https://doi.org/10.3390/met9030278
Chicago/Turabian StyleBorra, Chenna Rao, Thijs J. H. Vlugt, Jeroen Spooren, Peter Nielsen, Yongxiang Yang, and S. Erik Offerman. 2019. "Characterization and Feasibility Studies on Complete Recovery of Rare Earths from Glass Polishing Waste" Metals 9, no. 3: 278. https://doi.org/10.3390/met9030278





