Hydrogen Effect on the Cyclic Behavior of a Superelastic NiTi Archwire
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
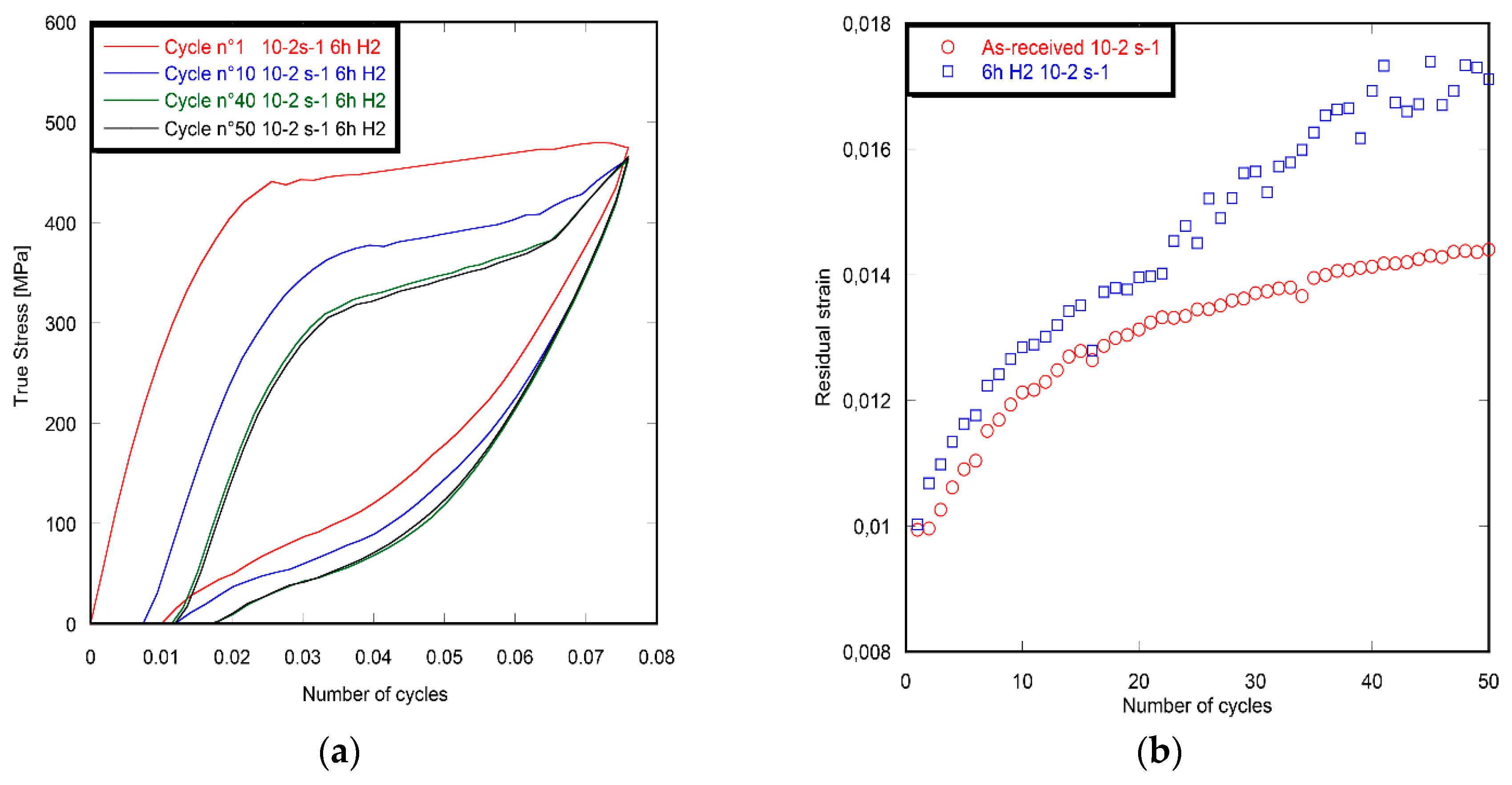
3.1. Strain Rate Effect
3.2. Annealing Effect
4. Conclusions
- After 6 h of hydrogen charging with a current density of 10 A/m2 and ageing, the cyclic tensile curves of the superelastic Ni–Ti SMA strongly depends on the strain rate. An embrittlement is detected in the 18th cycle for the imposed lower strain rate of 10−4 s−1. However, there is no embrittlement detected for the higher strain rate of 10−2 s−1. In addition, the dissipated energy density is tumbled for the low strain rate, compared to the as-received specimen. In contrast, no difference is detected for the higher strain rate. This behavior is attributed to the interaction between the hydrogen diffused into the volume and the thermo-mechanical mechanism of the nucleation and growth of the martensitic bands. This interaction is considered as strongly linked to the hydrogen diffused into the volume of the archwire and to the applied strain rates.
- Despite the annealing of the NiTi archwire for 1 h at 400 °C, causing the desorption of the diffused hydrogen, the material is fractured in a brittle manner in the plateau of the austenite-martensite transformation after 44 cycles of loading and unloading. This result is attributed to the development of internal stress in the subsurface of the parent phase during hydrogen charging and to the creation of cracks and local zones of plasticity after desorption.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herget, G.; Müllner, M.; Suck, J.B.; Schmidt, R.; Wipf, H. Phonon Spectra of the Memory Alloy NiTi. Europhys. Lett. 1989, 10, 49–54. [Google Scholar] [CrossRef]
- Waitz, T.; Kazykhanov, V.; Karnthaler, H.P. Martensitic phase transformations in nanocrystalline NiTi studied by TEM. Acta Mater. 2004, 52, 137–147. [Google Scholar] [CrossRef]
- Sharma, N.; Jangra, K.; Raj, T. Applications of Nickel-Titanium Alloy. J. Eng. Technol. 2015, 5, 1. [Google Scholar] [CrossRef]
- Castleman, L.S.; Motzkin, S.M.; Alicandri, F.P. Biocompatibility of nitinol alloy as an endotracheal implant material. J. Biomed. Mater. Res. 1976, 10, 695–731. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.R.; Verma, P.R. Strip-electro-mechanical yield model for transversely situated two semi-permeable collinear cracks in piezoelectric strip. Theor. Appl. Fract. Mech. 2016, 81, 32–49. [Google Scholar] [CrossRef]
- Lagoudas, D.C. Shape Memory Alloys: Modeling and Engineering Applications; Springer-Verlag: Berlin, Germany, 2008. [Google Scholar]
- Sarraj, R.; Letaief, W.E.; Hassine, T.; Gamaoun, F. Modeling of rate dependency of mechanical behavior of superelastic NiTi alloy under cyclic loading. Int. J. Adv. Manuf. Technol. 2019, 100, 2715–2724. [Google Scholar] [CrossRef]
- Petrini, L.; Migliavacca, F. Biomedical Applications of Shape Memory Alloys. J. Metall. 2011, 2011, 501483. [Google Scholar] [CrossRef]
- Bartzela, T.N.; Senn, C.; Wichelhaus, A. Load-deflection characteristics of superelastic nickel-titanium wires. Angle Orthod. 2007, 77, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Bewerse, C.; Gall, K.R.; McFarland, G.J.; Zhu, P.; Brinson, L.C. Local and global strains and strain ratios in shape memory alloys using digital imagecorrelation. Mater. Sci. Eng. A 2013, 568, 134–142. [Google Scholar] [CrossRef]
- Eaton-Evans, J.; Dulieu-Barton, J.M.; Little, E.G.; Brown, I.A. A New Approach to Stress Analysis of Vascular Devices Using High Resolution Thermoelastic Stress Analysis. Appl. Mech. Mater. 2009, 5–6, 63–70. [Google Scholar] [CrossRef]
- Delpueyo, D.; Balandraud, X.; Grédiac, M. Applying infrared thermography to analyse martensitic microstructures in a Cu–Al–Be shape-memory alloy subjected to a cyclic loading. Mater. Sci. Eng. A 2011, 528, 8249–8258. [Google Scholar] [CrossRef]
- Helbert, G.; Saint-Sulpice, L.; Chirani, S.A.; Dieng, L.; Lecompte, T.; Calloch, S.; Pilvin, P. Experimental characterisation of three-phase NiTi wires under tension. Mech. Mater. 2014, 79, 85–101. [Google Scholar] [CrossRef]
- Brinson, L.C.; Schmidt, I.; Lammering, R. Stress-induced transformation behavior of a polycrystalline NiTi shape memory alloy: Micro and macromechanical investigations via in situ optical microscopy. J. Mech. Phys. Solids 2004, 52, 1549–1571. [Google Scholar] [CrossRef]
- Dayananda, G.N.; Rao, M.S. Effect of strain rate on properties of superelastic NiTi thin wires. Mater. Sci. Eng. A 2008, 486, 96–103. [Google Scholar] [CrossRef]
- Nemat-Nasser, S.; Guo, W.-G. Superelastic and cyclic response of NiTi SMA at various strain rates and temperatures. Mech. Mater. 2006, 38, 463–474. [Google Scholar] [CrossRef]
- Tobushi, H.; Shimeno, Y.; Hachisuka, T.; Tanaka, K. Influence of strain rate on superelastic properties of TiNi shape memory alloy. Mech. Mater. 1998, 30, 141–150. [Google Scholar] [CrossRef]
- Letaief, W.E.; Hassine, T.; Gamaoun, F. In situ stress relaxation mechanism of a superelastic NiTi shape memory alloy under hydrogen charging. Philos. Mag. Lett. 2017, 97, 50–57. [Google Scholar] [CrossRef]
- Huang, H.-H.; Chiu, Y.H.; Lee, T.H.; Wu, S.C.; Yang, H.W.; Su, K.H.; Hsu, C.C. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials 2003, 24, 3585–3592. [Google Scholar] [CrossRef]
- Mirjalili, M.; Momeni, M.; Ebrahimi, N.; Moayed, M.H. Comparative study on corrosion behaviour of Nitinol and stainless steel orthodontic wires in simulated saliva solution in presence of fluoride ions. Mater. Sci. Eng. C 2013, 33, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Wang, C.-C.; Huang, T.-K.; Chen, L.-K.; Chou, M.-Y.; Huang, H.-H. Corrosion resistance of titanium-containing dental orthodontic wires in fluoride-containing artificial saliva. J. Alloys Compd. 2009, 488, 482–489. [Google Scholar] [CrossRef]
- Gamaoun, F.; Skhiri, I.; Bouraoui, T.; Zineb, T.B. Hydrogen effect on the austenite-martensite transformation of the cycled Ni-Ti alloy. J. Intell. Mater. Syst. Struct. 2014, 25, 980–988. [Google Scholar] [CrossRef]
- Gamaoun, F.; Ltaief, M.; Bouraoui, T.; Zineb, T.B. Effect of hydrogen on the tensile strength of aged Ni-Ti superelastic alloy. J. Intell. Mater. Syst. Struct. 2011, 22, 2053–2059. [Google Scholar] [CrossRef]
- Yokoyama, K.; Tomita, M.; Sakai, J. Hydrogen embrittlement behavior induced by dynamic martensite transformation of Ni-Ti superelastic alloy. Acta Mater. 2009, 57, 1875–1885. [Google Scholar] [CrossRef]
- Yokoyama, K.; Watabe, S.; Hamada, K.; Sakai, J.; Asaoka, K.; Nagumo, M. Susceptibility to delayed fracture of Ni-Ti superelastic alloy. Mater. Sci. Eng. A 2003, 341, 91–97. [Google Scholar] [CrossRef]
- Yokoyama, K.; Ogawa, T.; Takashima, K.; Asaoka, K.; Sakai, J. Hydrogen embrittlement of Ni-Ti superelastic alloy aged at room temperature after hydrogen charging. Mater. Sci. Eng. A 2007, 466, 106–113. [Google Scholar] [CrossRef]
- Gamaoun, F.; Hassine, T. Ageing effect and rate dependency of a NiTi shape memory alloy after hydrogen charging. J. Alloy Compd. 2014, 615, s680–s683. [Google Scholar] [CrossRef]
- Gamaoun, F.; Hassine, T.; Bouraoui, T. Strain rate response of a Ni–Ti shape memory alloy after hydrogen charging. Philos. Mag. Lett. 2014, 94, 30–36. [Google Scholar] [CrossRef]
- Letaief, W.E.; Hassine, T.; Gamaoun, F. Rate Dependency During Relaxation of Superelastic Orthodontic NiTi Alloys After Hydrogen Charging. Shape Mem. Superelasticity 2016, 2, 121–127. [Google Scholar] [CrossRef]
- Letaief, W.E.; Hassine, T.; Gamaoun, F. A coupled model between hydrogen diffusion and mechanical behavior of superelastic NiTi alloys. Smart Mater. Struct. 2017, 26, 075001. [Google Scholar] [CrossRef]
- Sarraj, R.; Hassine, T.; Gamaoun, F. Mechanical behavior of NiTi arc wires under pseudoelastic cycling and cathodically hydrogen charging. Mater. Res. Express 2018, 5, 015704. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, K.; Kaneko, K.; Ogawa, T.; Moriyama, K.; Asaoka, K.; Sakai, J. Hydrogen embrittlement of work-hardened Ni-Ti alloy in fluoride solutions. Biomaterials 2005, 26, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Rozenak, P.; Eliezer, D. Phase changes related to hydrogen-induced cracking in austenitic stainless steel. Acta Metall. 1987, 35, 2329–2340. [Google Scholar] [CrossRef]
- Gustiono, D.; Sakaguchi, N.; Shibayama, T.; Kinoshita, H.; Takahashi, H. Plane and Cross-Sectional TEM Observation to Clarify the Effect of Damage Region by Ion Implantation on Induced Phase Transformation in Austenitic 301 Stainless Steel. Mater. Trans. 2004, 45, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wang, L.; Yu, Z.; Hei, Z. A comparative study on microstructure of the plasma-nitrided layers on austenitic stainless steel and pure Fe. Surf. Coat. Technol. 2005, 192, 220–224. [Google Scholar]
- Runciman, A.; Chen, K.C.; Pelton, A.R.; Trépanier, C. Effects of Hydrogen on the Phases and Transition Temperatures of NiTi. Int. Conf. Shape Mem. Superelastic Technol. 2006, 185–196. [Google Scholar] [CrossRef]
- Rozenak, P.; Loew, A. Stress distributions due to hydrogen concentrations in electrochemically charged and aged austenitic stainless steel. Corros. Sci. 2008, 50, 3021–3030. [Google Scholar] [CrossRef]
- Tomita, M.; Yokoyama, K.; Asaoka, K.; Sakai, J. Hydrogen thermal desorption behavior of Ni-Ti superelastic alloy subjected to tensile deformation after hydrogen charging. Mater. Sci. Eng. A 2008, 476, 308–315. [Google Scholar] [CrossRef]
- He, Y.J.; Sun, Q.P. Macroscopic equilibrium domain structure and geometric compatibility in elastic phase transition of thin plates. Int. J. Mech. Sci. 2010, 52, 198–211. [Google Scholar] [CrossRef]
- He, Y.J.; Sun, Q.P. Rate-dependent domain spacing in a stretched NiTi strip. Int. J. Solids Struct. 2010, 47, 2775–2783. [Google Scholar] [CrossRef] [Green Version]
- Ammar, O.; Dieng, L.; Haddar, N. Modeling of strain rate effect on the pseudoelastic behavior of NiTi SMA using a simple thermomechanical coupling model. Mech. Mater. 2018, 124, 7–17. [Google Scholar] [CrossRef]
- Morin, C.; Moumni, Z.; Zaki, W. Thermomechanical coupling in shape memory alloys under cyclic loadings: Experimental analysis and constitutive modeling. Int. J. Plast. 2011, 27, 1959–1980. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, P.; He, Y.; Yu, T.; Sun, Q. Experimental study on rate dependence of macroscopic domain and stress hysteresis in NiTi shape memory alloy strips. Int. J. Mech. Sci. 2010, 52, 1660–1670. [Google Scholar] [CrossRef]
- Rozenak, P.; Zevin, L.; Eliezer, D. Hydrogen effects on phase transformations in austenitic stainless steels. J. Mater. Sci. 1984, 19, 567–573. [Google Scholar] [CrossRef]
- Rozenak, P.; Zevin, L.; Eliezer, D. Internal stresses in austenitic steels cathodically charged with hydrogen. J. Mater. Sci. Lett. 1983, 2, 63–66. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Hänninen, H.; Tarasenko, A.V.; Tereshchenko, A.S.; Ullakko, K. Phase transformations and relaxation phenomena caused by hydrogen in stable austenitic stainless steels. Acta Metall. Mater. 1995, 43, 559–568. [Google Scholar] [CrossRef]
- Rozenak, P.; Bergman, R. X-ray phase analysis of martensitic transformations in austenitic stainless steels electrochemically charged with hydrogen. Mater. Sci. Eng. A 2006, 437, 366–378. [Google Scholar] [CrossRef]
- Narita, N.; Altstetter, C.J.; Birnbaum, H.K. Hydrogen-related phase transformations in austenitic stainless steels. Metall. Trans. A 1982, 13, 1355–1365. [Google Scholar] [CrossRef]
- Goodier, J.N. The thermal stress in a strip due to variation of temperature along the length and through the thickness. J. Appl. Phys. 1936, 7, 156–159. [Google Scholar] [CrossRef]










| Imposed Stain Rate | Type of Specimen | ||
|---|---|---|---|
| 10−4 s−1 | As received | Hydrogen charged and aged | Hydrogen charged, aged and annealed |
| 10−2 s−1 | As received | Hydrogen charged and aged | Hydrogen charged, aged and annealed |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarraj, R.; Kessentini, A.; Hassine, T.; Algahtani, A.; Gamaoun, F. Hydrogen Effect on the Cyclic Behavior of a Superelastic NiTi Archwire. Metals 2019, 9, 316. https://doi.org/10.3390/met9030316
Sarraj R, Kessentini A, Hassine T, Algahtani A, Gamaoun F. Hydrogen Effect on the Cyclic Behavior of a Superelastic NiTi Archwire. Metals. 2019; 9(3):316. https://doi.org/10.3390/met9030316
Chicago/Turabian StyleSarraj, Rihem, Amir Kessentini, Tarek Hassine, Ali Algahtani, and Fehmi Gamaoun. 2019. "Hydrogen Effect on the Cyclic Behavior of a Superelastic NiTi Archwire" Metals 9, no. 3: 316. https://doi.org/10.3390/met9030316





