Effect of Manganese on Microstructure and Corrosion Behavior of the Mg-3Al Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.3. Thermodynamic Calculations
3. Results and Discussion
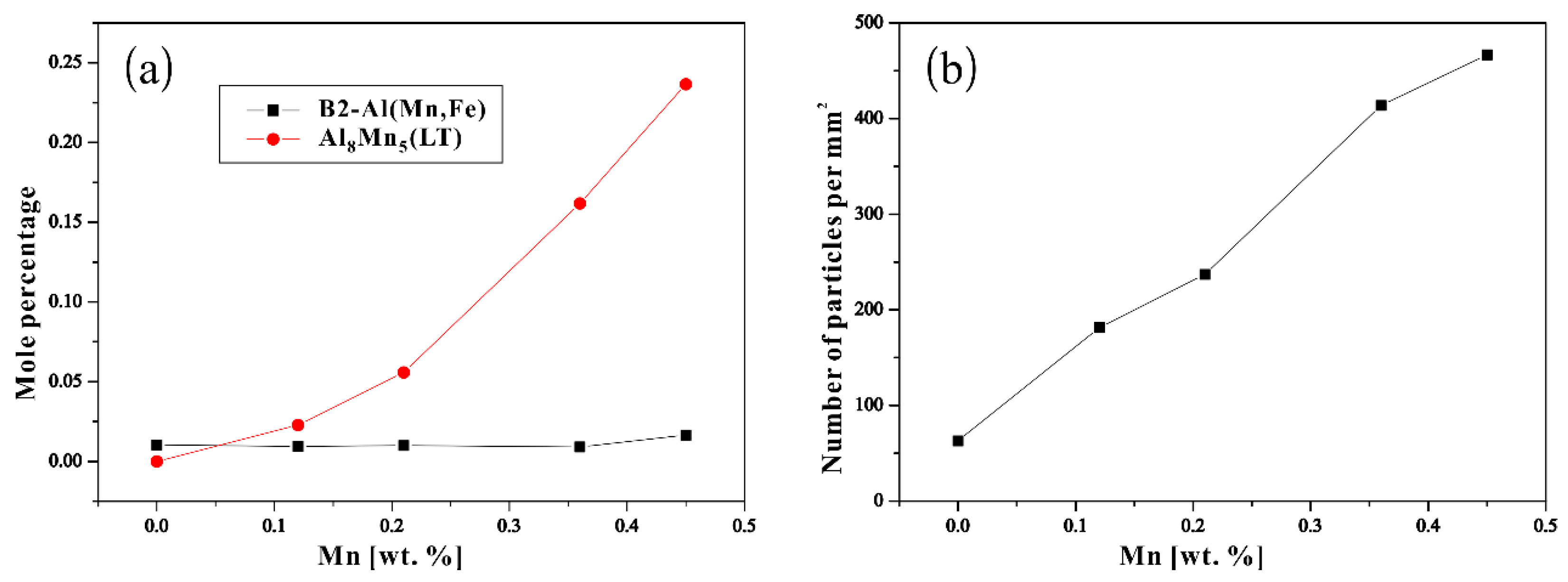
3.1. Microstructure Observation
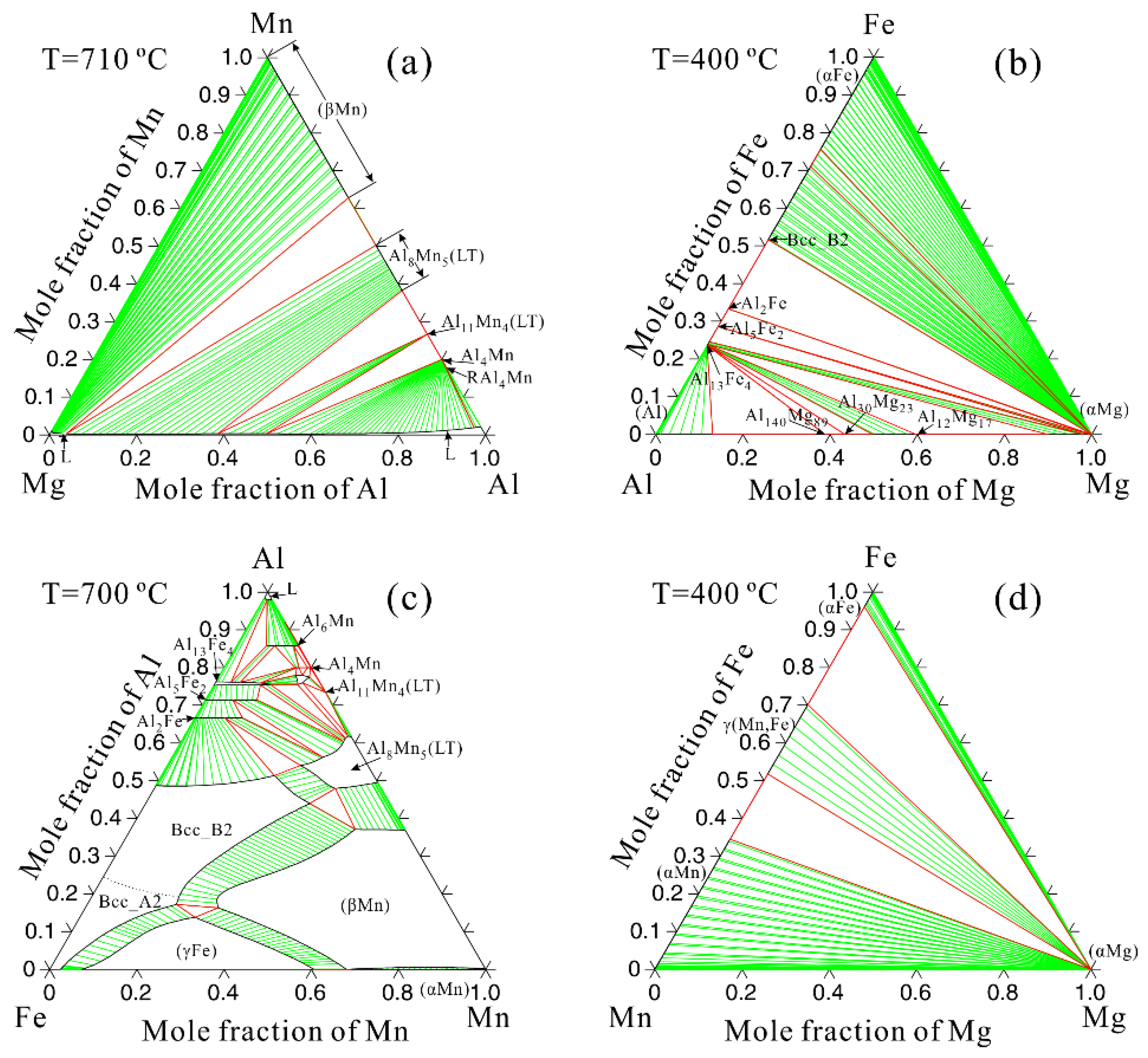
3.2. Thermodynamic Calculation
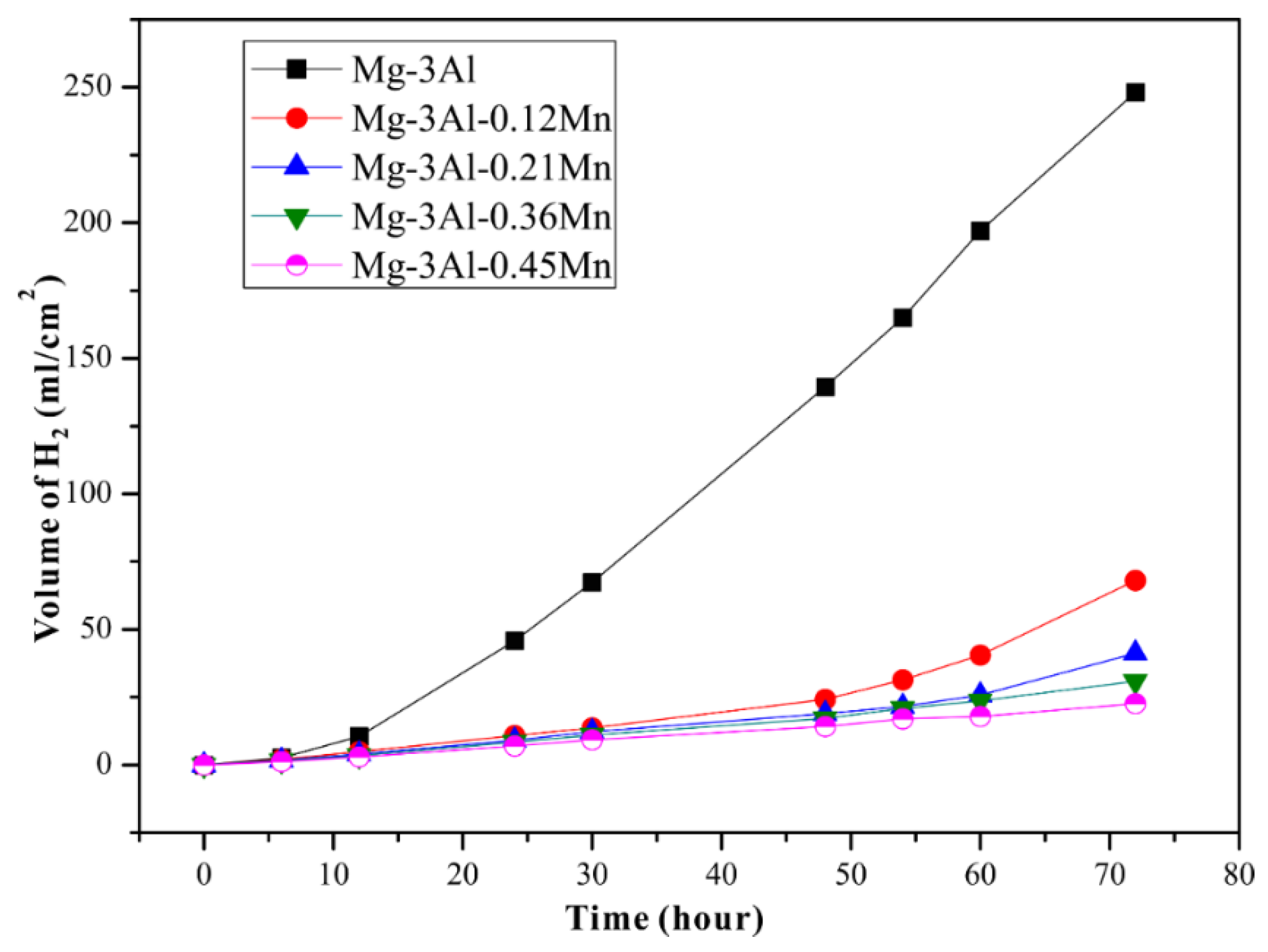
3.3. Electrochemical Tests and Corrosion Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Kulekci, M.K. Magnesium and its alloys applications in automotive industry. Int. J. Adv. Manuf. Technol. 2007, 39, 851–865. [Google Scholar] [CrossRef]
- Kumar, D.S.; Sasanka, C.T.; Ravindra, K.; Suman, K. Magnesium and its alloys in automotive applications—A review. Am. J. Mater. Sci. Technol. 2015, 4, 12–30. [Google Scholar] [CrossRef]
- Pekguleryuz, M.O.; Kaya, A.A. Creep resistant magnesium alloys for powertrain applications. Adv. Eng. Mater. 2003, 5, 866–878. [Google Scholar] [CrossRef]
- La, M.; Zhou, H.J.; Li, N.; Xin, Y.C.; Sha, R.; Bao, S.H.; Jin, P. Improved performance of Mg–Y alloy thin film switchable mirrors after coating with a superhydrophobic surface. Appl. Surf. Sci. 2017, 403, 23–28. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, Z.M.; Cao, F.Y.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Cao, H.H.; Huo, W.T.; Ma, S.F.; Zhang, Y.S.; Zhou, L. Microstructure and corrosion behavior of composite coating on pure Mg acquired by sliding friction treatment and micro-arc oxidation. Materials 2018, 11, 1232. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Shi, Z.M.; Soltan, A.; Johnston, S.; Dargusch, M.S. Understanding the Corrosion of Mg and Mg Alloys. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elservier: Oxford, UK, 2018; pp. 515–534. [Google Scholar]
- Liu, W.J.; Cao, F.H.; Chang, L.R.; Zhang, Z.; Zhang, J.Q. Effect of rare earth element Ce and La on corrosion behavior of AM60 magnesium alloy. Corros. Sci. 2009, 51, 1334–1343. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Edyvean, R.G.J.; Sellars, C.M.; Jones, H. Effect of additions of Mn, Ce, Nd, and Si on rate of dissolution of splat quenched Mg–Al and Mg–Zn alloys in 3% NaCl solution. Mater. Sci. Technol. 1990, 6, 469–474. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wang, J.; Qiu, X.; Zhang, D.P.; Tian, Z.; Niu, X.D.; Tang, D.X.; Meng, J. Effect of Nd on the microstructure, mechanical properties and corrosion behavior of die-cast Mg–4Al-based alloy. J. Alloys Compd. 2008, 464, 556–564. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Mendis, C.L.; del Olmo, R.; Matykina, E.; Hort, N.; Merino, M.C.; Pardo, A. Corrosion of Mg–9Al alloy with minor alloying elements (Mn, Nd, Ca, Y and Sn). Mater. Des. 2017, 130, 48–58. [Google Scholar] [CrossRef]
- Arrabal, R.; Mingo, B.; Pardo, A.; Matykina, E.; Mohedano, M.; Merino, M.C.; Rivas, A.; Maroto, A. Role of alloyed Nd in the microstructure and atmospheric corrosion of as-cast magnesium alloy AZ91. Corros. Sci. 2015, 97, 38–48. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.L.; Wang, L.D.; Wu, Y.M.; Wang, L.M. Electrochemical corrosion behavior of Mg–5Al–0.4Mn–xNd in NaCl solution. Corros. Sci. 2009, 51, 1328–1333. [Google Scholar] [CrossRef]
- Cai, S.H.; Lei, T.; Li, N.F.; Feng, F.F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Nam, N.D. Corrosion behavior of Mg-5Al based magnesium alloy with 1 wt.% Sn, Mn and Zn additions in 3.5 wt.% NaCl solution. J. Magn. Alloys 2014, 2, 190–195. [Google Scholar] [CrossRef]
- Baek, S.M.; Kang, J.S.; Shin, H.J.; Yim, C.D.; You, B.S.; Ha, H.Y.; Park, S.S. Role of alloyed Y in improving the corrosion resistance of extruded Mg–Al–Ca-based alloy. Corros. Sci. 2017, 118, 227–232. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdellahi, M.; Hamzah, E.; Ismail, A.F.; Bahmanpour, M. Modelling corrosion rate of biodegradable magnesium-based alloys: The case study of Mg–Zn–RE–xCa (x = 0, 0.5, 1.5, 3 and 6 wt.%) alloys. J. Alloys Compd. 2016, 687, 630–642. [Google Scholar] [CrossRef]
- Nam, N.D.; Mathesh, M.; Forsyth, M.; Jo, D.S. Effect of manganese additions on the corrosion behavior of an extruded Mg–5Al based alloy. J. Alloys Compd. 2012, 542, 199–206. [Google Scholar] [CrossRef]
- Metalnikov, P.; Ben-Hamu, G.; Templeman, Y.; Shin, K.S.; Meshi, L. The relation between Mn additions, microstructure and corrosion behavior of new wrought Mg–5Al alloys. Mater. Charact. 2018, 145, 101–115. [Google Scholar] [CrossRef]
- Wan, D.Q.; Wang, J.C.; Wang, G.F.; Chen, X.Y.; Lin, L.; Feng, Z.G.; Yang, G.C. Effect of Mn on damping capacities, mechanical properties, and corrosion behaviour of high damping Mg–3wt.%Ni based alloy. Mater. Sci. Eng. A 2008, 494, 139–142. [Google Scholar]
- Cho, D.H.; Lee, B.W.; Park, J.Y.; Cho, K.M.; Park, I.M. Effect of Mn addition on corrosion properties of biodegradable Mg-4Zn-0.5Ca-xMn alloys. J. Alloys Compd. 2017, 695, 1166–1174. [Google Scholar] [CrossRef]
- Ha, H.Y.; Kim, H.J.; Baek, S.M.; Kim, B.; Sohn, S.D.; Shin, H.J.; Jeong, H.Y.; Park, S.H.; Yim, C.D.; You, B.S.; et al. Improved corrosion resistance of extruded Mg–8Sn–1Zn–1Al alloy by microalloying with Mn. Scr. Mater. 2015, 109, 38–43. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, M.Z.; Fan, L.Q.; Wang, H.Y.; Feng, C.; Meng, H. Effects of Mn on Corrosion Resistant Property of AZ91 Alloys. Rare Met. Mater. Eng. 2014, 43, 278–282. [Google Scholar]
- Song, G.L.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Hillis, J.E.; Murray, R.W. Finishing alternatives for high purity magnesium alloys. In Proceedings of the SDCE 14th International Die Casting Congress and Exposition, Toronto, ON, Canada, 11–14 May 1987. Paper No. G-T87-003. [Google Scholar]
- Hillis, J.E.; Shook, S.O. Composition and performance of an improved magnesium AS41 alloy. In Proceedings of the SAE International Congress and Exposition, Detroit, MI, USA, 1 February 1989. SAE Technical Paper 890205. [Google Scholar]
- Hillis, J.E.; Reichek, K. High Purity Magnesium AM60 Alloy: The Critical Contaminant Limits and the Salt Water Corrosion Performance. In Proceedings of the SAE International Congress and Exposition, Detroit, MI, USA, 1 February 1986. SAE Technical Paper 860288. [Google Scholar]
- Liu, M.; Uggowitzer, P.J.; Nagasekhar, A.V.; Schmutz, P.; Easton, M.; Song, G.L.; Atrens, A. Calculated phase diagrams and the corrosion of die-cast Mg–Al alloys. Corros. Sci. 2009, 51, 602–619. [Google Scholar] [CrossRef]
- Mercer, W.E.; Hillis, J.E. The critical contaminant limits and salt water corrosion performance of magnesium AE42 alloy. In Proceedings of the SAE International Congress and Exposition, Detroit, MI, USA, 1 February 1992. SAE Technical Paper 920073. [Google Scholar]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Han, G.; Liu, X.F. Phase control and formation mechanism of Al–Mn(–Fe) intermetallic particles in Mg–Al-based alloys with FeCl3 addition or melt superheating. Acta Mater. 2016, 114, 54–66. [Google Scholar] [CrossRef]
- Danaie, M.; Asmussen, R.M.; Jakupi, P.; Shoesmith, D.W.; Botton, G.A. The cathodic behaviour of Al–Mn precipitates during atmospheric and saline aqueous corrosion of a sand-cast AM50 alloy. Corros. Sci. 2014, 83, 299–309. [Google Scholar] [CrossRef]
- Ye, H.Z.; Liu, X.Y. In situ formation behaviors of Al8Mn5 particles in Mg–Al alloys. J. Alloys Compd. 2006, 419, 54–60. [Google Scholar] [CrossRef]
- Pawar, S.; Zhou, X.; Hashimoto, T.; Thompson, G.E.; Scamans, G.; Fan, Z. Investigation of the microstructure and the influence of iron on the formation of Al8Mn5 particles in twin roll cast AZ31 magnesium alloy. J. Alloys Compd. 2015, 628, 195–198. [Google Scholar] [CrossRef]
- Pan, F.S.; Feng, Z.X.; Zhang, X.Y.; Tang, A.T. The Types and Distribution Characterization of Al–Mn Phases in the AZ61 Magnesium Alloy. Procedia Eng. 2012, 27, 833–839. [Google Scholar] [CrossRef]
- Lun Sin, S.; Dubé, D.; Tremblay, R. Characterization of Al–Mn particles in AZ91D investment castings. Mater. Charact. 2007, 58, 989–996. [Google Scholar] [CrossRef]
- Pawar, S.; Zhou, X.; Thompson, G.E.; Scamans, G.; Fan, Z. The Role of Intermetallics on the Corrosion Initiation of Twin Roll Cast AZ31 Mg Alloy. J. Electrochem. Soc. 2015, 162, C442–C448. [Google Scholar] [CrossRef]
- Laser, T.; Nürnberg, M.R.; Janz, A.; Hartig, C.; Letzig, D.; Schmid-Fetzer, R.; Bormann, R. The influence of manganese on the microstructure and mechanical properties of AZ31 gravity die cast alloys. Acta Mater. 2006, 54, 3033–3041. [Google Scholar] [CrossRef]
- Wang, R.M.; Eliezer, A.; Gutman, E.M. An investigation on the microstructure of an AM50 magnesium alloy. Mater. Sci. Eng. A 2003, 355, 201–207. [Google Scholar] [CrossRef]
- Liu, C.P.; Pan, F.S.; Wang, W.Q. Phase analysis of Al-Mn compounds in the AZ magnesium alloys. Mater. Sci. Forum 2007, 546–549, 395–398. [Google Scholar] [CrossRef]
- Han, G.; Ma, G.L.; Liu, X.F. Effect of manganese on the microstructure of Mg–3Al alloy. J. Alloys Compd. 2009, 486, 136–141. [Google Scholar] [CrossRef]
- Medved, J.; Mrvar, P. Thermal Analysis of the Mg–Al Alloys. Mater. Sci. Forum 2006, 508, 603–608. [Google Scholar] [CrossRef]
- Voncina, M.; Petric, M.; Mrvar, P.; Medved, J. Thermodynamic characterization of solidification and defects that occur in Mg-alloy AM60. J. Min. Metall. Sect. B 2017, 53, 107–114. [Google Scholar] [CrossRef]
- Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Birbilis, N. CALPHAD simulation of the Mg–(Mn, Zr)–Fe system and experimental comparison with as-cast alloy microstructures as relevant to impurity driven corrosion of Mg-alloys. Mater. Chem. Phys. 2014, 143, 1082–1091. [Google Scholar] [CrossRef]
- Xu, G.L.; Zhang, L.G.; Liu, L.B.; Du, Y.; Zhang, F.; Xu, K.; Liu, S.H.; Tan, M.Y.; Jin, Z.P. Thermodynamic database of multi-component Mg alloys and its application to solidification and heat treatment. J. Magn. Alloys 2016, 4, 249–264. [Google Scholar] [CrossRef]
- Chen, H.L.; Chen, Q.; Engström, A. Development and applications of the TCAL aluminum alloy database. Calphad 2018, 62, 154–171. [Google Scholar] [CrossRef]
- Schmid-Fetzer, R.; Zhang, F. The light alloy Calphad databases PanAl and PanMg. Calphad 2018, 61, 246–263. [Google Scholar] [CrossRef]
- Shi, R.H.; Luo, A.A. Applications of CALPHAD modeling and databases in advanced lightweight metallic materials. Calphad 2018, 62, 1–17. [Google Scholar] [CrossRef]
- Dahle, A.K.; Lee, Y.C.; Nave, M.D.; Schaffer, P.L.; StJohn, D.H. Development of the as-cast microstructure in magnesium–aluminium alloys. J. Light Met. 2001, 1, 61–72. [Google Scholar] [CrossRef]
- Khan, S.A.; Miyashita, Y.; Mutoh, Y.; Sajuri, Z.B. Influence of Mn content on mechanical properties and fatigue behavior of extruded Mg alloys. Mater. Sci. Eng. A 2006, 420, 315–321. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2014, 60, 169–194. [Google Scholar] [CrossRef]
- Zheng, W.S.; He, S.; Selleby, M.; He, Y.L.; Li, L.; Lu, X.G.; Ågren, J. Thermodynamic assessment of the Al–C–Fe system. Calphad 2017, 58, 34–49. [Google Scholar] [CrossRef]
- Zheng, W.; Mao, H.; Lu, X.G.; He, Y.; Li, L.; Selleby, M.; Ågren, J. Thermodynamic investigation of the Al-Fe-Mn system over the whole composition and wide temperature ranges. J. Alloys Compd. 2018, 742, 1046–1057. [Google Scholar] [CrossRef]
- Balanetskyy, S.; Pavlyuchkov, D.; Velikanova, T.; Grushko, B. The Al-rich region of the Al–Fe–Mn alloy system. J. Alloys Compd. 2015, 619, 211–220. [Google Scholar] [CrossRef]
- Lindahl, B.B.; Selleby, M. The Al–Fe–Mn system revisited-An updated thermodynamic description using the most recent binaries. Calphad 2013, 43, 86–93. [Google Scholar] [CrossRef]
- Lindahl, B.B.; Burton, B.P.; Selleby, M. Ordering in ternary BCC alloys applied to the Al–Fe–Mn system. Calphad 2015, 51, 211–219. [Google Scholar] [CrossRef]
- Qiu, K.; Wang, R.C.; Peng, C.Q.; Lu, X.X.; Wang, N.G. Polynomial regression and interpolation of thermodynamic data in Al–Si–Mg–Fe system. Calphad 2015, 48, 175–183. [Google Scholar] [CrossRef]
- Wu, P.P.; Xu, F.J.; Deng, K.K.; Han, F.Y.; Zhang, Z.Z.; Gao, R. Effect of extrusion on corrosion properties of Mg-2Ca-xAl (x = 0, 2, 3, 5) alloys. Corros. Sci. 2017, 127, 280–290. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, M.; Liu, Z.K. Contribution of first-principles energetics to Al–Mg thermodynamic modeling. Calphad 2005, 29, 303–311. [Google Scholar] [CrossRef]
- Gröbner, J.; Mirkovic, D.; Ohno, M.; Schmid-Fetzer, R. Experimental Investigation and Thermodynamic Calculation of Binary Mg–Mn Phase Equilibria. J. Phase Equilib. Diffus. 2005, 26, 234–239. [Google Scholar] [CrossRef]
- Ansara, I.; Dinsdale, A.T.; Rand, M.H. COST 507: Thermochemical Database for Light Metal Alloys; Office for Official Publications of the European Communities: Brussels, Belgium, 1998; Volume 2, p. 195. [Google Scholar]
- Du, Y.; Wang, J.; Zhao, J.R.; Schuster, J.C.; Weitzer, F.; Schmid-Fetzer, R.; Ohno, M.; Xu, H.; Liu, Z.K.; Shang, S.L.; et al. Reassessment of the Al–Mn system and a thermodynamic description of the Al–Mg–Mn system. Int. J. Mater. Res. 2007, 98, 855–871. [Google Scholar] [CrossRef]
- Sundman, B.; Ohnuma, I.; Dupin, N.; Kattner, U.R.; Fries, S.G. An assessment of the entire Al–Fe system including D03 ordering. Acta Mater. 2009, 57, 2896–2908. [Google Scholar] [CrossRef]
- Huang, W.M. An assessment of the Fe-Mn system. Calphad 1989, 13, 243–252. [Google Scholar] [CrossRef]
- Djurovic, D.; Hallstedt, B.; von Appen, J.; Dronskowski, R. Thermodynamic assessment of the Fe–Mn–C system. Calphad 2011, 35, 479–491. [Google Scholar] [CrossRef]
- Liu, S.H. Order-Disorder Phase Transition, Topology of Phase Diagrams and Their Applications during Solidification of Al Alloys. Ph.D. Thesis, Central South University, Changsha, China, 2010. [Google Scholar]
- Wang, P.S.; Zhao, J.R.; Du, Y.; Xu, H.H.; Gang, T.; Fen, J.C.; Zhang, L.J.; He, C.Y.; Liu, S.H.; Ouyang, H.W. Experimental investigation and thermodynamic calculation of the Fe–Mg–Mn and Fe–Mg–Ni systems. Int. J. Mater. Res. 2011, 102, 6–16. [Google Scholar] [CrossRef]
- Cao, P.; Qian, M.; StJohn, D.H. Effect of manganese on grain refinement of Mg–Al based alloys. Scr. Mater. 2006, 54, 1853–1858. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, M.; Fan, Z.; Zhou, X.; Thompson, G.E. The effect of Al8Mn5 intermetallic particles on grain size of as-cast Mg–Al–Zn AZ91D alloy. Intermetallics 2010, 18, 1683–1689. [Google Scholar] [CrossRef]
- Revie, R.W. Uhlig’s Corrosion Handbook, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2011; p. 830. [Google Scholar]
- Kim, H.J.; Kim, B.; Baek, S.M.; Sohn, S.D.; Shin, H.J.; Jeong, H.Y.; Yim, C.D.; You, B.S.; Ha, H.Y.; Park, S.S. Influence of alloyed Al on the microstructure and corrosion properties of extruded Mg–8Sn–1Zn alloys. Corros. Sci. 2015, 95, 133–142. [Google Scholar] [CrossRef]
- Liu, J.R.; Chen, H.K.; Zhao, L.; Huang, W.D. Oxidation behaviour of molten magnesium and AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres. Corros. Sci. 2009, 51, 129–134. [Google Scholar] [CrossRef]
- Pettersen, G.; Øvrelid, E.; Tranell, G.; Fenstad, J.; Gjestland, H. Characterisation of the surface films formed on molten magnesium in different protective atmospheres. Mater. Sci. Eng. A 2002, 332, 285–294. [Google Scholar] [CrossRef]
- Zeng, G.; Xian, J.W.; Gourlay, C.M. Nucleation and growth crystallography of Al8Mn5 on B2-Al(Mn,Fe) in AZ91 magnesium alloys. Acta Mater. 2018, 153, 364–376. [Google Scholar] [CrossRef]
- Crisan, A.D.; Vasiliu, F.; Nicula, R.; Bartha, C.; Mercioniu, I.; Crisan, O. Thermodynamic, structural and magnetic studies of phase transformations in MnAl nanocomposite alloys. Mater. Charact. 2018, 140, 1–8. [Google Scholar] [CrossRef]
- Balanetskyy, S.; Meisterernst, G.; Heggen, M.; Feuerbacher, M. Reinvestigation of the Al–Mn–Pd alloy system in the vicinity of the T-and R-phases. Intermetallics 2008, 16, 71–87. [Google Scholar] [CrossRef]
- Braun, P.B.; Goedkoop, J.A. An X-ray and neutron diffraction investigation of the magnetic phase Al0.89Mn1.11. Acta Cryst. 1963, 16, 737–740. [Google Scholar] [CrossRef]
- Koch, A.J.J.; Hokkeling, P.; vd. Steeg, M.G.; de Vos, K.J. New Material for Permanent Magnets on a Base of Mn and Al. J. Appl. Phys. 1960, 31, S75–S77. [Google Scholar] [CrossRef]
- Krendelsberger, N.; Weitzer, F.; Schuster, J.C. On the constitution of the system Al–Mn–Si. Metall. Mater. Trans. A 2002, 33, 3311–3319. [Google Scholar] [CrossRef]
- Grushko, B.; Stafford, G. A Cs–Cl-type phase in electrodeposited Al–Mn alloys. Scr. Metall. Mater. 1994, 31, 1711–1716. [Google Scholar] [CrossRef]
- Hafner, J.; Hobbs, D. Understanding the complex metallic element Mn. II. Geometric frustration in β-Mn, phase stability, and phase transitions. Phys. Rev. B 2003, 68, 014408–014423. [Google Scholar] [CrossRef]
- Nishidate, K.; Baba, M.; Sato, T.; Nishikawa, K. Molecular-dynamics studies on the shock-induced phase transition of a MgF2 crystal. Phys. Rev. B 1995, 52, 3170–3176. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yim, C.D.; You, B.S. Grain refining mechanism in Mg–Al base alloys with carbon addition. Scr. Mater. 2007, 57, 691–694. [Google Scholar] [CrossRef]
- Choi, H.Y.; Kim, W.J. Development of the highly corrosion resistant AZ31 magnesium alloy by the addition of a trace amount of Ti. J. Alloys Compd. 2016, 664, 25–37. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Zhang, M.L.; Yang, P.P.; Yang, L.H.; Cao, D.X.; Li, J.Q. One-step synthesis of lamellar molybdate pillared hydrotalcite and its application for AZ31 Mg alloy protection. Solid State Sci. 2009, 11, 376–381. [Google Scholar] [CrossRef]
- Feng, H.; Liu, S.H.; Du, Y.; Lei, T.; Zeng, R.C.; Yuan, T.C. Effect of the second phases on corrosion behavior of the Mg-Al-Zn alloys. J. Alloys Compd. 2017, 695, 2330–2338. [Google Scholar] [CrossRef]
- Wei, Y.H.; Zhang, L.X.; Ke, W. Evaluation of corrosion protection of carbon black filled fusion-bonded epoxy coatings on mild steel during exposure to a quiescent 3% NaCl solution. Corros. Sci. 2007, 49, 287–302. [Google Scholar] [CrossRef]
- Ma, Y.L.; Zhang, J.; Yang, M.B. Research on microstructure and alloy phases of AM50 magnesium alloy. J. Alloys Compd. 2009, 470, 515–521. [Google Scholar] [CrossRef]
- Andreatta, F.; Apachitei, I.; Kodentsov, A.A.; Dzwonczyk, J.; Duszczyk, J. Volta potential of second phase particles in extruded AZ80 magnesium alloy. Electrochim. Acta 2006, 51, 3551–3557. [Google Scholar] [CrossRef]











| Alloys | Al | Mn | Fe | Cu | Ni | Si | Fe/Mn | Mg |
|---|---|---|---|---|---|---|---|---|
| Mg-3Al | 2.97 | 0.01 | 0.013 | 0.001 | 0.006 | 0.001 | 1.300 | Balance |
| Mg-3Al-0.12Mn | 2.98 | 0.12 | 0.010 | 0.001 | 0.015 | 0.001 | 0.083 | Balance |
| Mg-3Al-0.21Mn | 2.86 | 0.21 | 0.010 | 0.001 | 0.030 | 0.001 | 0.048 | Balance |
| Mg-3Al-0.36Mn | 2.97 | 0.36 | 0.008 | 0.003 | 0.019 | 0.001 | 0.022 | Balance |
| Mg-3Al-0.45Mn | 2.96 | 0.45 | 0.015 | 0.002 | 0.018 | 0.001 | 0.033 | Balance |
| Phase | Other Name | Crystal System | Pearson Symbol | Space Group | Lattice Parameters | Reference | |
|---|---|---|---|---|---|---|---|
| a, b, c/Å | α, β, γ | ||||||
| Al8Mn5(LT) | γ2 D810-Al8Mn5 | Hexagonal | 12.54, 12.54, 15.74 | α = β = 90°, γ = 120° | [75] | ||
| Al8Mn5(HT) | γ1 D82-Al8Mn5 | Cubic | 8.89, 8.89, 8.89 | α = β = γ = 90° | [74,76] | ||
| τ–Al0.89Mn1.11 | τ | Tetragonal | 2.77, 2.77, 3.54 | α = β = γ = 90° | [77,78] | ||
| Al11Mn4(LT) | - | Triclinic | 5.11, 8.87, 5.06 | α = 89°, β = 101°, γ = 105° | [79] | ||
| B2-Al(Mn,Fe) | Bcc_B2, B2-AlMn | Cubic | 3.08, 3.08, 3.08 | α = β = γ = 90° | [74,80] | ||
| (βMn) | - | Cubic | 32 | 6.29, 6.29, 6.29 | α = β = γ = 90° | [81] | |
| MgF2 | - | Tetragonal | 4.63, 4.63, 3.06 | α = β = γ = 90° | [82] | ||
| Phase | Fe Range (at.%) | Morphologies | Alloys |
|---|---|---|---|
| Al8Mn5(LT) | 0.2–4 | Micro-plates Rods/ribbons Dice-like particles Polyhedral particles | Mg-3Al-xMn (x = 0.12, 0.21, 0.36, 0.45) |
| τ–Al0.89Mn1.11 | 0.5–5.9 | Micro-plates Polyhedral particles | Mg-3Al-xMn (x = 0.36, 0.45) |
| B2-Al(Mn,Fe) | 12.5–17.3 | Micro-plates Polyhedral particles | Mg-3Al-xMn (x = 0.12, 0.45) |
| Al-Fe phase | 15.3–17.3 | Irregular blocky particles | Mg-3Al |
| Alloys | icorr/uA·cm−2 | Ecorr/VSCE | Eb/VSCE | (Eb − Ecorr)/mV |
|---|---|---|---|---|
| Mg-3Al | 410.1 | −1.560 | - | - |
| Mg-3Al-0.12Mn | 112.5 | −1.545 | - | - |
| Mg-3Al-0.21Mn | 51.3 | −1.546 | - | - |
| Mg-3Al-0.36Mn | 18.6 | −1.485 | −1.471 | 14 |
| Mg-3Al-0.45Mn | 15.8 | −1.512 | −1.417 | 95 |
| Specimen | Rs /Ω·cm−2 | Cc /μF·cm−2 | Rc /Ω·cm−2 | CPE | Rct /Ω·cm−2 | L /H·cm−2 | Rdiff /Ω·cm−2 | |
|---|---|---|---|---|---|---|---|---|
| C /μF·cm−2 | n (0–1) | |||||||
| Mg-3Al | 4.81 | 6.62 | 12.75 | 3.05 | 1 | 21.34 | 14.46 | 78.9 |
| Mg-3Al-0.12Mn | 2.91 | 8.38 | 10.26 | 11.12 | 0.9123 | 37.03 | 26.42 | 86.4 |
| Mg-3Al-0.21Mn | 1.03 | 7.34 | 29.14 | 51.34 | 0.3518 | 53.95 | 58.68 | 255.4 |
| Mg-3Al-0.36Mn | 2.99 | 5.99 | 22.01 | 6.23 | 0.8646 | 95.21 | 49.58 | 279.5 |
| Mg-3Al-0.45Mn | 4.73 | 7.08 | 20.76 | 49.23 | 0.5554 | 106.54 | 58.09 | 341.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Liu, S.; Zeng, G.; Li, X.; Lei, T.; Li, Y.; Du, Y. Effect of Manganese on Microstructure and Corrosion Behavior of the Mg-3Al Alloys. Metals 2019, 9, 460. https://doi.org/10.3390/met9040460
Yao S, Liu S, Zeng G, Li X, Lei T, Li Y, Du Y. Effect of Manganese on Microstructure and Corrosion Behavior of the Mg-3Al Alloys. Metals. 2019; 9(4):460. https://doi.org/10.3390/met9040460
Chicago/Turabian StyleYao, Sheng, Shuhong Liu, Guang Zeng, Xiaojing Li, Ting Lei, Yunping Li, and Yong Du. 2019. "Effect of Manganese on Microstructure and Corrosion Behavior of the Mg-3Al Alloys" Metals 9, no. 4: 460. https://doi.org/10.3390/met9040460





