Influence of the Alkaline Reserve of Chloride-Contaminated Mortars on the 6-Year Corrosion Behavior of Corrugated UNS S32304 and S32001 Stainless Steels
Abstract
:1. Introduction
2. Experimental
- -
- HH: Non-carbonated samples manufactured without chlorides and exposed to high relative humidity (between 90% and 95%).
- -
- PI: Non-carbonated samples manufactured without chlorides and partially immersed in 3.5% (w/w) NaCl solution and at high relative humidity. In this case, the level of the solution was kept coinciding with the middle of the exposed length of the bars embedded in the mortar.
- -
- HH-PC: Partially carbonated samples manufactured without chlorides and exposed to high relative humidity (90–95%).
- -
- PI-PC: Partially carbonated samples manufactured without chlorides, partially immersed in 3.5% (w/w) NaCl solution and at high relative humidity. In this case, the level of the solution was also kept coinciding with the middle of the exposed length of the bars embedded in the mortar.
3. Results and Discussion
4. Conclusions
- S32304 steel was confirmed to be more corrosion resistant than S32001 (with lower Cr and Ni contents).
- The durability of S32001 in environments with very high chloride content can be limited, though other factors as the alkalinity of the cover also have a high impact.
- In mortars that keep their alkalinity, S32304 does not seem to have any corrosion risks for mortar chloride contents up to 1.8% (w/w).The decrease in the alkaline reserve of the mortars—with only a small decrease in their pH—could affect the corrosion behavior of both studied corrugated lean duplex stainless steels in chloride-rich environments.
- The duplex structure of the stainless steels makes the corrosion proceed by selective corrosion of the phases. Austenite corrodes preferentially except in the most-strained areas of the corrugated surface, where ferrite dissolves selectively.
Author Contributions
Funding
Conflicts of Interest
References
- Pachón-Montaño, A.; Sánchez-Montero, J.; Andrade, C.; Fullea, J.; Moreno, E.; Matres, V. Threshold concentration of chlorides in concrete for stainless steel reinforcement: Classic austenitic and new duplex stainless steel. Constr. Build. Mater. 2018, 186, 495–502. [Google Scholar] [CrossRef]
- Bautista, A.; Paredes, E.C.; Velasco, F.; Alvarez, S.M. Corrugated stainless steels embedded in mortar for 9 years: Corrosion results of non-carbonated, chloride-contaminated samples. Constr. Build. Mater. 2015, 93, 350–359. [Google Scholar] [CrossRef]
- Bautista, A.; Alvarez, S.M.; Paredes, E.C.; Velasco, F.; Guzmán, S. Corrugated stainless steels embedded in carbonated mortars with and without chlorides: 9-Year corrosion results. Constr. Build. Mater. 2015, 95, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, S.; Bastidas, D.M.; Ryan, M.P.; Criado, M.; McPhail, D.S.; Morris, R.J.H.; Bastidas, J.M. Low energy SIMS characterization of passive oxide films formed on a low-nickel stainless steel in alkaline media. Appl. Surf. Sci. 2014, 288, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Bautista, A.; Blanco, G.; Velasco, F.; Gutierrez, A.; Soriano, L.; Palomares, F.J.; Takenouti, H. Changes in the passive layer of corrugated austenitic stainless steel of low nickel content due to exposure to simulated pore solutions. Corros. Sci. 2009, 51, 785–792. [Google Scholar] [CrossRef]
- Gardner, L.; Ng, K.T. Temperature development in structural stainless steel sections exposed to fire. Fire Saf. J. 2006, 41, 185–203. [Google Scholar] [CrossRef]
- Pérez-Quiroz, J.T.; Terán, J.; Herrera, M.J.; Martínez, M.; Genescá, J. Assessment of stainless steel reinforcement for concrete structures rehabilitation. J. Constr. Steel Res. 2008, 64, 1317–1324. [Google Scholar] [CrossRef]
- Callaghan, B.G. The performance of a 12% Cr chromium steel in concrete in severe marine environments. Corros. Sci. 1993, 35, 1535–1541. [Google Scholar] [CrossRef]
- Sedar, M.; Meral, C.; Kunz, M.; Bjegovic, D.; Wenk, H.-R.; Monteiro, P.J.M. Spatial distribution of crystalline corrosion products formed during corrosion of stainless steel in concrete. Cem. Concr. Res. 2015, 71, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Kouril, M.; Novak, P.; Bojko, M. Threshold chloride concentration for stainless steel activation in concrete pore solutions. Cem. Concr. Res. 2010, 40, 431–436. [Google Scholar] [CrossRef]
- Itty, P.-A.; Sedar, M.; Meral, C.; Parkinson, D.; MacDowell, A.A.; Bjegovic, D.; Monteiro, P.J.M. In situ 3D monitoring of corrosion on carbon steel and ferritic stainless steel embedded in cement paste. Corros. Sci. 2014, 83, 409–418. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.; Dong, C.; Xiao, K.; Li, X. Electrochemical and passivation behavior investigation of ferritic stainless steel in simulated concrete pore media. Data Brief 2015, 5, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolini, L.; Pedeferri, P. Laboratory and field experience on the use of stainless steels to improve the durability of reinforced concrete. Corros. Rev. 2002, 20, 129–152. [Google Scholar] [CrossRef]
- Bautista, A.; Blanco, G.; Velasco, F. Corrosion behaviour of low-nickel austenitic stainless steels reinforcements: A comparative study in simulated pore solutions. Cem. Concr. Res. 2006, 36, 1922–1930. [Google Scholar] [CrossRef]
- Moser, R.; Singh, P.M.; Kahn, L.F.; Kurtis, K.E.; González-Niño, D.; McClelland, Z.B. Crevice corrosion and environmentally assisted cracking of high-strength duplex stainless steels in simulated concrete pore solutions. Constr. Build. Mater. 2019, 203, 366–376. [Google Scholar] [CrossRef]
- Fajardo, S.; Bastidas, D.M.; Criado, M.; Bastidas, J.M. Electrochemical study of a new-low nickel stainless teel in carbonated solution in the presence of chlorides. Electrochim. Acta 2014, 129, 160–170. [Google Scholar] [CrossRef]
- Freire, L.; Novoa, X.R.; Pena, G.; Vivier, V. On the corrosion mechanism of AISI 204Cu stainless steel in chlorinated alkaline media. Corros. Sci. 2008, 50, 3205–3212. [Google Scholar] [CrossRef]
- Moser, R.D.; Singh, P.M.; Kahn, L.F.; Kurtis, K.E. Chloride-induced corrosion resistance of high-strength stainless steels in simulated alkaline and carbonated concrete pore solutions. Corros. Sci. 2012, 57, 241–253. [Google Scholar] [CrossRef]
- Sedar, M.; Zulj, L.V.; Bjegovic, D. Long-term corrosion behaviour of stainless reinforcing steel in mortar exposed in chloride environment. Corros. Sci. 2013, 69, 149–157. [Google Scholar] [CrossRef]
- Alvarez, S.M.; Bautista, A.; Velasco, F. Corrosion resistance of corrugated lean duplex stainless steel in simulated concrete pore solutions. Corros. Sci. 2011, 53, 1748–1755. [Google Scholar] [CrossRef]
- Medina, E.; Medina, J.M.; Cobo, A.; Bastidas, D.M. Evaluation of mechanical and structural behavior of austenitic and duplex stainless steel reinforcements. Constr. Build. Mater. 2015, 78, 1–15. [Google Scholar] [CrossRef]
- Briz, E.; Biezma, M.V.; Bastidas, D.M. Stress corrosion cracking of new 2001 lean–duplex stainless steel reinforcements in chloride contained concrete pore solution: An electrochemical study. Constr. Build. Mater. 2018, 192, 1–8. [Google Scholar] [CrossRef]
- Bertolini, L.; Gastaldi, M. Corrosion resistance of low-nickel duplex stainless steel rebars. Mater. Corros. 2011, 62, 120–129. [Google Scholar] [CrossRef]
- Gastaldi, M.; Bertolini, L. Effect of temperature on the corrosion behavior of new-low nickel stainless steel in concrete. Cem. Concr. Res. 2014, 56, 52–60. [Google Scholar] [CrossRef]
- Duarte, R.G.; Castela, A.S.; Neves, R.; Freire, L.; Montemor, M.F. Corrosion behaviour of stainless steel rebars embedded in concrete: An electrochemical impedance spectroscopy study. Electrochim. Acta 2014, 124, 218–224. [Google Scholar] [CrossRef]
- Mundra, S.; Criado, M.; Bernal, S.A.; Provis, J.L. Chloride-induced corrosion of steel rebars in simulated pore solutions of alkali-activated concretes. Cem. Concr. Res. 2017, 100, 385–397. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Otero, E.; Feliu, S.; Bautista, A.; Ramírez, E.; Rodríguez, P.; López, W. Some considerations on the effect of chloride ions on the corrosion of steel reinforcements embedded in concrete structures. Mag. Concr. Res. 1998, 50, 189–199. [Google Scholar] [CrossRef]
- Paul, S.C.; Panda, B.; Huang, Y.; Garg, A.; Peng, X. An empirical model design for evaluation and estimation of carbonation depth in concrete. Measurement 2018, 124, 205–210. [Google Scholar] [CrossRef]
- Angulo-Ramírez, D.E.; Mejía de Gutiérrez, R.; Valencia-Saavedra, W.G.; de Medeiros, M.H.F.; Hoppe-Filho, J. Carbonation of hybrid concrete with high blast furnace slag content and its impact on structural steel corrosion. Mater. Constr. 2019, 69, e182. [Google Scholar] [CrossRef]
- Alonso, M.C.; Luna, F.J.; Criado, M. Corrosion behavior of duplex stainless steelreinforcement in ternary binder concrete exposed to natural chloride penetration. Constr. Build. Mater. 2019, 199, 385–395. [Google Scholar] [CrossRef]
- RILEM. Draft recommendation for repair strategies for concrete structures damaged by reinforcement corrosion. Mater. Struct. 1994, 27, 415–438. [Google Scholar] [CrossRef]
- Czarnecki, L.; Woyciechowski, P. Concrete carbonation as a limited process and its relevance to concrete cover thickness. ACI Mater. J. 2012, 109, 275–282. [Google Scholar]
- Dhir, R.K.; Jones, M.R.; Ahmed, H.E.H. Determination of total and soluble chlorides in concrete. Cem. Concr. Res. 1990, 20, 579–590. [Google Scholar] [CrossRef]
- Song, H.-K.; Kwon, S.J. Permeability characteristics of carbonated concrete considering capillary pore structure. Cem. Concr. Res. 2007, 37, 909–915. [Google Scholar] [CrossRef]
- Bautista, A.; Paredes, E.C.; Alvarez, S.M.; Velasco, F. Welded, sandblasted, stainless steel corrugated bars in non-carbonated and carbonated mortars: A 9-year corrosion study. Corros. Sci. 2016, 103, 363–372. [Google Scholar] [CrossRef]
- Paredes, E.C.; Bautista, A.; Velasco, F.; Alvarez, S.M. Welded, pickled stainless steel reinforcements: Corrosion results after 9 years in mortar. Mag. Concr. Res. 2016, 68, 1099–1109. [Google Scholar] [CrossRef]
- Page, C.L. Mechanism of corrosion protection in reinforced-concrete marine structures. Nature 1975, 258, 514–515. [Google Scholar] [CrossRef]
- Glass, G.K.; Yang, R.; Dickhaus, T.; Buenfeld, N.R. Backscattered electron imaging of the steel-concrete interface. Corros. Sci. 2001, 43, 605–610. [Google Scholar] [CrossRef]
- Chen, F.; Chun-Qing, L.; Baji, H.; Baogue, M. Quantification of steel-concrete interface in reinforced concrete using Backscattered Electron imaging technique. Contr. Build. Mater. 2018, 179, 420–429. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A. Electrochemical polarization I. A theoretical analysis of the shape of the polarization curves. J. Electrochem. Soc. 1957, 104, 56–58. [Google Scholar] [CrossRef]
- Andrade, C.; Gonzalez, J.A. Quantitative measurements of corrosion rate of reinforcing steel embedded in concrete using polarization resistance measurements. Mater. Corros. 1978, 29, 515–519. [Google Scholar] [CrossRef]
- Stefanoni, M.; Angst, U.; Elsener, B. Corrosion rate of carbon steel in carbonated concrete—A critical review. Cem. Concr. Res. 2018, 103, 35–48. [Google Scholar] [CrossRef]
- Song, H.W.; Saraswathy, V. Corrosion monitoring of reinforced concrete structures—A review. Int. J. Electrochem. Sci. 2007, 2, 1–28. [Google Scholar]
- Revert, A.B.; Hornbostel, K.; De Weerdt, K.; Geiker, M.R. Macrocell corrosion in carbonated Portland and Portland-fly ash concrete—Contribution and mechanism. Cem. Concr. Res. 2019, 116, 273–283. [Google Scholar] [CrossRef]
- Paredes, E.C.; Bautista, A.; Alvarez, S.M.; Velasco, F. Influence of the forming process of corrugated stainless steels on their corrosion behaviour in simulated pore solutions. Corros. Sci. 2012, 58, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Monrrabal, G.; Bautista, A.; Guzman, S.; Gutierrez, C.; Velasco, F. Influence of the cold working induced martensite on the electrochemical behavior of AISI 304 stainless steel surfaces. J. Mater. Res. Technol. 2019, 8, 1135–1346. [Google Scholar] [CrossRef]
- Bautista, A.; Alvarez, S.M.; Velasco, F. Selective corrosion of duplex stainless steel bars in acid. Part II: Effect of the surface strain and numerical analysis. Mater. Corros. 2015, 66, 357–365. [Google Scholar] [CrossRef]
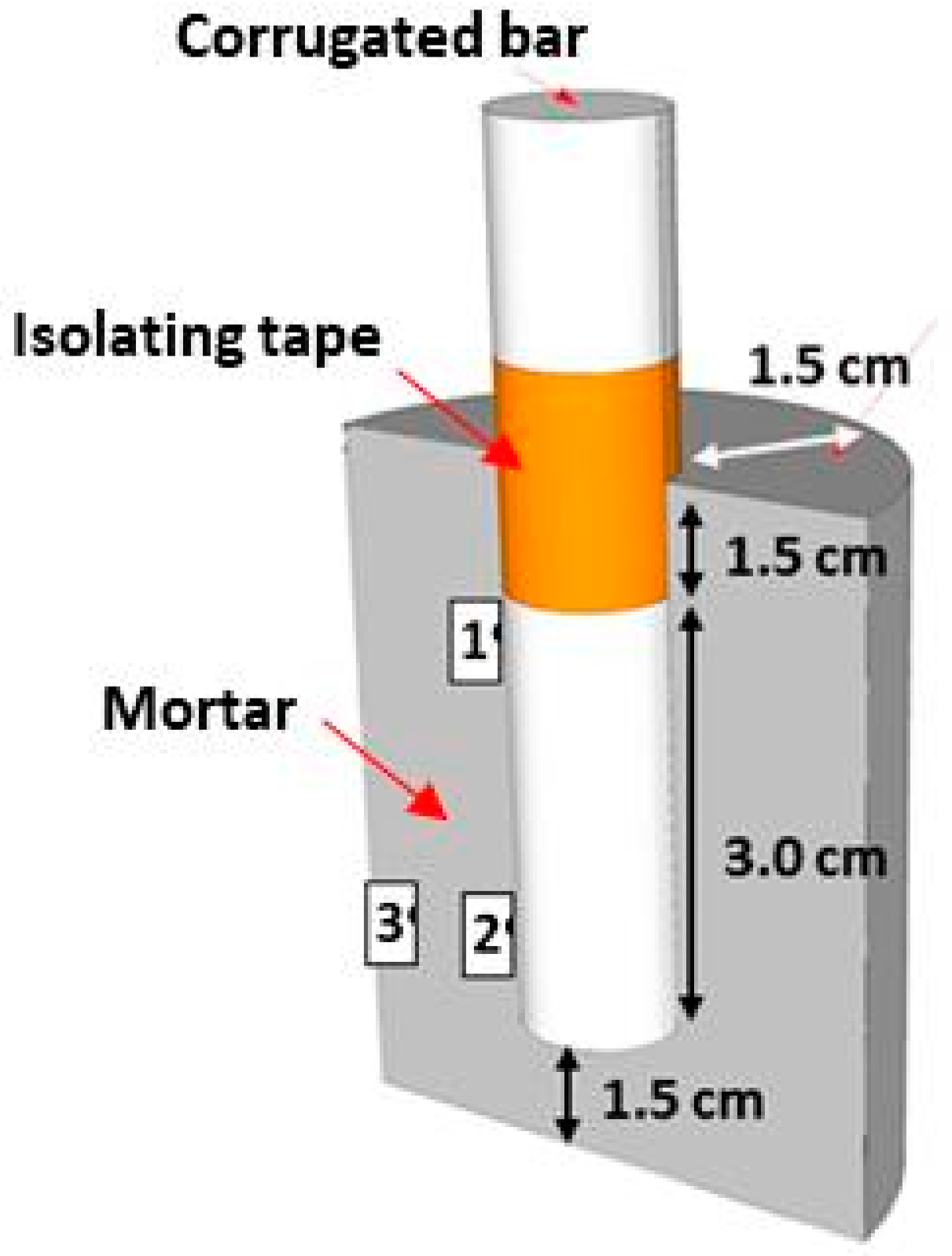




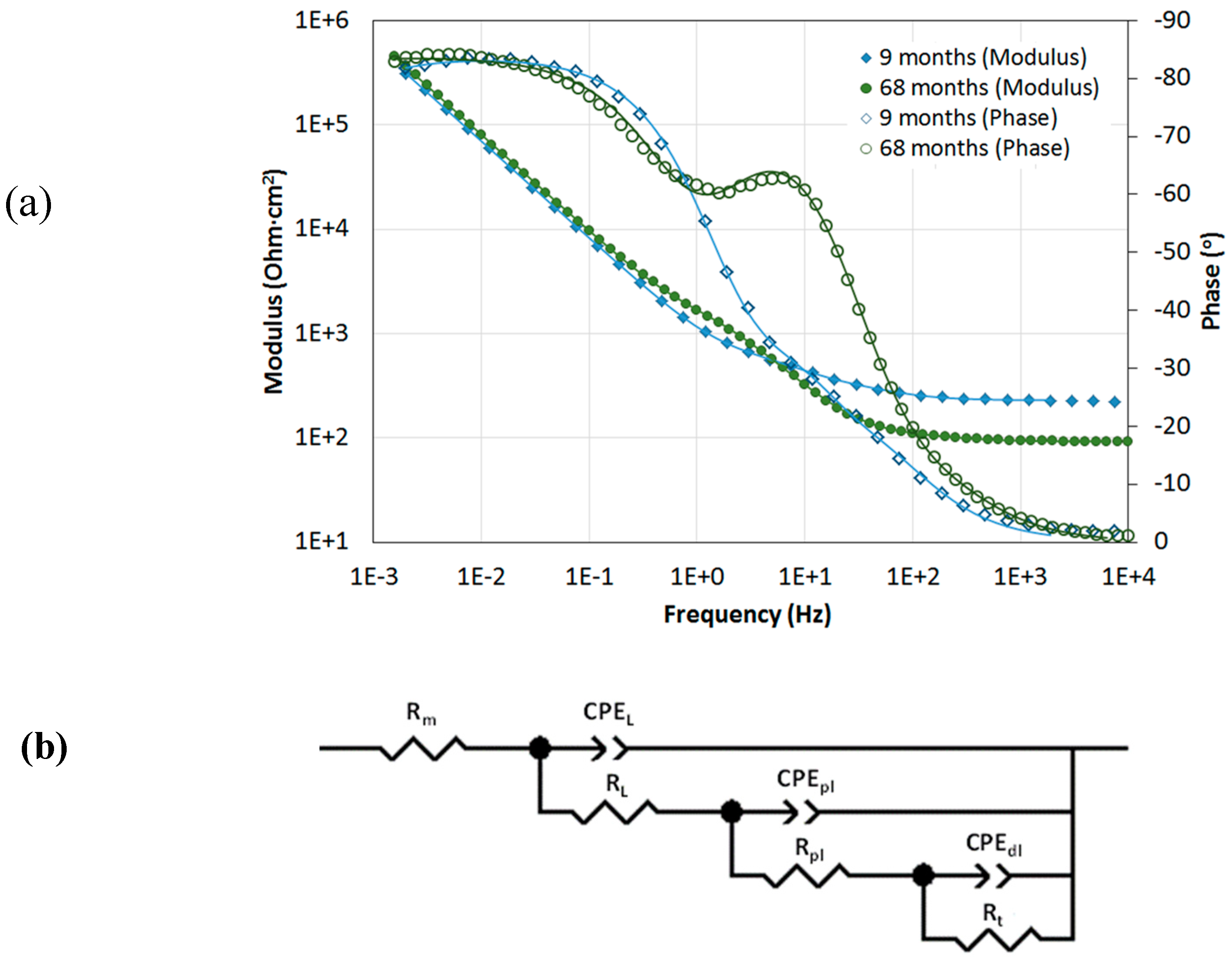



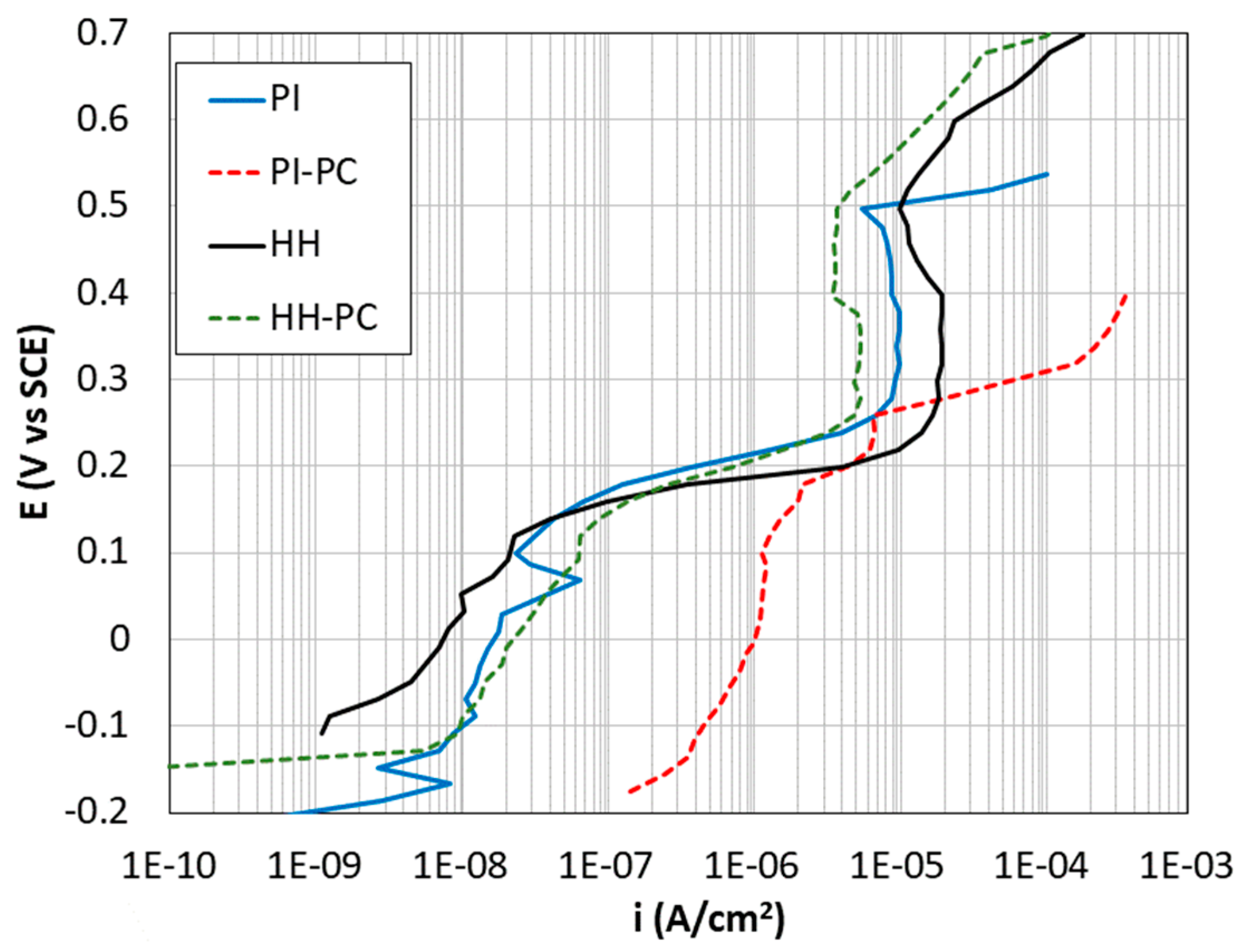


| UNS Grade | Ultimate Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) |
|---|---|---|---|
| S32304 | 769 | 568 | 38 |
| S32001 | 824 | 553 | 44 |
| UNS Grade | Chemical Composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | S | Si | Mn | Cr | Ni | Mo | N | Cu | Fe | |
| S32304 | 0.017 | 0.002 | 0.57 | 1.68 | 23.7 | 4.32 | 0.24 | 0.153 | 0.186 | Bal. |
| S32001 | 0.025 | 0.002 | 0.75 | 4.39 | 20.6 | 1.74 | 0.22 | 0.124 | 0.073 | Bal. |
| EIS Simulation Parameters | Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 15 | 24 | 36 | 42 | 50 | 60 | 68 | 73 | ||
| Rm (Ω·cm2) | 268 ± 36 | 202 ± 70 | 247 ± 146 | 263 ± 112 | 202 ± 79 | 240 ± 86 | 256 ± 92 | 218 ± 108 | 205 ± 73 | |
| RL (Ω·cm2) | 112 ± 42 | 65 ± 27 | 225 ± 119 | 527 ± 112 | 482 ± 85 | 802 ± 531 | 838 ± 497 | 1159 ± 355 | 1379 ± 286 | |
| CPEL (μF·cm−2·sn−1) | 30 ± 19 | 34 ± 17 | 20 ± 12 | 19 ± 3 | 22 ± 3 | 30 ± 11 | 34 ± 10 | 30 ± 5 | 29 ± 10 | |
| nL | 0.76 ± 0.14 | 0.78 ± 0.12 | 0.81 ±0.11 | 0.72 ± 0.05 | 0.74 ± 0.03 | 0.75 ± 0.08 | 0.78 ± 0.08 | 0.69 ± 0.07 | 0.69 ± 0.03 | |
| Rpl (kΩ·cm2) | S32304 | 0.4 ± 0.0 | 0.3 ± 0.1 | 0.5 ± 0.2 | 0.4 ± 0.0 | 0.6 ± 0.2 | 1.3 ± 0.6 | 1.5 ± 0.7 | 1.5 ± 0.5 | 1.6 ± 0.4 |
| S32001 | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.7 ± 0.1 | 0.6 ± 0.0 | 1.9 ± 1.6 | 1.8 ± 0.9 | 2.0 ± 1.3 | 3.2 ± 0.2 | |
| CPEpl (μF·cm−2·sn−1) | S32304 | 130 ± 19 | 141 ± 5 | 142 ± 49 | 89 ± 20 | 101 ± 16 | 111 ± 23 | 92 ± 26 | 106 ± 24 | 107 ± 16 |
| S32001 | 99 ± 10 | 88 ± 22 | 108 ± 64 | 32 ± 10 | 64 ± 7 | 65 ± 53 | 79 ± 23 | 81 ± 24 | 86 ± 9 | |
| npl | S32304 | 0.97 ± 0.03 | 0.97 ± 0.02 | 0.96 ± 0.00 | 0.96 ± 0.02 | 0.95 ± 0.01 | 0.96 ± 0.01 | 0.95 ± 0.02 | 0.96 ± 0.02 | 0.96 ± 0.02 |
| S32001 | 0.99 ± 0.01 | 0.95 ± 0.06 | 0.96 ± 0.02 | 0.89 ± 0.01 | 0.95 ± 0.01 | 0.92 ± 0.12 | 0.97 ± 0.03 | 0.99 ± 0.01 | 0.99 ± 0.01 | |
| Rt (MΩ·cm2) | S32304 | 14 ± 8 | 5.2 ± 3.1 | 13 ± 8 | 67 ± 24 | 16 ± 7.7 | 37 ± 5 | 28 ± 10 | 78 ± 20 | 43 ± 11 |
| S32001 | 5.2 ± 1 | 0.7 ± 0.3 | 5.3 ± 2.7 | 9.7 ± 2 | 8.2 ± 7 | 8.4 ± 2.6 | 16 ± 9.5 | 36 ± 27 | 19 ± 8.8 | |
| CPEdl (μF·cm−2·sn−1) | S32304 | 130 ± 33 | 127 ± 30 | 163 ± 20 | 170 ± 53 | 187 ± 22 | 141 ± 17 | 165 ± 24 | 160 ± 19 | 159 ± 20 |
| S32001 | 98 ± 20 | 140 ± 45 | 133 ± 48 | 171 ± 20 | 132 ± 6 | 106 ± 40 | 87 ± 22 | 75 ± 18 | 64 ± 1 | |
| ndl | S32304 | 0.94 ± 0.04 | 0.93 ± 0.02 | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.94 ± 0.02 | 0.94 ± 0.02 |
| S32001 | 0.98 ± 0.01 | 0.95 ± 0.01 | 0.95 ± 0.02 | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.94 ± 0.03 | 0.92 ± 0.05 | 0.93 ± 0.05 | 0.97 ± 0.03 | |
| EIS Simulation Parameters | Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 15 | 24 | 36 | 42 | 50 | 60 | 68 | 73 | ||
| Rm (Ω·cm2) | 268 ± 35 | 249 ± 62 | 247 ± 94 | 263 ± 59 | 202 ± 84 | 240 ± 89 | 256 ± 98 | 218 ± 87 | 205 ± 118 | |
| RL (Ω·cm2) | 102 ± 16 | 101 ± 69 | 112 ± 71 | 182 ± 92 | 153 ± 67 | 313 ± 74 | 257 ± 111 | 201 ± 172 | 611 ± 290 | |
| CPEL (μF·cm−2·sn−1) | 72 ± 41 | 29 ± 15 | 65 ± 40 | 44 ± 29 | 32 ± 25 | 22 ± 8 | 21 ± 12 | 15 ± 11 | 13 ± 14 | |
| nL | 0.74 ± 0.09 | 0.86 ± 0.16 | 0.83 ± 0.11 | 0.84 ± 0.07 | 0.80 ± 0.09 | 0.67 ± 0.12 | 0.66 ± 0.11 | 0.75 ± 0.10 | 0.64 ± 0.06 | |
| Rpl (kΩ·cm2) | S32304 | 0.8 ± 0.2 | 0.8 ± 0.5 | 0.8 ± 0.3 | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.5 ± 0.2 |
| S32001 | 0.6 ± 0.2 | 0.3 ± 0.1 | 0.9 ± 0.6 | 0.5 ± 0.0 | 0.4 ± 0.2 | 0.4 ± 0.3 | 0.8 ± 0.7 | 0.3 ± 0.0 | 0.9 ± 0.8 | |
| CPEpl (μF·cm−2·sn−1) | S32304 | 114 ± 18 | 118 ± 85 | 131 ± 23 | 126 ± 22 | 133 ± 19 | 70 ± 21 | 154 ± 80 | 140 ± 66 | 145 ± 75 |
| S32001 | 89 ± 9 | 110 ± 27 | 157 ± 89 | 106 ± 13 | 131 ± 29 | 92 ± 31 | 109 ± 72 | 152 ± 29 | 111 ± 83 | |
| npl | S32304 | 0.97 ± 0.05 | 0.97 ± 0.04 | 0.95 ± 0.08 | 0.97 ± 0.01 | 0.96 ± 0.00 | 0.93 ± 0.06 | 0.91 ± 0.03 | 0.92 ± 0.04 | 0.91 ± 0.06 |
| S32001 | 0.99 ± 0.02 | 0.96 ± 0.04 | 0.93 ± 0.06 | 0.97 ± 0.04 | 0.93 ± 0.02 | 0.96 ± 0.04 | 0.97 ± 0.06 | 0.91 ± 0.13 | 0.94 ± 0.09 | |
| Rt (MΩ·cm2) | S32304 | 7.3 ± 4.1 | 1.3 ± 1.0 | 3.8 ± 2.2 | 2.9 ± 0.3 | 15 ± 7 | 5 ± 2 | 10 ± 6.5 | 13 ± 9 | 4.7 ± 2.5 |
| S32001 | 1.1 ± 0.7 | 1.2 ± 0.8 | 17 ± 3 | 0.7 ± 0.1 | 0.1 ± 0.1 | 19 ± 10 | 16 ± 10 | 0.4 ± 0.1 | 0.2 ± 0.1 | |
| CPEdl (μF·cm−2·sn−1) | S32304 | 55 ± 2 | 79 ± 34 | 92 ± 33 | 129 ± 0 | 154 ± 6 | 170 ± 55 | 148 ± 34 | 158 ± 7 | 170 ± 32 |
| S32001 | 80 ± 50 | 101 ± 27 | 29 ± 26 | 110 ± 33 | 133 ± 79 | 80 ± 55 | 110 ± 85 | 118 ± 40 | 103 ± 90 | |
| ndl | S32304 | 0.98 ± 0.02 | 0.93 ± 0.07 | 0.95 ± 0.06 | 0.93 ± 0.00 | 0.93 ± 0.00 | 0.94 ± 0.02 | 0.94 ± 0.02 | 0.93 ± 0.01 | 0.92 ± 0.01 |
| S32001 | 0.92 ± 0.10 | 0.89 ± 0.06 | 1.00 ± 0.00 | 0.94 ± 0.04 | 0.94 ± 0.05 | 0.94 ± 0.06 | 0.95 ± 0.07 | 0.92 ± 0.12 | 0.95 ± 0.04 | |
| EIS Simulation Parameters | Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 15 | 24 | 36 | 42 | 50 | 60 | 68 | 73 | ||
| Rm (Ω·cm2) | 282 ± 53 | 227 ± 47 | 276 ± 98 | 234 ± 41 | 197 ± 25 | 261 ± 64 | 295 ± 50 | 205 ± 58 | 208 ± 59 | |
| RL (Ω·cm2) | 120 ± 31 | 72 ± 40 | 150 ± 25 | 55 ± 39 | 116 ± 96 | 90 ± 63 | 191 ± 95 | 84 ± 57 | 71 ± 21 | |
| CPEL (μF·cm−2·sn−1) | 33 ± 16 | 26 ± 23 | 39 ± 31 | 28 ± 19 | 34 ± 33 | 17 ± 12 | 39 ± 14 | 19 ± 22 | 14 ± 8 | |
| nL | 0.85 ± 0.07 | 0.86 ± 0.05 | 0.82 ± 0.10 | 0.87 ± 0.05 | 0.88 ± 0.12 | 0.80 ± 0.06 | 0.88 ± 0.02 | 0.83 ± 0.08 | 0.82 ± 0.07 | |
| Rpl (kΩ·cm2) | S32304 | 1.1 ± 0.2 | 1.0 ± 0.1 | 2.5 ± 0.4 | 2.1 ± 1.2 | 1.8 ± 1.3 | 3.7 ± 1.4 | 7.8 ± 1.4 | 2.9 ± 1.8 | 5.5 ± 3.3 |
| S32001 | 0.8 ± 0.5 | 0.7 ± 0.4 | 2.6 ± 0.6 | 6.5 ± 5.5 | 0.9 ± 0.7 | 1.8 ± 1.5 | 15.0 ± 8.1 | 2.6 ± 2.3 | 5.6 ± 1.0 | |
| CPEpl (μF·cm−2·sn−1) | S32304 | 54 ± 10 | 95 ± 18 | 68 ± 20 | 113 ± 5 | 121 ± 8 | 122 ± 9 | 78 ± 46 | 103 ± 24 | 77 ± 8 |
| S32001 | 61 ± 18 | 70 ± 18 | 75 ± 0 | 90 ± 35 | 62 ± 48 | 71 ± 13 | 95 ± 11 | 62 ± 26 | 42 ± 19 | |
| npl | S32304 | 0.89 ± 0.02 | 0.93 ± 0.01 | 0.91 ± 0.02 | 0.90 ± 0.00 | 0.94 ± 0.03 | 0.90 ± 0.00 | 0.90 ± 0.03 | 0.89 ± 0.02 | 0.88 ± 0.03 |
| S32001 | 0.96 ± 0.01 | 0.94 ± 0.07 | 0.91 ± 0.12 | 0.95 ± 0.02 | 0.94 ± 0.01 | 0.91 ± 0.05 | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.96 ± 0.05 | |
| Rt (MΩ·cm2) | S32304 | 30 ± 8 | 19 ± 13 | 68 ± 3 | 2 ± 0 | 17 ± 10 | 29 ± 15 | 60 ± 43 | 150 ± 77 | 270 ± 179 |
| S32001 | 10 ± 2 | 27 ± 20 | 12 ± 4 | 8 ± 5 | 14 ± 6 | 28 ± 7 | 330 ± 140 | 23 ± 6 | 77 ± 35 | |
| CPEdl (μF·cm−2·sn−1) | S32304 | 145 ± 10 | 97 ± 24 | 114 ± 25 | 100 ± 17 | 79 ± 32 | 75 ± 17 | 87 ± 28 | 92 ± 48 | 94 ± 25 |
| S32001 | 87 ± 20 | 83 ± 8 | 67 ± 25 | 74 ± 64 | 106 ± 31 | 96 ± 16 | 38 ± 18 | 78 ± 29 | 98 ± 9 | |
| ndl | S32304 | 0.89 ± 0.03 | 0.93 ± 0.01 | 0.91 ± 0.02 | 0.90 ± 0.00 | 0.94 ± 0.03 | 0.90 ± 0.00 | 0.90 ± 0.03 | 0.89 ± 0.02 | 0.88 ± 0.03 |
| S32001 | 0.96 ± 0.01 | 0.94 ± 0.07 | 0.95 ± 0.01 | 0.95 ± 0.02 | 0.94 ± 0.01 | 0.91 ± 0.05 | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.93 ± 0.01 | |
| EIS Simulation Parameters | Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 15 | 24 | 36 | 42 | 50 | 60 | 68 | 73 | ||
| Rm (Ω·cm2) | 312 ± 57 | 309 ± 70 | 442 ± 35 | 463 ± 54 | 324 ± 37 | 463 ± 101 | 549 ± 57 | 422 ± 73 | 472 ± 53 | |
| RL (Ω·cm2) | 127 ± 105 | 125 ± 51 | 324 ± 126 | 308 ± 139 | 186 ± 69 | 241 ± 70 | 346 ± 206 | 250 ± 223 | 271 ± 157 | |
| CPEL (μF·cm−2·sn−1) | 32 ± 30 | 28 ± 11 | 35 ± 15 | 28 ± 8 | 33 ± 15 | 17 ± 8 | 19 ± 8 | 17 ± 7 | 16 ± 9 | |
| nL | 0.87 ± 0.07 | 0.89 ± 0.03 | 0.88 ± 0.04 | 0.86 ± 0.05 | 0.87 ± 0.03 | 0.84 ± 0.02 | 0.85 ± 0.05 | 0.84 ± 0.06 | 0.83 ± 0.05 | |
| Rpl (kΩ·cm2) | S32304 | 1.1 ± 1.0 | 0.5 ± 0.2 | 1.8 ± 0.6 | 1.8 ± 0.8 | 1.1 ± 0.5 | 1.3 ± 0.6 | 1.6 ± 0.4 | 1.4 ± 0.6 | 2.3 ± 1.4 |
| S32001 | 0.5 ± 0.4 | 0.5 ± 0.2 | 3.1 ± 1.5 | 1.6 ± 1.0 | 1.5 ± 1.4 | 1.1 ± 0.3 | 0.7 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.1 | |
| CPEpl (μF·cm−2·sn−1) | S32304 | 84 ± 20 | 80 ± 6 | 81 ± 19 | 81 ± 28 | 96 ± 16 | 80 ± 12 | 79 ± 4 | 86 ± 9 | 88 ± 7 |
| S32001 | 67 ± 13 | 67 ± 15 | 85 ± 9 | 68 ± 15 | 83 ± 15 | 48 ± 11 | 63 ± 15 | 69 ± 19 | 60 ± 21 | |
| npl | S32304 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.93 ± 0.01 | 0.92 ± 0.01 | 0.93 ± 0.01 | 0.91 ± 0.01 | 0.92 ± 0.01 | 0.93 ± 0.01 | 0.92 ± 0.02 |
| S32001 | 0.95 ± 0.02 | 0.95 ± 0.01 | 0.93 ± 0.01 | 0.89 ± 0.02 | 0.92 ± 0.02 | 0.90 ± 0.02 | 0.91 ± 0.03 | 0.89 ± 0.02 | 0.89 ± 0.02 | |
| Rt (MΩ·cm2) | S32304 | 50 ± 27 | 32 ± 28 | 20 ± 22 | 130 ± 72 | 200 ± 130 | 80 ± 42 | 190 ± 120 | 82 ± 64 | 28 ± 10 |
| S32001 | 23 ± 14 | 23 ± 16 | 69 ± 48 | 140 ± 30 | 77 ± 97 | 24 ± 17 | 180 ± 97 | 40 ± 10 | 170 ± 105 | |
| CPEdl (μF·cm−2·sn−1) | S32304 | 77 ± 22 | 101 ± 20 | 84 ± 21 | 96 ± 12 | 82 ± 11 | 97 ± 14 | 91 ± 17 | 86 ± 7 | 78 ± 16 |
| S32001 | 112 ± 14 | 105 ± 21 | 69 ± 15 | 75 ± 20 | 79 ± 28 | 75 ± 7 | 85 ± 36 | 81 ± 26 | 86 ± 30 | |
| ndl | S32304 | 0.91 ± 0.00 | 0.91 ± 0.00 | 0.91 ± 0.01 | 0.91 ± 0.01 | 0.90 ± 0.00 | 0.90 ± 0.00 | 0.90 ± 0.01 | 0.91 ± 0.01 | 0.90 ± 0.00 |
| S32001 | 0.94 ± 0.00 | 0.92 ± 0.01 | 0.92 ± 0.01 | 0.87 ± 0.02 | 0.89 ± 0.02 | 0.88 ± 0.02 | 0.91 ± 0.03 | 0.90 ± 0.04 | 0.90 ± 0.03 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista, A.; Velasco, F.; Torres-Carrasco, M. Influence of the Alkaline Reserve of Chloride-Contaminated Mortars on the 6-Year Corrosion Behavior of Corrugated UNS S32304 and S32001 Stainless Steels. Metals 2019, 9, 686. https://doi.org/10.3390/met9060686
Bautista A, Velasco F, Torres-Carrasco M. Influence of the Alkaline Reserve of Chloride-Contaminated Mortars on the 6-Year Corrosion Behavior of Corrugated UNS S32304 and S32001 Stainless Steels. Metals. 2019; 9(6):686. https://doi.org/10.3390/met9060686
Chicago/Turabian StyleBautista, Asunción, Francisco Velasco, and Manuel Torres-Carrasco. 2019. "Influence of the Alkaline Reserve of Chloride-Contaminated Mortars on the 6-Year Corrosion Behavior of Corrugated UNS S32304 and S32001 Stainless Steels" Metals 9, no. 6: 686. https://doi.org/10.3390/met9060686





