Design for Novel Hot-Work Die Steel by Thermodynamic Calculation and Microstructural Examination
Abstract
:1. Introduction
2. Materials and Methods
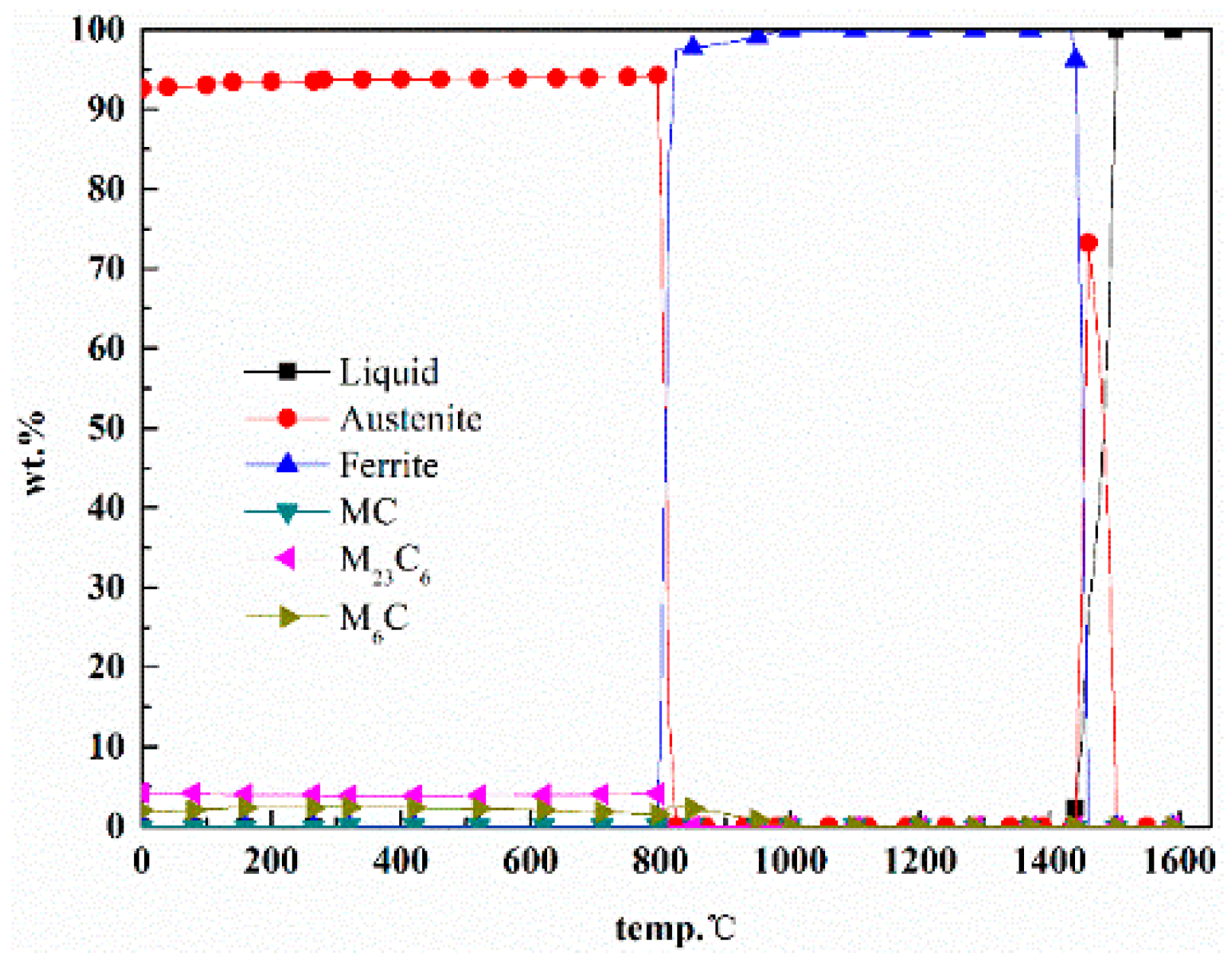
2.1. Phase Diagram Calculation and Alloy Design
2.2. Experimental Materials
2.3. The Process of Phase Diagram Calculation and Alloy Design
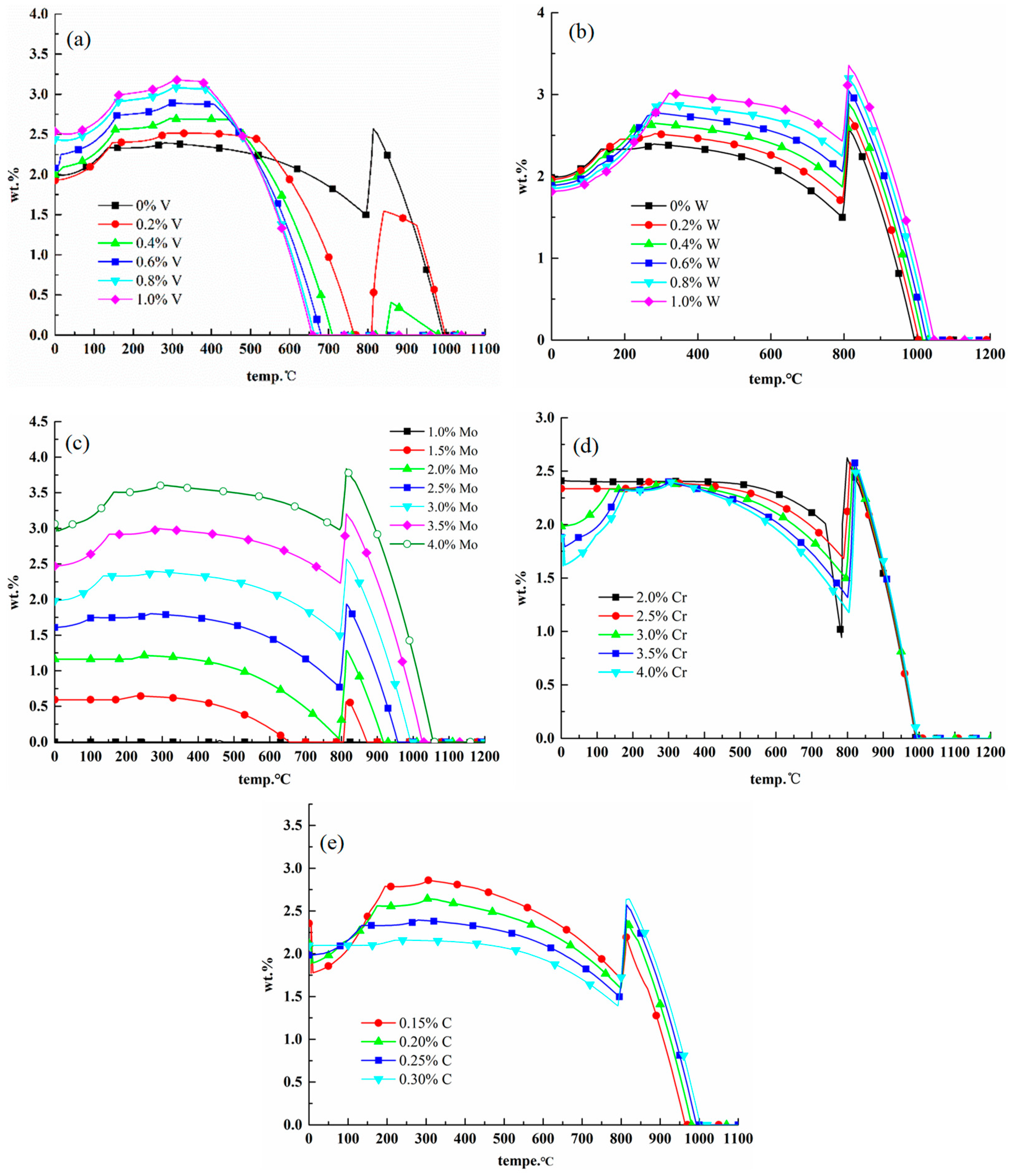
2.3.1. Effects of Elements on M23C6 Carbides
2.3.2. Effects of Elements on M6C Carbides
2.3.3. Effects of Elements on MC Carbides
2.3.4. Comparison of Precipitates between 25Cr3Mo2NiWVNb Steel and 25Cr3Mo3NiNb Steel
2.4. Microstructure
TEM Investigations
2.5. Mechanical Properties
3. Results and Discussion
4. Conclusions
- (1)
- In order to obtain MC and M2C carbides which are beneficial to thermal stability and high-temperature strength, the contents of V and W were increased while the contents of Mo and Cr were decreased. Meanwhile, Ni was added appropriately to improve the toughness and hardenability of the material.
- (2)
- Based on the thermodynamic calculation, a new material 25Cr3Mo2NiWVNb steel was designed. The new material contains a large amount of nano-sized short rod-shaped MC and M2C carbides (the lengths are 5–10 nm and 80–120 nm respectively). In contrast, the traditional material only contains the rod-shaped carbides with a diameter of 10–20 nm and a length about 80 nm.
- (3)
- The large number of finely dispersed MC carbides and M2C carbides is beneficial for stabilizing martensite lath at high temperatures, which further hinders the dislocation motion at high temperature. The high-temperature strength of the new material is about 30% higher than that of the traditional material.
Author Contributions
Funding
Conflicts of Interest
References
- Gu, J.; Li, J.; Huo, J. Effect of precipitation on hardening and toughening of nitrogen-alloyed H13 steel. Steel Res. Int. 2017, 88, 1700031. [Google Scholar] [CrossRef]
- Katoch, S.; Sehgal, R.; Singh, V. Effect of cryogenic treatment on the tribological behaviour of H11 hot die steel dry sliding against D3 steel. Tribol. Mater. Surf. Interfaces 2016, 10, 185–195. [Google Scholar] [CrossRef]
- Gu, J.; Li, J.; Chen, Y. Microstructure and strengthening-toughening mechanism of nitrogen-alloyed 4Cr5Mo2V hot-working die steel. Metals 2017, 7, 310. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Chen, S.; Li, J. Wear resistance of H13 and a new hot-work die steel at high temperature. J. Mater. Eng. Perform. 2016, 25, 2993–3006. [Google Scholar] [CrossRef]
- Barrau, O.; Boher, C.; Gras, R.; Rezai-Aria, F. Analysis of the friction and wear behaviour of hot work tool steel for forging. Wear 2003, 255, 1444–1454. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.X.; Wang, F.; Wang, S.Q.; Cui, X.H. Comparative research on the elevated-temperature wear resistance of a cast hot-working die steel. Mater. Des. 2009, 30, 3608–3614. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, Z.; Liu, X.; Dong, Z.; Fang, H. Understanding of fatigue crack growth behavior in welded joint of a new generation Ni-Cr-Mo-V high strength steel. Eng. Fract. Mech. 2018, 194, 224–239. [Google Scholar] [CrossRef]
- Schaffner, T.; Hartmaier, A.; Kokotin, V.; Pohl, M. Analysis of hydrogen diffusion and trapping in ultra-high strength steel grades. J. Alloys Compd. 2018, 746, 557–566. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Kodur, V.; Wang, B. Mechanical properties of high-strength Q690 steel at elevated temperature. J. Mater. Civ. Eng. 2018, 30, 4018062. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, X.; Liu, Y.; Li, C.; Liu, C.; Guo, Q. Carbide precipitation in Nb-V-Ti microalloyed ultra-high strength steel during tempering. Mater. Sci. Eng. A 2017, 683, 215–226. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Zhan, D.; Jiang, M. Precipitation behavior of VN in high nitrogen and vanadium micro-alloyed low carbon weathering steel. Trans. Indian Inst. Met. 2018, 71, 1607–1613. [Google Scholar] [CrossRef]
- Delagnes, D.; Lamesle, P.; Mathon, M.H.; Mebarki, N.; Levaillant, C. Influence of silicon content on the precipitation of secondary carbides and fatigue properties of a 5% Cr tempered martensitic steel. Mater. Sci. Eng. A 2005, 394, 435–444. [Google Scholar] [CrossRef]
- Ishii, R.; Tsuda, Y.; Yamada, M.; Kimura, K. Fine precipitates in high chromium heat resisting steels. Tetsu-to-Hagané 2009, 88, 36–43. [Google Scholar] [CrossRef]
- Mebarki, N.; Delagnes, D.; Lamesle, P.; Delmas, F.; Levaillant, C. Relationship between microstructure and mechanical properties of a 5% Cr tempered martensitic tool steel. Mater. Sci. Eng. A 2004, 387, 171–175. [Google Scholar] [CrossRef]
- Wu, D.; Wang, F.; Cheng, J.; Li, C. Effects of Nb and tempering time on carbide precipitation behavior and mechanical properties of Cr–Mo–V steel for brake discs. Steel Res. Int. 2018, 89, 1700491. [Google Scholar] [CrossRef]
- Wen, T.; Hu, X.; Song, Y.; Yan, D.; Rong, L. Carbides and mechanical properties in a Fe–Cr–Ni–Mo high-strength steel with different V contents. Mater. Sci. Eng. A 2013, 588, 201–207. [Google Scholar] [CrossRef]
- Michaud, P.; Delagnes, D.; Lamesle, P.; Mathon, M.H.; Levaillant, C. The effect of the addition of alloying elements on carbide precipitation and mechanical properties in 5% chromium martensitic steels. Acta Mater. 2007, 55, 4877–4889. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Wu, X.; Shi, N.; Li, J.; Min, N. Microstructure evolution and kinetic analysis of DM hot-work die steels during tempering. Mater. Sci. Eng. A 2011, 528, 5696–5700. [Google Scholar] [CrossRef]
- Wang, M.; Dong, H.; Wang, Q. Elevated-temperature properties of one long-life high-strength gun steel. Rare Met. 2004, 11, 67. [Google Scholar]
- Shi, Y.J.; Wu, X.C.; Li, J.W.; Min, N. Tempering stability of Fe-Cr-Mo-W-V hot forging die steels. Int. J. Min. Met. Mater. 2017, 24, 1145–1157. [Google Scholar] [CrossRef]
- Kelly, A.; Nicholson, R.B. Strengthening Methods in Crystals; Halstead Press Division, John Wiley & Sons: New York, NY, USA, 1971. [Google Scholar]
- Palmiere, E.J.; Garcia, C.I.; Deardo, A.J. The influence of niobium supersaturation in austenite on the static recrystallization behavior of low carbon microalloyed steels. Metall. Mater. Trans. A 1996, 27, 951–960. [Google Scholar] [CrossRef]
- Dutta, I.; Bourell, D.L. Influence of dislocation density and distribution on the aging behavior of 6061 Al SiCw composites. Acta Metall. Mater. 1990, 38, 2041–2049. [Google Scholar] [CrossRef]
- Rahmanifard, R.; Farhangi, H.; Novinrooz, A.J. Development of mechanical performance of 12YWT steel nanocomposite by addition of zirconium and tantalum. J. Alloys Compd. 2016, 657, 646–654. [Google Scholar] [CrossRef]











| Elements | C | Ni | Cr | Mo | Nb |
|---|---|---|---|---|---|
| wt% | 0.25 | 0.65 | 3 | 3 | 0.1 |
| Elements | C | Ni | Cr | Mo | V | W | Nb | Fe |
|---|---|---|---|---|---|---|---|---|
| Chemical compositions | 0.29 | 1.45 | 2.50 | 2.18 | 0.46 | 0.58 | 0.05 | Bal. |
| Elements | C | Ni | Cr | Mo | Nb | Fe |
|---|---|---|---|---|---|---|
| Chemical compositions | 0.28 | 0.65 | 3.00 | 2.9 | 0.08 | Bal. |
| Heat Treatment Process | Heat Treatment Process System |
|---|---|
| Annealing process | Annealed at 870 °C for 2 h, cooled to 500 °C at a cooling rate of 30 °C/h, and then cooled down to room temperature in the air |
| Quenching process | Austenitization temperature at 1020 °C holding for 1 h, followed by an immediate water-quench to room temperature |
| Tempering process | Tempered at 640 °C for 2.5 h after quenching, cooled down to room temperature in the air |
| Elements | C | Si | Mn | Ni | Cr | V | Mo | W | Nb |
|---|---|---|---|---|---|---|---|---|---|
| wt% | 0.27–0.32 | ≤0.10 | ≤0.10 | 1.3–1.5 | 2.0–3.0 | 0.3–1.0 | 1.5–2.5 | 0.40–0.70 | 0.03–0.1 |
| Balanced Phase | 25Cr3Mo3NiNb (Alloy 1) | 25Cr3Mo2NiWVNb (Alloy 2) | ||||
|---|---|---|---|---|---|---|
| Content at 500 °C | Content at 600 °C | Content at 700 °C | Content at 500 °C | Content at 600 °C | Content at 700 °C | |
| MC | 0.0987% | 0.109% | 0.117% | 0.787% | 0.81% | 0.557% |
| M6C | 2.26% | 2.11% | 1.85% | 1.4% | 1.05% | 0% |
| M23C6 | 3.84% | 3.91% | 4.05% | 3.92% | 3.73% | 3.32% |
| M2C | 0 | 0 | 0 | 0 | 0.239% | 1.283% |
| Steel | Ultimate Tensile Strength/MPa | Yield Strength/MPa | Elongation/% | Hardness/HRC |
|---|---|---|---|---|
| Alloy 1 | 1180 | 920 | 18.5 | 35.5 |
| Alloy 2 | 1374 | 1178 | 17 | 43 |
| Materials | Test Temperature/°C | Ultimate Tensile Strength/MPa | Yield Strength/MPa | Elongation/% |
|---|---|---|---|---|
| Alloy 1 | 700 | 380 | 277 | 24.0 |
| Alloy 2 | 700 | 495 | 385 | 21.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, J.; Yao, Z.; Xie, G.; Lian, Y.; Ma, M.; Zhao, C.; Huang, J. Design for Novel Hot-Work Die Steel by Thermodynamic Calculation and Microstructural Examination. Metals 2019, 9, 805. https://doi.org/10.3390/met9070805
Zhang Z, Zhang J, Yao Z, Xie G, Lian Y, Ma M, Zhao C, Huang J. Design for Novel Hot-Work Die Steel by Thermodynamic Calculation and Microstructural Examination. Metals. 2019; 9(7):805. https://doi.org/10.3390/met9070805
Chicago/Turabian StyleZhang, Zunjun, Jishan Zhang, Zhihao Yao, Guoliang Xie, Yong Lian, Minyu Ma, Chao Zhao, and Jinfeng Huang. 2019. "Design for Novel Hot-Work Die Steel by Thermodynamic Calculation and Microstructural Examination" Metals 9, no. 7: 805. https://doi.org/10.3390/met9070805





