Thermodynamics and Agglomeration Behavior on Spinel Inclusion in Al-Deoxidized Steel Coupling with Mg Treatment
Abstract
:1. Introduction
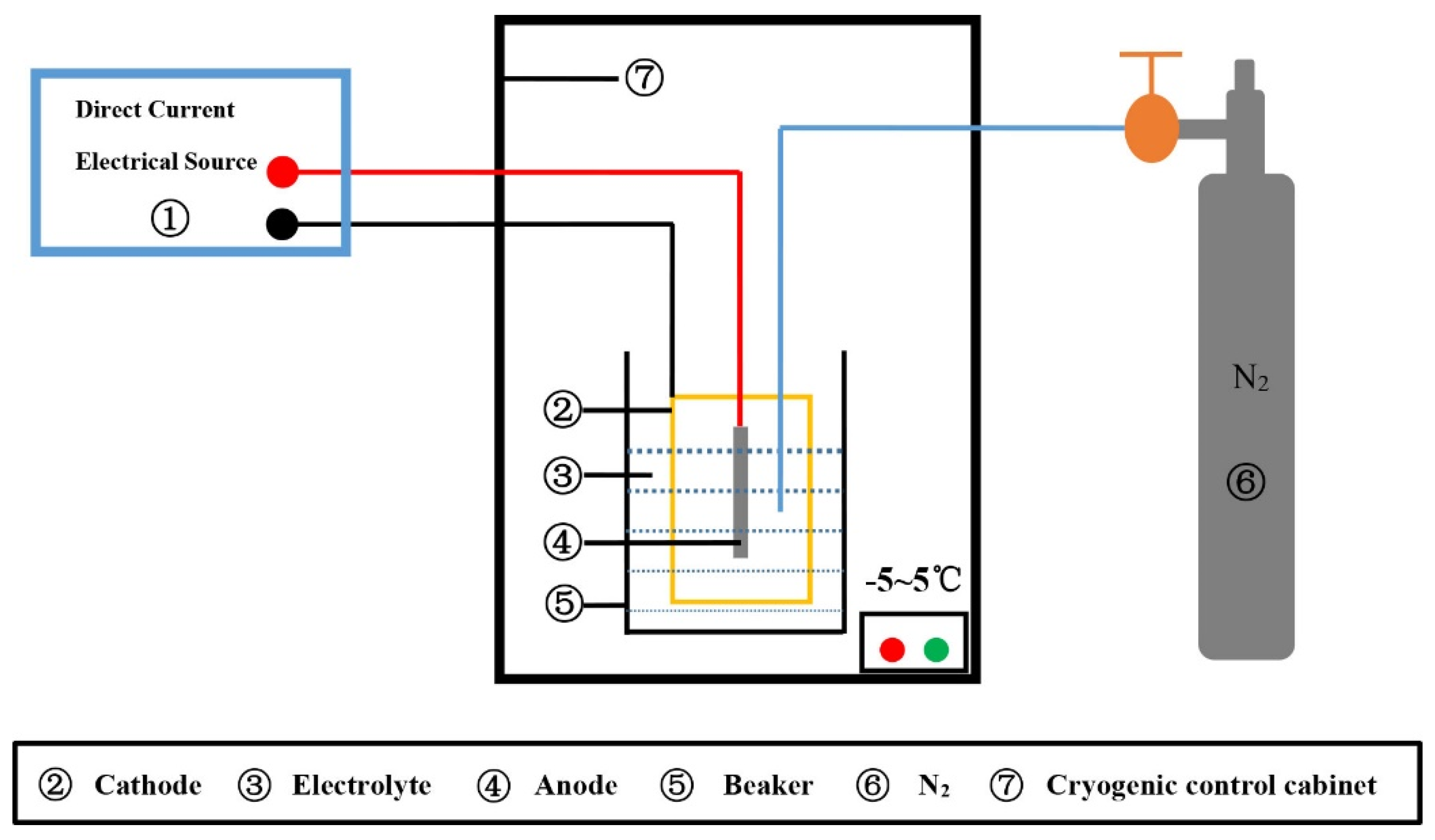
2. Experimental
2.1. Materials and Processes
2.2. Chemical Composition Analysis
2.3. Inclusion Samples Preparation
3. Results
3.1. Experimental Steel Composition
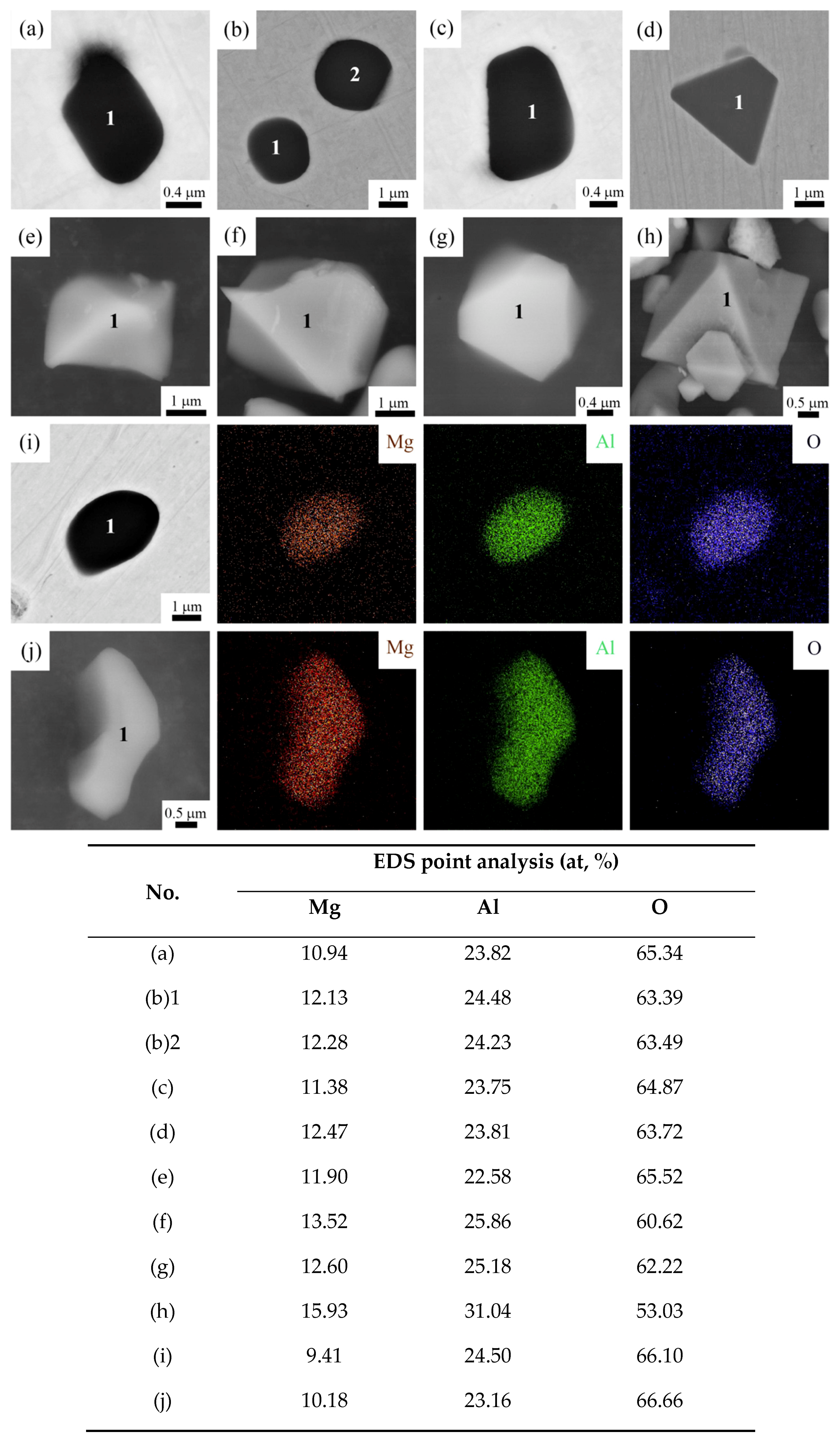
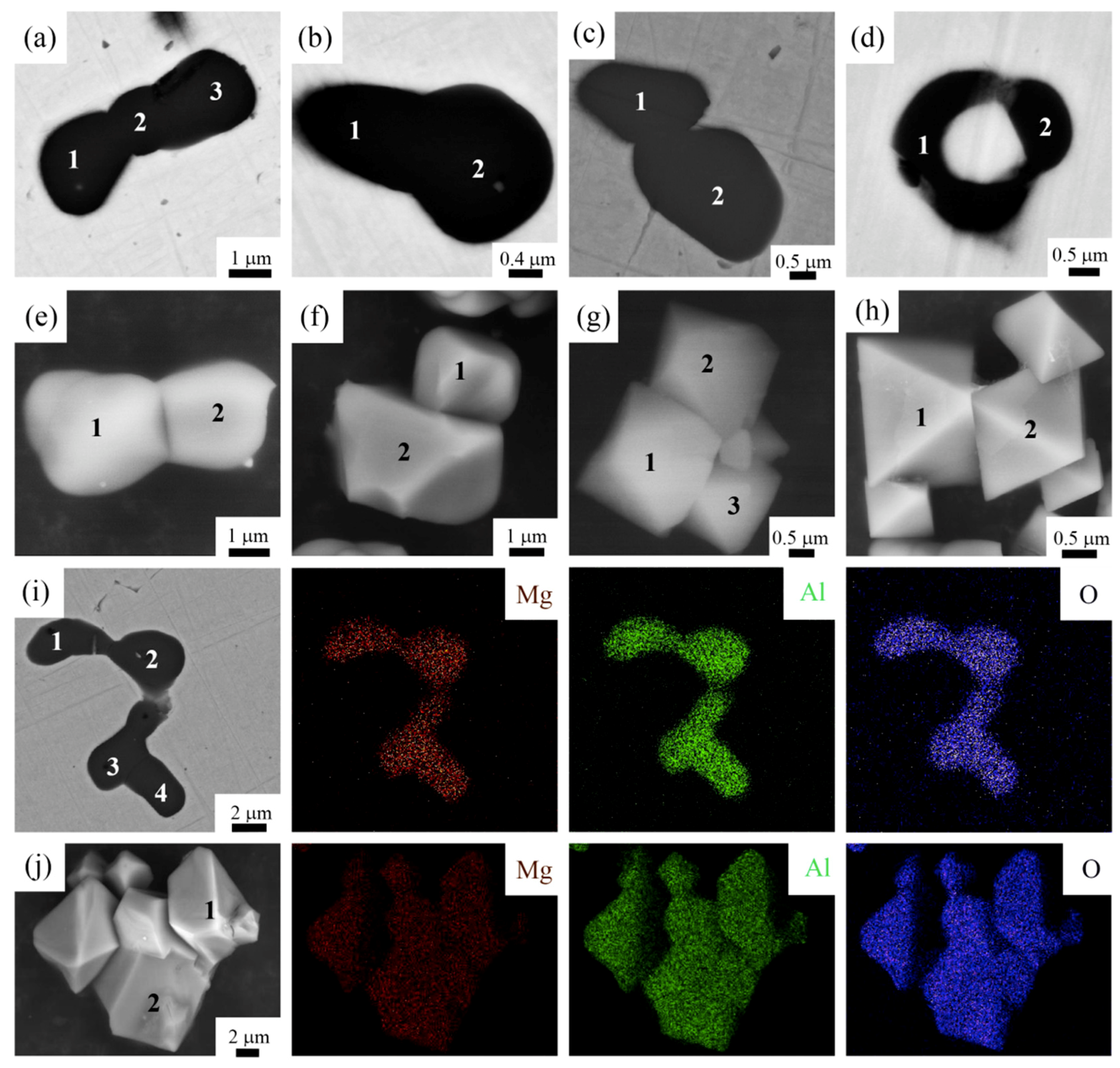
3.2. 2D and 3D Morphologies of Single and Aggregated MgAl2O4 Inclusions
3.3. 2D and 3D Morphologies of MgAl2O4-MnS Inclusion
4. Discussion
4.1. Thermodynamics of the Formation of MgAl2O4 Inclusion
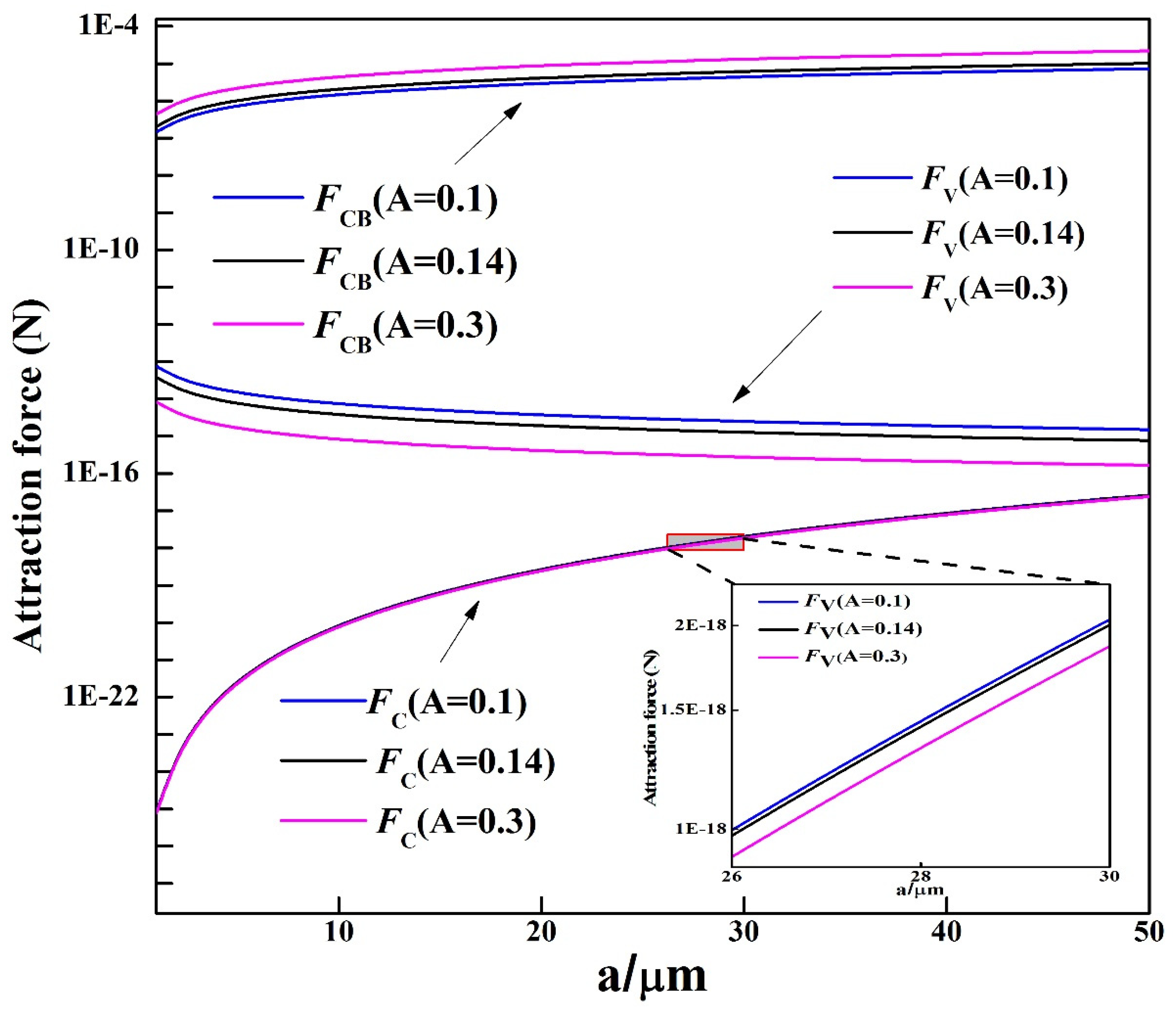
4.2. Mechanism on the Agglomeration of MgAl2O4 Inclusions
4.3. Mechanism on the Formation of MgAl2O4-MnS Inclusion
5. Conclusions
- (1)
- The Al-deoxidation coupling with Mg treated experiment has been carried out, the 3D morphologies of octahedral MgAl2O4, and agglomerated MgAl2O4, MgAl2O4-MnS inclusion particles were observed by non-aqueous electrolysis extraction method and FESEM.
- (2)
- Thermodynamic calculation shows MgAl2O4 precipitates in the liquid phase. The phase transformation follows liquid + Al2O3 + MgAl2O4 → liquid + MgAl2O4 → liquid + MgO + MgAl2O4 → liquid + MgO with the Mg content increasing when the Al content is a constant in molten steel.
- (3)
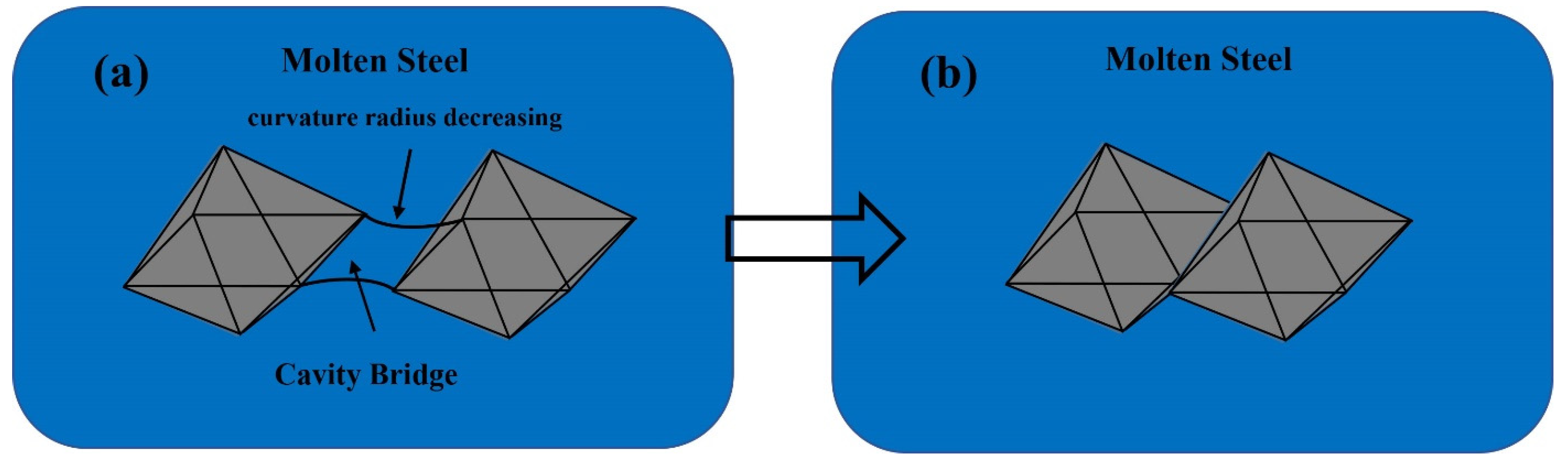
- The cavity bridge force acts as the dominant force leading to the agglomeration of two MgAl2O4 particles in the molten steel. Two MgAl2O4 inclusions can connect with each other due to the action of the cavity bridge force.
- (4)
- Thermodynamic calculations show that the precipitation temperature of MnS inclusions is lower than the solidus temperature, so MgAl2O4-MnS inclusions can be formed. The mismatch degree of MgAl2O4-MnS interface indicates that MgAl2O4 inclusion can provide heterogeneous nucleation sites for MnS, while MnS can nucleate on (110) and (100) plane of MgAl2O4 inclusion easily.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Min, Y.; Li, X.; Yu, Z.; Liu, C.; Jiang, M.F. Characterization of the acicular ferrite in Al-deoxidized low-carbon steel combined with Zr and Mg additions. Steel Res Int. 2016, 87, 1503–1510. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, D.; Yang, Q.; An, J.; Huang, Z.; Fu, J. Exploration of morphology evolution of the inclusions in Mg-treated 16MnCrS5 steel. Ironmak. Steelmak. 2018, 1–10. [Google Scholar] [CrossRef]
- Ma, W.; Bao, Y.; Wang, M.; Zhao, L. Effect of Mg and Ca treatment on behavior and particle size of inclusions in bearing steels. ISIJ Int. 2014, 54, 536–542. [Google Scholar] [CrossRef]
- Wen, B.; Song, B.; Pan, N.; Hu, Q.; Mao, J. Effect of SiMg alloy on inclusions and microstructures of 16Mn steel. Ironmak. Steelmak. 2011, 38, 577–583. [Google Scholar] [CrossRef]
- Chang, C.; Jung, I.; Park, S.C.; Kim, H.S.; Lee, H.G. Effect of Mg on the evolution of non-metallic inclusions in Mn-Si-Ti deoxidised steel during solidification:experiments and thermodynamic calculations. Ironmak. Steelmak. 2005, 32, 251–257. [Google Scholar] [CrossRef]
- Zhang, T.S.; Liu, C.J.; Jiang, M.F. Effect of Mg on behavior and particle size of inclusions in Al-Ti deoxidized molten steels. Metall. Mater. Trans. B 2016, 47B, 2253–2262. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Shi, C.B.; Li, J. Evolution of Al2O3 inclusions by magnesium treatment in H13 hot work die steel. Ironmak. Steelmak. 2017, 44, 128–133. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Wang, C.; Gong, W.; Wang, H.D. Evolution of inclusions and change of as-cast microstructure with Mg addition in high carbon and high chromium die steel. Ironmak. Steelmak. 2015, 42, 669–674. [Google Scholar] [CrossRef]
- Kim, H.S.; Chang, C.H.; Lee, H.G. Evolution of inclusions and resultant microstructural change with Mg addition in Mn/Si/Ti deoxidized steels. Scripta Mater. 2005, 53, 1253–1258. [Google Scholar] [CrossRef]
- Yang, J.; Yamasaki, T.; Kuwabara, M. Behavior of inclusions in deoxidation process of molten steel with in situ produced Mg vapor. ISIJ Int. 2007, 47, 699–708. [Google Scholar] [CrossRef]
- Takata, R.; Yang, J.; Kuwabara, M. Characteristics of inclusions generated during Al-Mg complex deoxidation of molten steel. ISIJ Int. 2007, 47, 1379–1386. [Google Scholar] [CrossRef]
- Du, G.; Li, J.; Wang, Z.B.; Shi, C.B. Effect of magnesium addition on behavior of collision and agglomeration between solid inclusion particles on H13 steel melts. Steel Res. Int. 2017, 88, 1600185. [Google Scholar] [CrossRef]
- Kimura, S.; Nakajima, K.; Mizoguchi, S. Behavior of alumina-magnesia complex inclusions and magnesia inclusions on the surface of molten low-carbon steels. Metall. Mater. Trans. B 2001, 32B, 79–85. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Shi, C.B.; Wang, L.L.; Wu, Z.; Wang, H. Effect of trace magnesium on carbide improvement in H13 steel. Can. Metall. Quart. 2016, 55, 321–327. [Google Scholar] [CrossRef]
- Isobe, K. Effect of Mg addition on solidification structure of low carbon steel. ISIJ Int. 2010, 50, 1972–1980. [Google Scholar] [CrossRef]
- Chen, P.J.; Zhu, C.Y.; Li, G.Q.; Dong, Y.W.; Zhang, Z.C. Effect of sulphur concentration on precipitation behaviors of MnS-containing inclusions in GCr15 bearing steels after LF refining. ISIJ Int. 2017, 57, 1019–1028. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.B.; Gaye, H.R. Thermodynamics of the formation of MgO-Al2O3-TiOx inclusions in Ti-stabilized 11Cr ferritic stainless steel. Metall. Mater. Trans. B 2008, 39B, 853–861. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Wang, S.; Zhang, Q.; Zhang, C. Research of microalloy elements to induce intragranular acicular ferrite in shipbuilding steel. Ironmak. Steelmak. 2019, 46, 499–507. [Google Scholar] [CrossRef]
- Wu, Z.; Zheng, W.; Li, G.; Matsuura, H.; Tsukihashi, F. Effect of inclusions’ behavior on the microstructure in Al-Ti deoxidized and magnesium-treated steel with different aluminum contents. Metall. Mater. Trans. B 2015, 46, 1226–1241. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Ning, B.; Li, Y. Effects of magnesium on wear resistance of H13 steel. Mater. Trans. 2014, 55, 1104–1108. [Google Scholar] [CrossRef]
- Song, M.; Hu, C.; Song, B.; Zhu, H.; Xue, Z.; Xu, R. Effect of Ti-Mg treatment on the impact toughness of heat affected zone in 0.15% C-1.31% Mn steel. Steel Res. Int. 2018, 89, 1700355. [Google Scholar] [CrossRef]
- Taniguchi, S.; Kikuchi, A. Mechanisms of collision and coagulation between fine particles in fluid. Tetsu-to-Hagané 1992, 78, 527–535. [Google Scholar] [CrossRef]
- Visser, J. On hamaker constants: A comparison between hamaker constants and lifshitz-van der Waals constants. Adv. Colloid Interface Sci. 1972, 3, 331–363. [Google Scholar] [CrossRef]
- Sasai, K. Interaction between alumina inclusions in molten steel due to cavity bridge force. ISIJ Int. 2016, 56, 1013–1022. [Google Scholar] [CrossRef]
- Paunov, V.N.; Kralchevsky, P.A.; Denkov, N.D.; Nagayama, K. Lateral capillary forces between floating submillimeter particles. J. Colloid Interface Sci. 1993, 157, 100–112. [Google Scholar] [CrossRef]
- Poirier, D.R.; Yin, H.B.; Suzuki, M.; Emi, T. Interfacial Properties of Dilute Fe-O-S Melts on Alumina Substrates. ISIJ Int. 1998, 38, 229–238. [Google Scholar] [CrossRef]
- Chan, D.Y.C.; Henry, H.J.D., Jr.; White, L.R. The interaction of colloidal particles collected at fluid interfaces. J. Colloid Interf. Sci. 1981, 79, 410–418. [Google Scholar] [CrossRef]
- Mu, W.Z.; Dogan, N.; Coley, K.S. Agglomeration of non-metallic inclusions at the steel/Ar interface: Model Application. Mater. Trans. B 2017, 48B, 2092–2103. [Google Scholar] [CrossRef]
- Wang, L.Z.; Yang, S.F.; Li, J.S.; Zhang, S.; Ju, J.T. Effect of Mg addition on the refinement and homogenized distribution of inclusions in steel with different Al contents. Metall. Mater. Trans. B 2017, 48B, 805–818. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jo, J.O.; Chung, T.I.; Kim, D.S.; Pak, J.J. Thermodynamics of titanium, nitrogen and TiN formation in liquid iron. ISIJ Int. 2007, 47, 1082–1089. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.F.; Duan, H.J.; Zhang, Y.; Luo, Y.; Conejo, A.N. Extraction, thermodynamic analysis, and precipitation mechanism of MnS-TiN complex inclusions in low-sulfur steels. Metall. Mater. Trans. A 2016, 47A, 3015–3025. [Google Scholar] [CrossRef]
- Luo, S.; Wang, B.Y.; Wang, Z.H.; Jiang, D.B.; Wang, W.L.; Zhu, M.Y. Morphology of solidification structure and MnS inclusion in high carbon steel continuously cast bloom. ISIJ Int. 2017, 57, 2000–2009. [Google Scholar] [CrossRef]
- Passerini, L. Ricerche sugli spinelli.-II. I composti. CuAl2O4, MgAl2O4, Mg Fe2O4, ZnAl2O4, ZnCr2O4, Zn Fe2O4, MnFe2O4. Gazz. Chim. Ital. 1930, 60, 389–399. [Google Scholar]
- Ott, H. Die strukturen von MnO, MnS, AgF, NiS, SnJ4, SrCk, BaF2. Z. Krist.- Cryst. Mater. 1926, 63, 222–230. [Google Scholar] [CrossRef]
- Bramfiti, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]





| Type | C | Si | Mn | P | S | Ni | Mg | Al |
|---|---|---|---|---|---|---|---|---|
| Pure Iron | <0.005 | 0.01 | 0.046 | 0.0032 | 0.013 | |||
| Ni-Mg alloy | 0.012 | 0.001 | 0.002 | 0.0003 | 79.6747 | 20.31 | ||
| Al Wire | 99.99 |
| Elements | C | Si | Mn | P | S | Al(s) | Al(t) | Mg | Ni | T.O |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.0032 | 0.003 | 0.043 | 0.022 | 0.012 | 0.016 | 0.019 | 0.0008 | 0.955 | 0.0107 |
| Inclusion | Crystal System | Lattice Parameter (nm) |
| MgAl2O4 | Cubic | 0.805 [33] |
| MnS | Cubic | 0.524 [34] |
| Case | [hkl]s | [hkl]n | d[hkl]s | d[hkl]n | θ,deg | Mismatch (δ) |
|---|---|---|---|---|---|---|
| (110)MnS//(110)MgAl2O4 | [–111] | [001] | 7.41 | 8.05 | - | - |
| [001] | [–112] | 9.076 | 9.859 | 0 | 7.94% | |
| [–110] | [–110] | 5.24 | 5.692 | - | - | |
| (100)MnS//(100)MgAl2O4 | [011] | [011] | 5.24 | 5.692 | - | - |
| [010] | [010] | 7.41 | 8.05 | 0 | 7.94% | |
| [01–1] | [01–1] | 5.24 | 5.692 | - | - | |
| (111)MnS//(111)MgAl2O4 | [–110] | [–110] | 3.705 | 5.692 | - | - |
| [–12–1] | [–12–1] | 6.418 | 9.859 | 0 | 38.60% | |
| [01–1] | [01–1] | 3.705 | 5.692 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Wang, G.; Yuan, X.; Jin, P.; Sridhar, S. Thermodynamics and Agglomeration Behavior on Spinel Inclusion in Al-Deoxidized Steel Coupling with Mg Treatment. Metals 2019, 9, 900. https://doi.org/10.3390/met9080900
Cao L, Wang G, Yuan X, Jin P, Sridhar S. Thermodynamics and Agglomeration Behavior on Spinel Inclusion in Al-Deoxidized Steel Coupling with Mg Treatment. Metals. 2019; 9(8):900. https://doi.org/10.3390/met9080900
Chicago/Turabian StyleCao, Lei, Guocheng Wang, Xinghu Yuan, Pengliang Jin, and Seetharaman Sridhar. 2019. "Thermodynamics and Agglomeration Behavior on Spinel Inclusion in Al-Deoxidized Steel Coupling with Mg Treatment" Metals 9, no. 8: 900. https://doi.org/10.3390/met9080900




