Carbide Precipitation, Dissolution, and Coarsening in G18CrMo2–6 Steel
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Preparation and Heat Treatments
2.2. Sample Preparation and Characterization
3. Results and Discussion
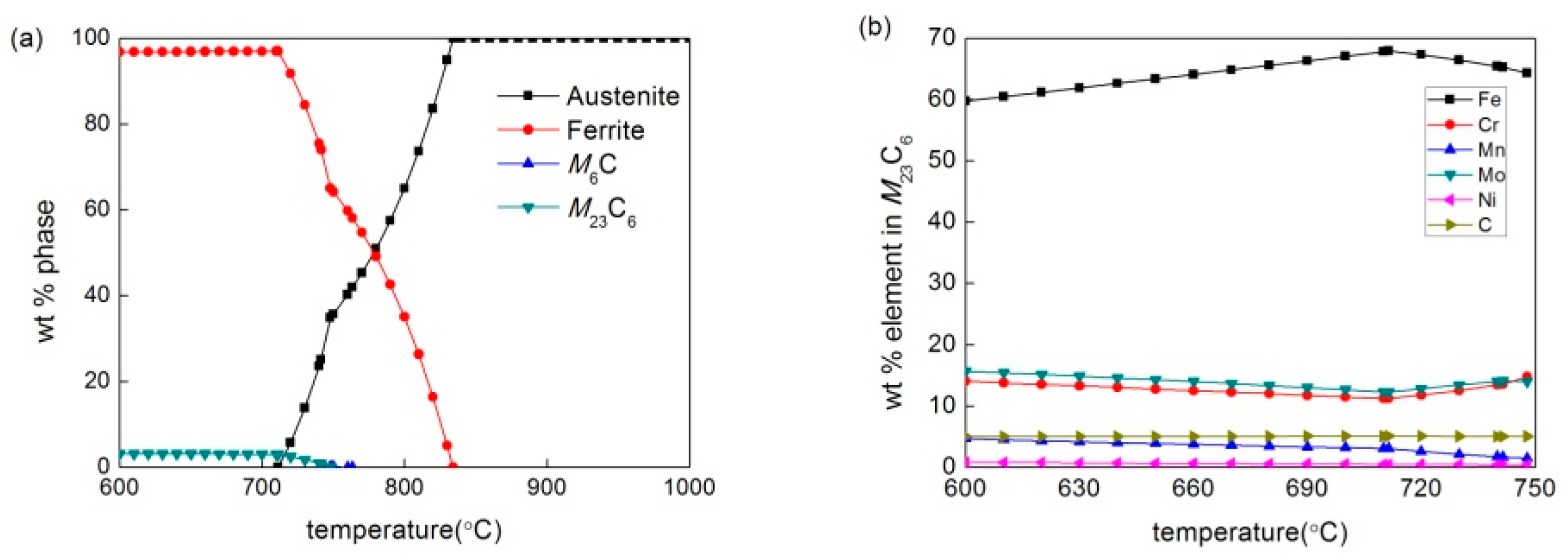
3.1. Phase Diagram Calculation
3.2. Characterization of Microstructural Evolution
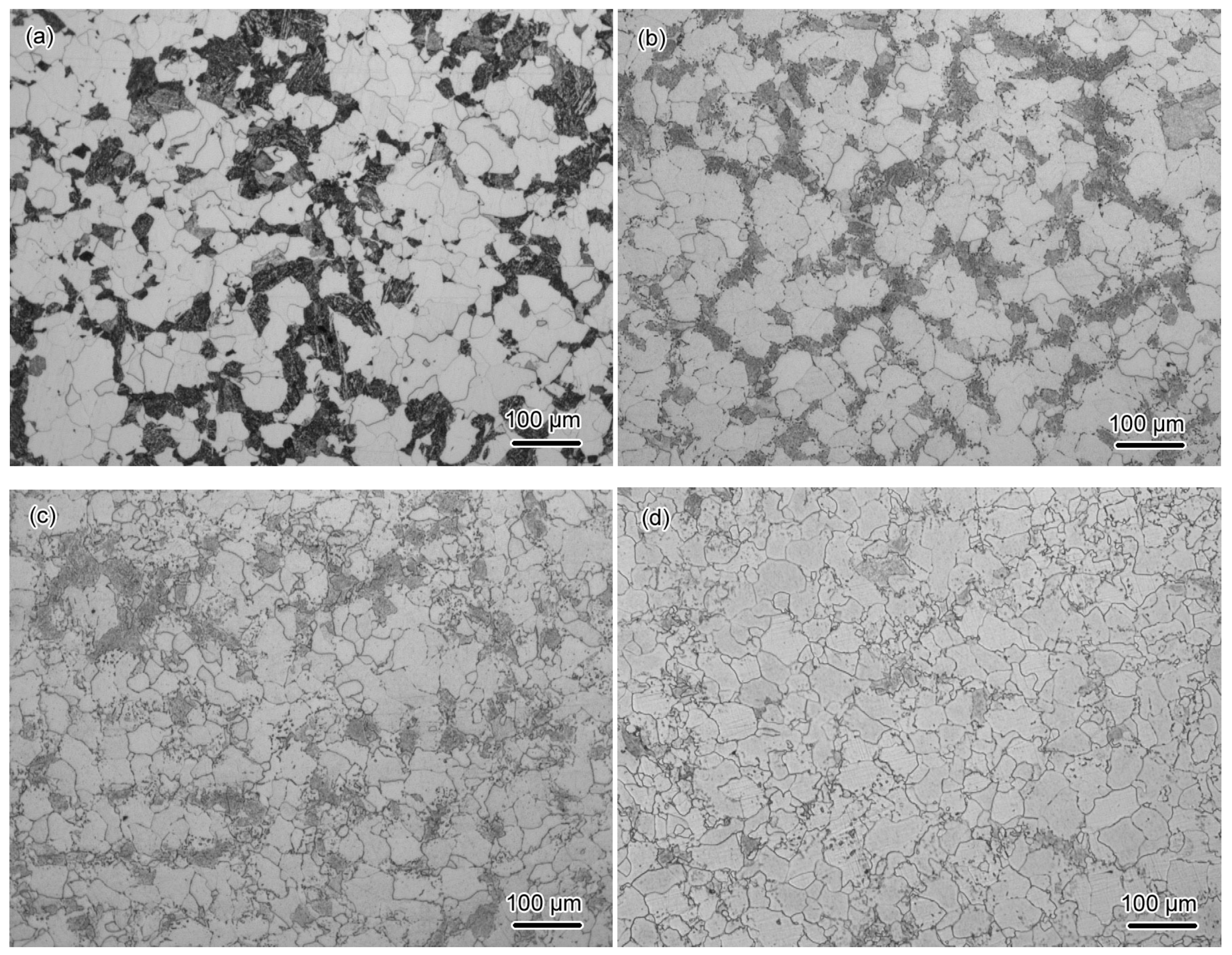
3.2.1. Characterization of Microstructural Evolution by OM
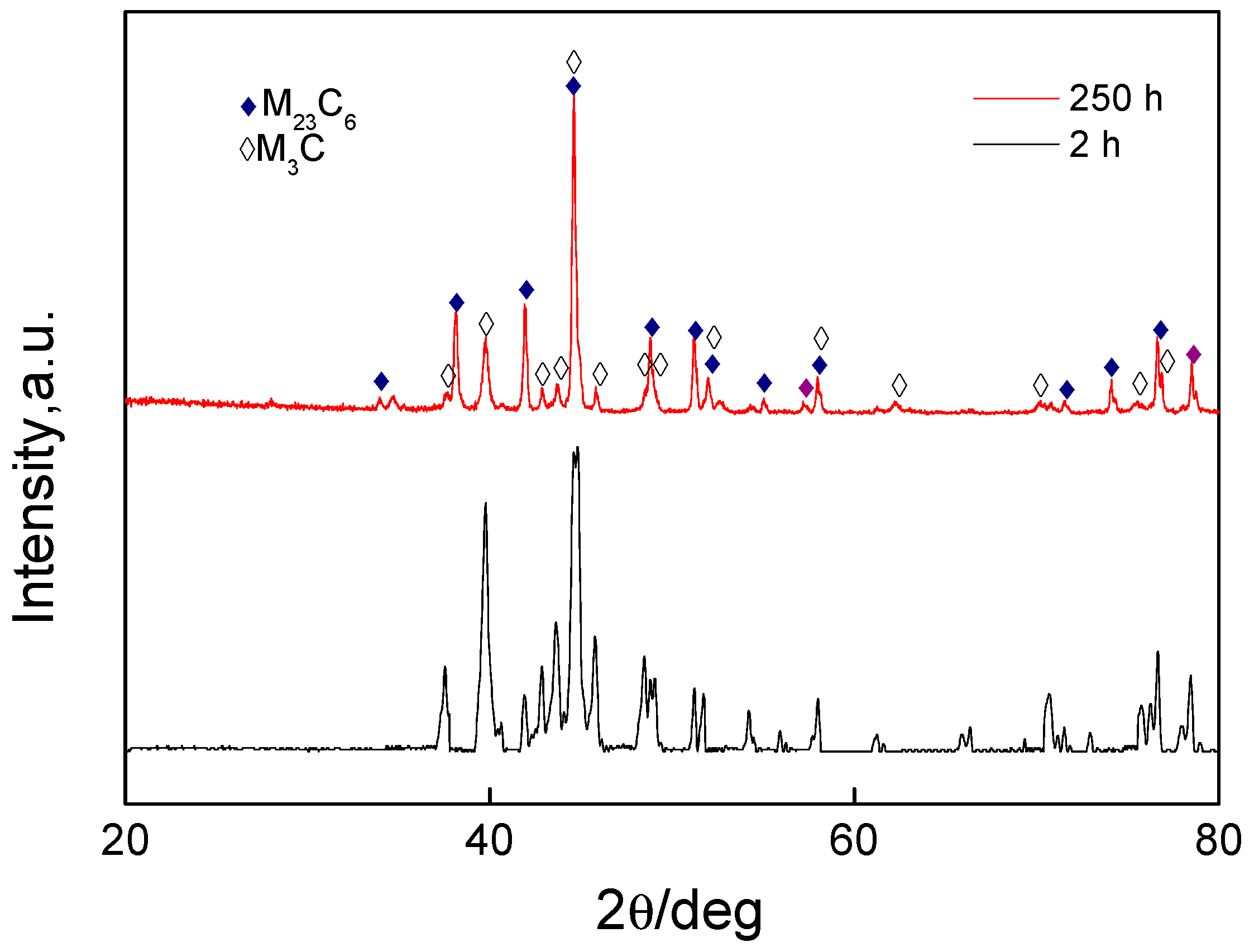
3.2.2. Characterization of Microstructural Evolution by SEM and XRD
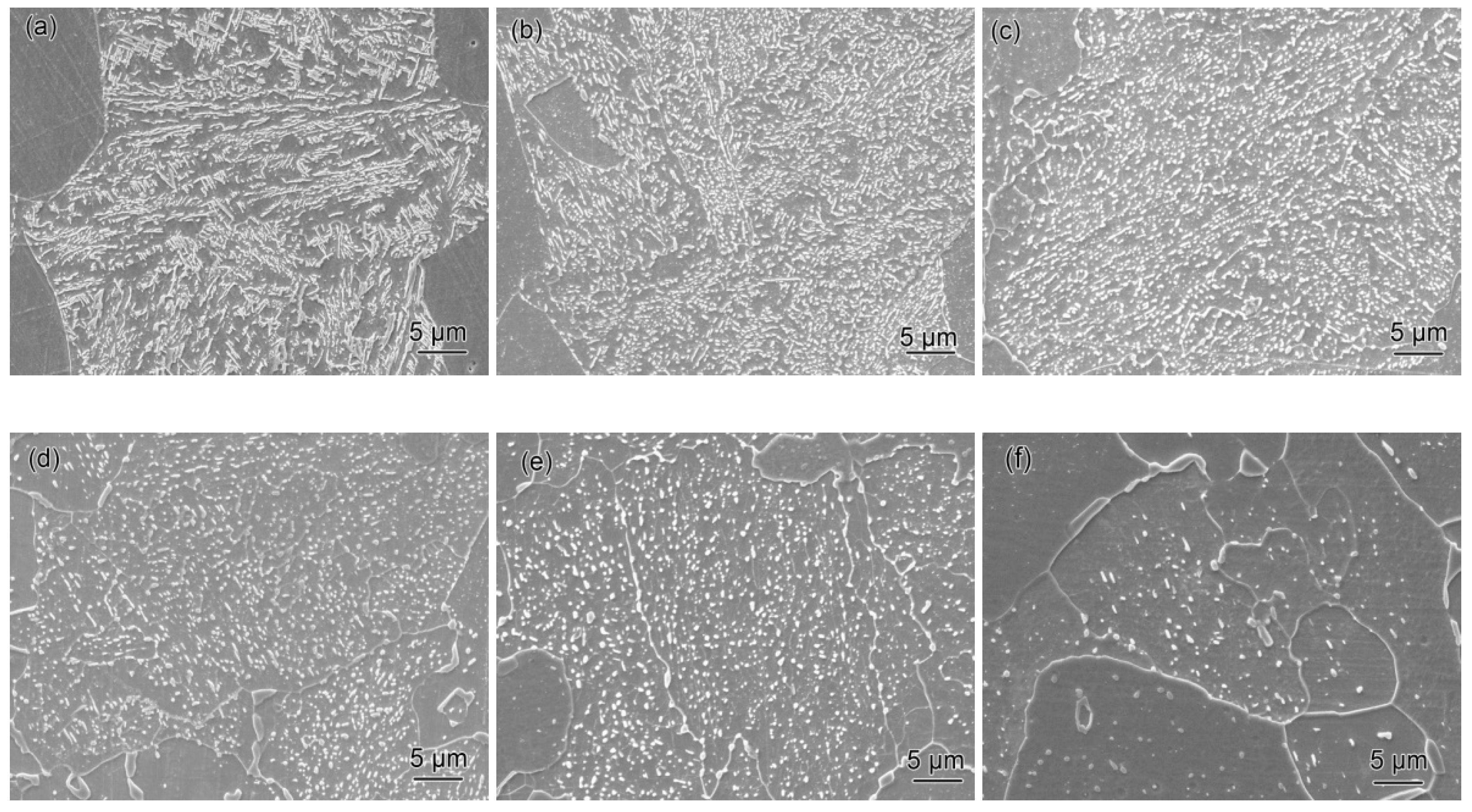
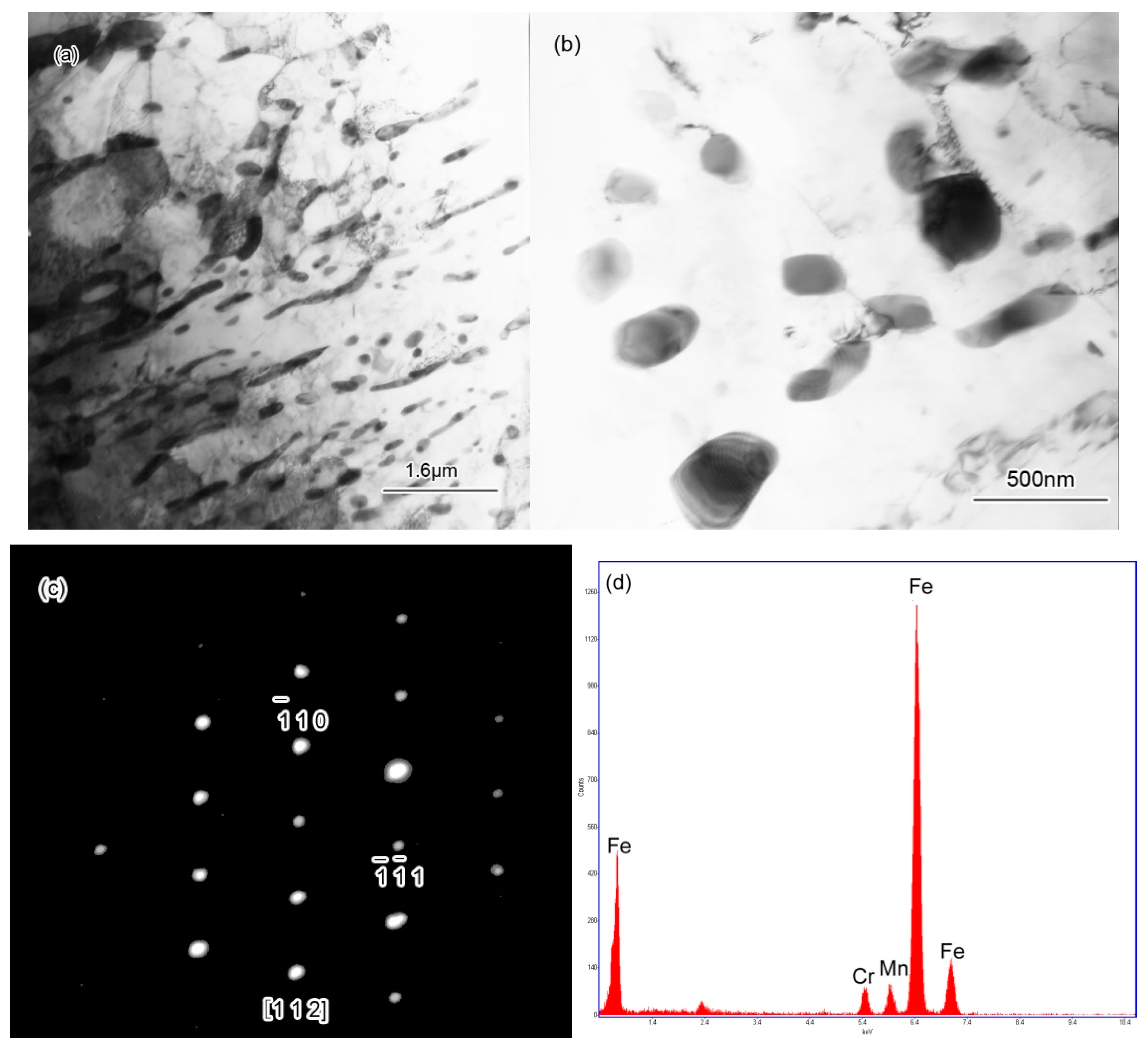
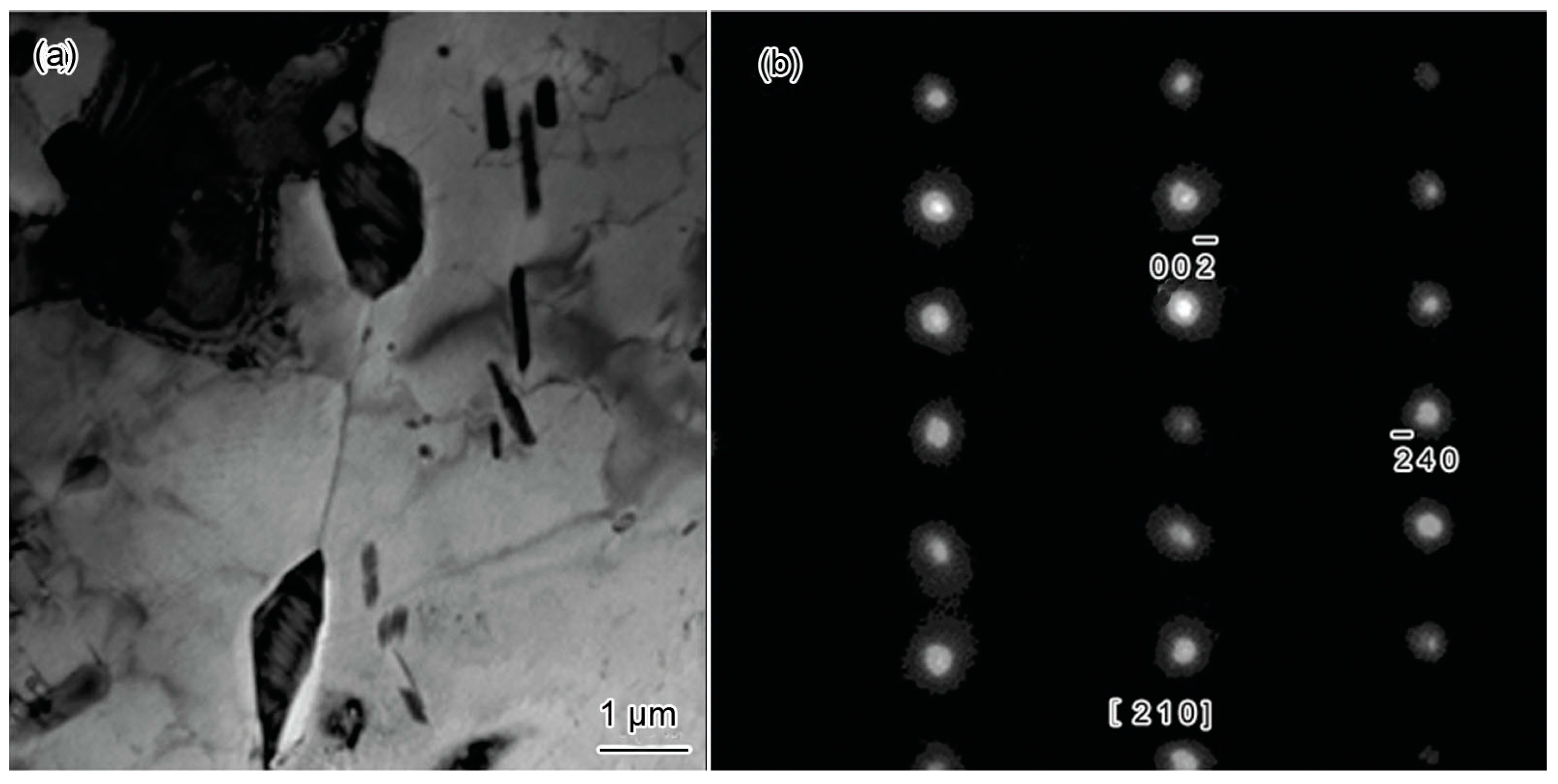
3.2.3. Characterization of Microstructural Evolution by TEM
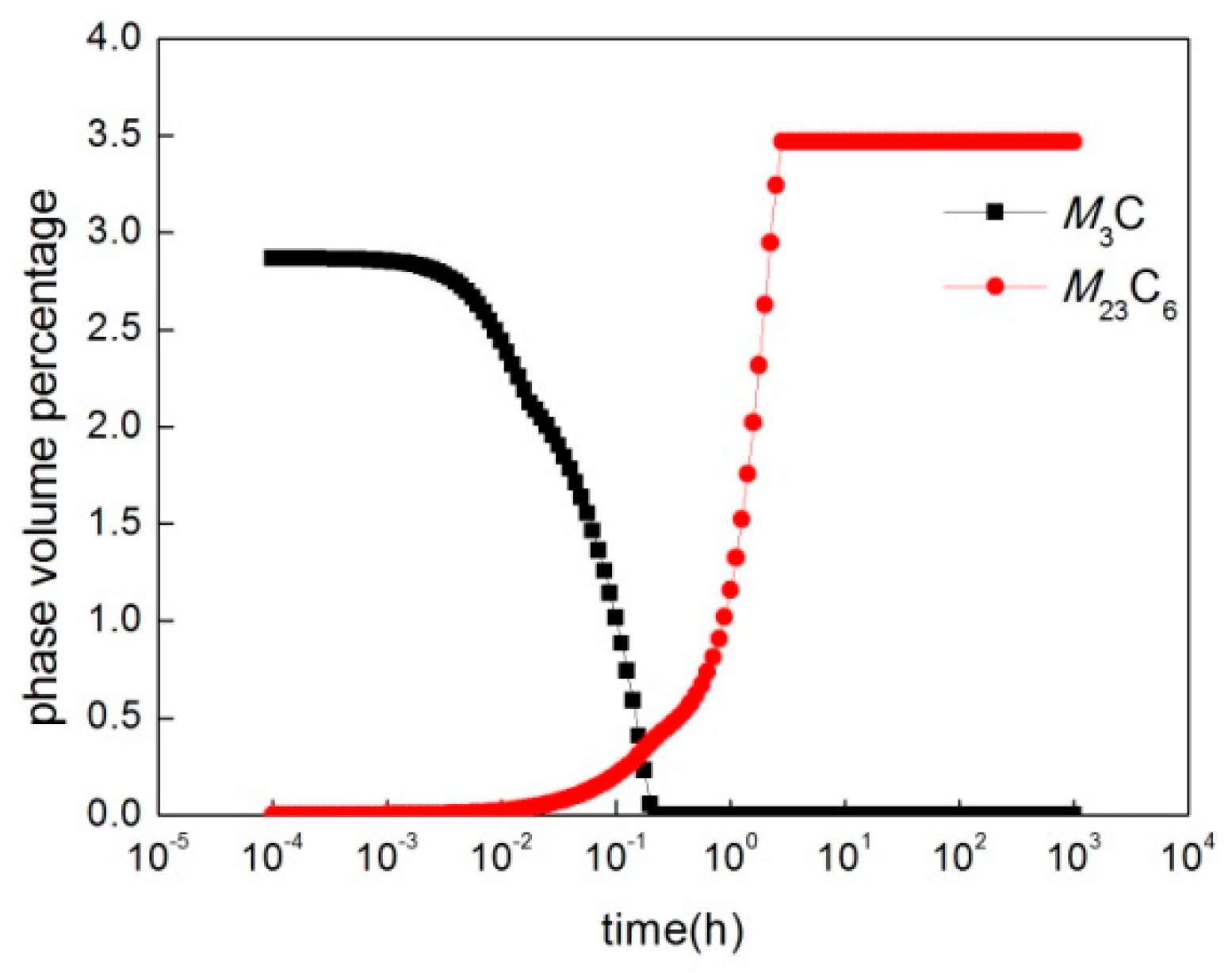
3.3. Kinetic Simulation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsai, M.; Chiou, C.; Yang, J. Microstructural evolution of simulated heat-affected zone in modified 2.25Cr–1Mo steel during high temperature exposure. J. Mater. Sci. 2003, 38, 2373–2391. [Google Scholar] [CrossRef]
- Tsai, M.; Yang, J. Microstructural degeneration of simulated heat-affected zone in 2.25Cr–1Mo steel during high-temperature exposure. Mater. Sci. Eng. A 2003, 340, 15–32. [Google Scholar] [CrossRef]
- Yang, H.; Kim, S. A study on the mechanical strength change of 2.25Cr–1Mo steel by thermal aging. Mater. Sci. Eng. A 2001, 319, 316–320. [Google Scholar] [CrossRef]
- Janovec, J.; Svoboda, M.; Kroupa, A.; Výrostková, A. Thermal-induced evolution of secondary phases in Cr–Mo–V low alloy steels. J. Mater. Sci. 2006, 41, 3425–3433. [Google Scholar] [CrossRef]
- Janovec, J.; Svoboda, M.; Výrostková, A.; Kroupa, A. Time–temperature–precipitation diagrams of carbide evolution in low alloy steels. Mater. Sci. Eng. A 2005, 402, 288–293. [Google Scholar] [CrossRef]
- Tao, P.; Zhang, C.; Yang, Z.-G.; Hiroyuki, T. Evolution and Coarsening of Carbides in 2.25Cr–1Mo Steel Weld Metal During High Temperature Tempering. J. Iron Steel Res. Int. 2010, 17, 74–78. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, P.; Li, D.; Li, Y. The evolutions of microstructure and mechanical properties of 2.25Cr-1Mo-0.25V steel with different initial microstructures during tempering. Mater. Sci. Eng. A 2017, 699, 165–175. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, P.; Li, D.; Li, Y. Influence of the decomposition behavior of retained austenite during tempering on the mechanical properties of 2.25Cr-1Mo-0.25 V steel. Mater. Sci. Eng. A 2019, 742, 540–552. [Google Scholar] [CrossRef]
- Agren, J. Local equilibrium and prediction of diffusional transformations. Scand. J. Metall. 1991, 20, 86–92. [Google Scholar]
- Ågren, J. Computer simulations of diffusional reactions in complex steels. ISIJ Int. 1992, 32, 291–296. [Google Scholar] [CrossRef]
- Bjärbo, A.; Hättestrand, M. Complex carbide growth, dissolution, and coarsening in a modified 12 pct chromium steel—An experimental and theoretical study. Metall. Mater. Trans. A 2001, 32, 19–27. [Google Scholar] [CrossRef]
- Prat, O.; Garcia, J.; Rojas, D.; Carrasco, C.; Kaysser-Pyzalla, A. Investigations on coarsening of MX and M23C6 precipitates in 12% Cr creep resistant steels assisted by computational thermodynamics. Mater. Sci. Eng. A 2010, 527, 5976–5983. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, C.; Yang, Z. Control of precipitation behavior in reduced activation steels by intermediate heat treatment. Mater. Sci. Eng. A 2011, 528, 6764–6768. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, G.; Hu, B.; Wang, J.; Ma, W. Coarsening behavior for M23C6 carbide in 12% Cr-reduced activation ferrite/martensite steel: experimental study combined with DICTRA simulation. J. Mater. Sci. 2013, 48, 5410–5419. [Google Scholar] [CrossRef]
- Zhu, N.; He, Y.; Liu, W.; Li, L.; Huang, S.; Vleugels, J.; van der Biest, O. Modeling of nucleation and growth of M23C6 carbide in multi-component Fe-based alloy. J. Mater. Sci. Technol. 2011, 27, 725–728. [Google Scholar] [CrossRef]
- Dahlgren, R. Prediction of Near Surface M3C after Hard Turning of SAE 52100 Steel. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2012. [Google Scholar]
- Gustafson, Å.; Hättestrand, M. Coarsening of precipitates in an advanced creep resistant 9% chromium steel—quantitative microscopy and simulations. Mater. Sci. Eng. A 2002, 333, 279–286. [Google Scholar] [CrossRef]
- Yin, Y.F.; Faulkner, R.G. Simulations of precipitation in ferritic steels. Met. Sci. J. 2003, 19, 91–98. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, N.; Li, D.; Zhang, J.; Luo, Y.; Zhang, R. Effect of microstructure evolution on strength and impact toughness of G18CrMo2–6 heat-resistant steel during tempering. Mater. Sci. Eng. A 2014, 604, 103–110. [Google Scholar] [CrossRef]
- Tian, Y.L.; Kraft, R.W. Mechanisms of pearlite spheroidization. Metall. Trans. A 1987, 18, 1403–1414. [Google Scholar] [CrossRef]
| Fe | Cr | Mn | Mo | Ni | Si | C |
|---|---|---|---|---|---|---|
| Bal. | 0.6 | 0.75 | 0.6 | 0.46 | 0.45 | 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Jia, P.; Liu, Y.; Qi, H. Carbide Precipitation, Dissolution, and Coarsening in G18CrMo2–6 Steel. Metals 2019, 9, 916. https://doi.org/10.3390/met9090916
Li Z, Jia P, Liu Y, Qi H. Carbide Precipitation, Dissolution, and Coarsening in G18CrMo2–6 Steel. Metals. 2019; 9(9):916. https://doi.org/10.3390/met9090916
Chicago/Turabian StyleLi, Zhenjiang, Pengju Jia, Yujing Liu, and Huiping Qi. 2019. "Carbide Precipitation, Dissolution, and Coarsening in G18CrMo2–6 Steel" Metals 9, no. 9: 916. https://doi.org/10.3390/met9090916





