Investigation of Mechanical Tests for Hydrogen Embrittlement in Automotive PHS Steels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogen Charging
2.3. Slow Strain Rate Tests
2.4. Four-Point Bending Tests
2.5. Permeation Tests
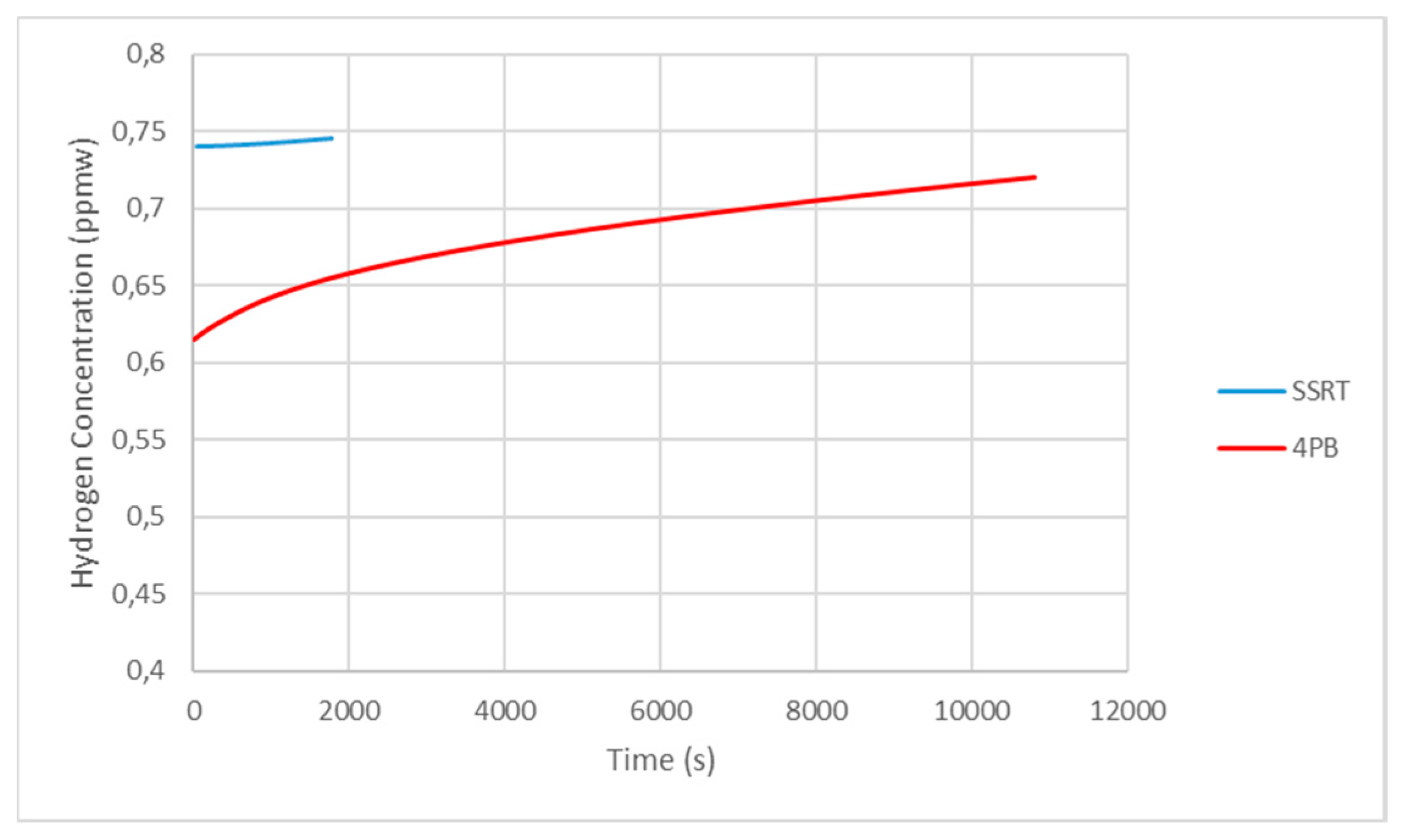
2.6. Hydrogen Measurement
3. Results
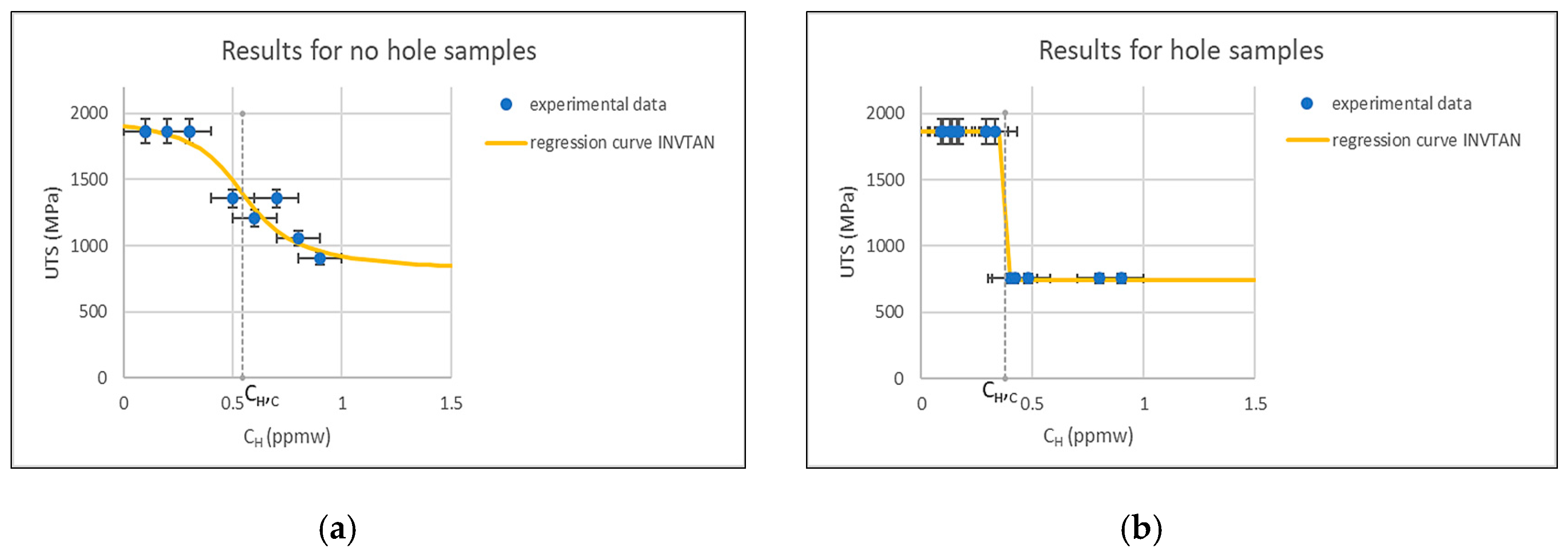
3.1. Slow Strain Rate Tests
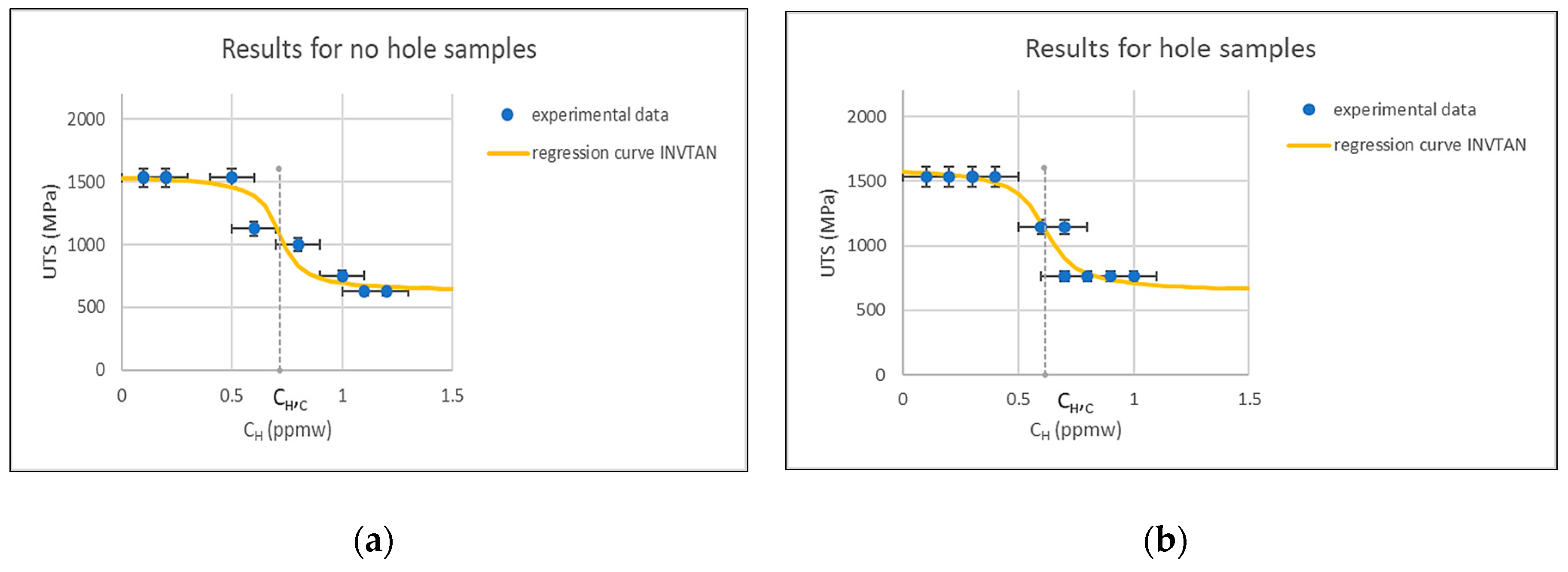
3.2. Four-Point Bending Tests
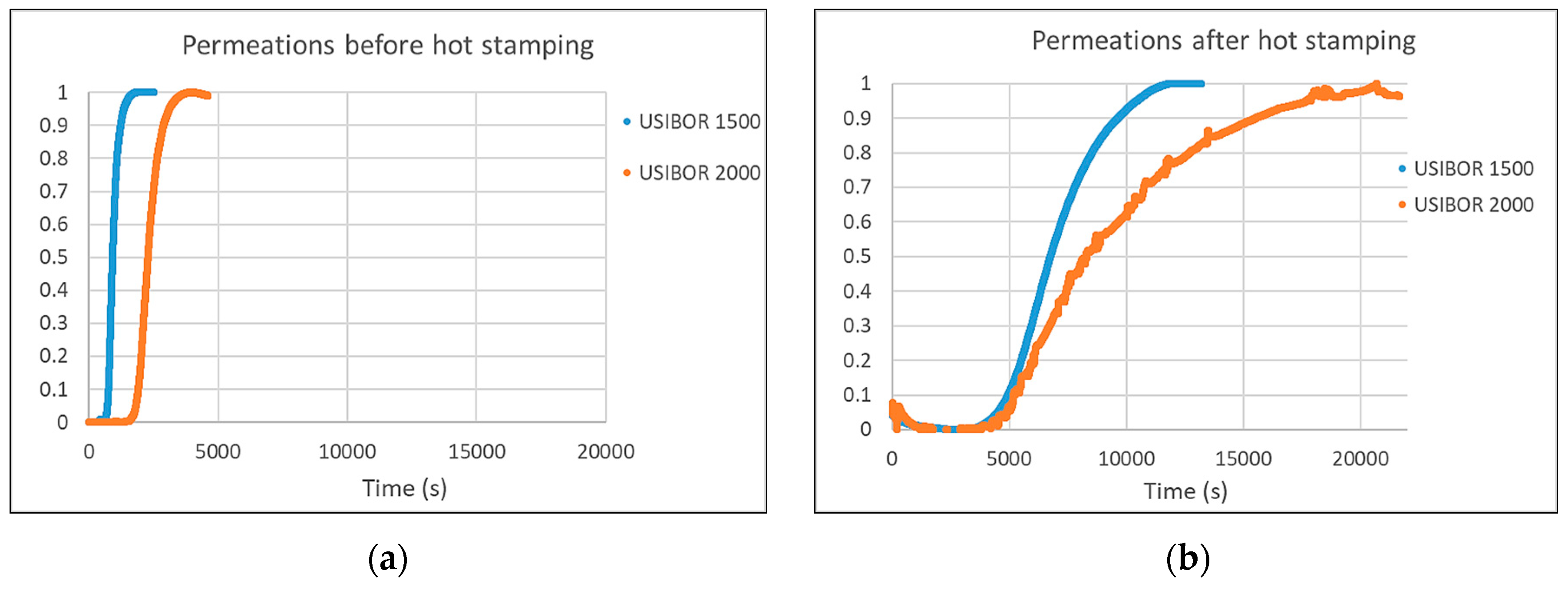
3.3. Permeation Tests
3.4. SEM Images
4. Discussion
5. Conclusions
- These steels are sensitive to hydrogen delayed fracture and the grade 2000 is considerably more sensitive than grade 1500.
- The 4 PB tests with the hole were the most severe because the failure occurred with a minimum average concentration.
- The hydrogen diffusion strongly decreased after the hot stamping and quenching process because of the martensite formation; moreover, because of the greater amount of carbon, grade 2000 presented slower diffusion.
- The differences in critical hydrogen concentration values for the mechanical tests were due to two factors:
- The effect of the deformation rate in the SSRT, which provided hydrogen with little time to diffuse near the crack tip.
- The presence of the hole in the 4 PB samples induced stress gradients that, coupled to time, created hydrogen accumulation near the crack tip.
- Finally, the 4 PB samples with the hole were absolutely the most realistic at simulating the risk of hydrogen embrittlement for this type of material. However, the SSRT is a quick method to compare the behavior of different materials in the presence of hydrogen.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- San Marchi, C.; Michler, T.; Nibur, K.A.; Somerday, B.P. On the physical differences between tensile testing of type 304 and 316 austenitic stainless steels with internal hydrogen and in external hydrogen. Int. J. Hydrogen Energy 2010, 35, 9736–9745. [Google Scholar] [CrossRef]
- Djukic, M.B.; Sijacki Zeravcic, V.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Lynch, S.P. Hydrogen Embrittlement (HE) phenomena and mechanisms. In Stress Corrosion Cracking: Theory and Practice; Raja, V.S., Shoji, T., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 90–130. [Google Scholar]
- Arcelor Mittal. Available online: https://automotive.arcelormittal.com/usibor_ductibor (accessed on 22 April 2019).
- Takaji, S.; Toji, Y. Application of NH4SCN Aqueous Solution to Hydrogen Embrittlement Resistance Evolution of Ultra-High Strength Steels. ISIJ Int. 2012, 52, 329–331. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, H.K.; Lee, C.W.; Yoo, B.G.; Jung, H.Y. Baking Effect on Desorption of Diffusible Hydrogen and Hydrogen Embrittlement on Hot-Stamped Boron Martensitic Steel. Metals 2019, 9, 636. [Google Scholar] [CrossRef]
- ASTM G129:2000. Standard Practice for Slow Strain Rate Testing to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking; ASTM: West Conshohocken, PA, USA, 2000. [Google Scholar]
- SEP 1970:2011. Test of the Resistance of Advanced High Strength Steel (AHSS) for Automotive Applications against Production Related Hydrogen Induced Brittle Fracture; Verlag Stahleisen GmbH: Düsseldorf, Germany, 2011. [Google Scholar]
- ASTM F1624:2018. Standard Test Method for Measurement of Hydrogen Embrittlement Threshold in Steel by the Incremental Step Loading Technique; ASTM: West Conshohocken, PA, USA, 2000. [Google Scholar]
- ISO 17081:2004. Method of Measurement of Hydrogen Permeation and Determination of Hydrogen Uptake and Transport in Metals by an Electrochemical Technique; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- Valentini, R. Method for Permeation Hydrogen Measurements. European Patent EP2912452, 7 December 2012. [Google Scholar]
- Lasia, A.; Gregoire, D. General Model of Electrochemical Hydrogen Absorption into Metals. J. Electrochem. Soc. 1995, 142, 3393–3399. [Google Scholar] [CrossRef]
- Georges, C.; Sturel, T.; Drillet, P.; Mataigne, J.M. Absorption/Desorption of Diffusible Hydrogen in Aluminized Boron Steel. ISIJ Int. 2013, 53, 1295–1304. [Google Scholar] [CrossRef] [Green Version]
- Lovicu, G.; Bottazzi, M.; D’Aiuto, F.; De Sanctis, M.; Di Matteo, A.; Santus, C.; Valentini, R. Hydrogen Embrittlement of Automotive Advanced High-Strength Steels. Metall. Mater. Trans. 2012, 43A, 4075–4087. [Google Scholar] [CrossRef]
- Cherubini, A.; Bacchi, L.; Corsinovi, S.; Beghini, M.; Valentini, R. Hydrogen Embrittlement in Advanced High Strength Steels: A new investigation approach. In Proceedings of the 22nd European Conference on Fracture, Belgrade, Serbia, 26–31 August 2018; Elsevier: Amsterdam, The Netherlands, 1880. [Google Scholar]
- McNabb, A.; Foster, P.K. A new analysis of the diffusion of hydrogen in iron and ferritic steels. Trans. Metall. Soc. AIME 1963, 227, 618–627. [Google Scholar]
- Brocks, W.; Felkenberg, R.; Scheider, I. Coupling aspects in the simulation of hydrogen-induced stress corrosion cracking. Procedia IUTAM 2012, 3, 11–24. [Google Scholar] [CrossRef]
- Beck, W.; Subramanyan, P.K.; Williams, F.S. Interpretation of Some Hydrogen Embrittlement Phenomena. Corrosion 1971, 27, 115–118. [Google Scholar] [CrossRef]
- Fu, L.; Fang, H. Formation Criterion of Hydrogen-Induced Cracking in Steel Based on Fracture Mechanics. Metals 2018, 8, 940. [Google Scholar] [CrossRef]
- Olden, V.; Alvaro, A.; Akselsen, O.M. Hydrogen diffusion and hydrogen influenced critical stress intensity in an API X70 pipeline steel welded joint-Experiments and FE simulations. Int. J. Hydrogen Energy 2012, 37, 11474–11486. [Google Scholar] [CrossRef]
- Valentini, R.; Tedesco, M.M.; Corsinovi, S.; Bacchi, L. Delayed Fracture in Automotive Advanced High Strength Steel: A New Investigation Approach. Steel Res. Int. 2019. accepted. [Google Scholar] [CrossRef]
- Beghini, M.; Benamati, G.; Bertini, L.; Recapito, I.; Valentini, R. Effect of hydrogen on the ductility reduction of F82H martensitic steel after different heat treatments. J. Nucl. Mater. 2001, 288, 1–6. [Google Scholar] [CrossRef]
- Pressouyre, G.M.; Faure, F.M. Quantitative Analysis of Critical Concentrations for Hydrogen Induced Cracking. In Proceedings of the Second National Symposium on Test Methods for Hydrogen Embrittlement: Prevention and Control, Los Angeles, CA, USA, 24–26 May 1985; ASTM: West Conshohocken, PA, USA, 1988. [Google Scholar]








| Material | C | Si | Mn | P | S | Al | B | Ti | Cr |
|---|---|---|---|---|---|---|---|---|---|
| USIBOR 1500® | 0.19 | 0.21 | 1.13 | 0.015 | 0.008 | 0.0038 | 0.003 | 0.031 | 0.19 |
| USIBOR 2000® | 0.38 | 0.19 | 1.21 | 0.013 | 0.006 | 0.032 | 0.0032 | 0.024 | 0.28 |
| Material | Rp,02 (MPa) | Rm (MPa) | A50 (%) |
|---|---|---|---|
| 1500 Grade | 1252 | 1485 | 7.3 |
| 2000 Grade | 1510 | 1881 | 6.2 |
| Material | Diffusion Coefficient (m2/s) | |
|---|---|---|
| Before Hot Stamping | After Hot Stamping | |
| 1500 Grade | 3.39 × 10−10 | 4.50 × 10−11 |
| 2000 Grade | 1.36 × 10−10 | 3.25 × 10−11 |
| Material | USIBOR 1500® | USIBOR 2000® |
|---|---|---|
| 4 PB Hole | 0.61 | 0.37 |
| 4 PB No Hole | 0.71 | 0.54 |
| SSRT | 0.74 | 0.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, R.; Tedesco, M.M.; Corsinovi, S.; Bacchi, L.; Villa, M. Investigation of Mechanical Tests for Hydrogen Embrittlement in Automotive PHS Steels. Metals 2019, 9, 934. https://doi.org/10.3390/met9090934
Valentini R, Tedesco MM, Corsinovi S, Bacchi L, Villa M. Investigation of Mechanical Tests for Hydrogen Embrittlement in Automotive PHS Steels. Metals. 2019; 9(9):934. https://doi.org/10.3390/met9090934
Chicago/Turabian StyleValentini, Renzo, Michele Maria Tedesco, Serena Corsinovi, Linda Bacchi, and Michele Villa. 2019. "Investigation of Mechanical Tests for Hydrogen Embrittlement in Automotive PHS Steels" Metals 9, no. 9: 934. https://doi.org/10.3390/met9090934





